Abstract
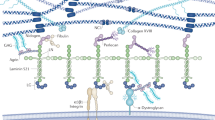
The glomerular basement membrane (GBM) is the central, non-cellular layer of the glomerular filtration barrier that is situated between the two cellular components—fenestrated endothelial cells and interdigitated podocyte foot processes. The GBM is composed primarily of four types of extracellular matrix macromolecule—laminin-521, type IV collagen α3α4α5, the heparan sulphate proteoglycan agrin, and nidogen—which produce an interwoven meshwork thought to impart both size-selective and charge-selective properties. Although the composition and biochemical nature of the GBM have been known for a long time, the functional importance of the GBM versus that of podocytes and endothelial cells for establishing the glomerular filtration barrier to albumin is still debated. Together with findings from genetic studies in mice, the discoveries of four human mutations affecting GBM components in two inherited kidney disorders, Alport syndrome and Pierson syndrome, support essential roles for the GBM in glomerular permselectivity. Here, we explain in detail the proposed mechanisms whereby the GBM can serve as the major albumin barrier and discuss possible approaches to circumvent GBM defects associated with loss of permselectivity.
Key Points
-
The glomerular basement membrane (GBM) is the extracellular matrix component of the glomerular filtration barrier; it is flanked by the podocyte and glomerular endothelial cell layers
-
The major GBM components are laminin-521, type IV collagen α3α4α5, nidogen, and the heparan sulphate proteoglycan agrin
-
Mutations in COL4 genes that result in absence of the type IV collagen α3α4α5 network cause Alport syndrome, a hereditary nephritis accompanied by hearing defects
-
Mutations in laminin β2 (LAMB2) cause Pierson syndrome, a congenital nephrotic syndrome with associated eye and neurologic abnormalities
-
Studies using mouse models of Pierson and Alport syndromes have shown that the defective GBM is more permeable to macromolecules than is the normal GBM, suggesting that it has a role in permselectivity
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Miner, J. H. Organogenesis of the kidney glomerulus: focus on the glomerular basement membrane. Organogenesis 7, 75–82 (2011).
Yurchenco, P. D. & Patton, B. L. Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 15, 1277–1294 (2009).
Miner, J. H. Building the glomerulus: a matricentric view. J. Am. Soc. Nephrol. 16, 857–861 (2005).
Kruegel, J., Rubel, D. & Gross, O. Alport syndrome—insights from basic and clinical research. Nat. Rev. Nephrol. 9, 170–178 (2013).
Matejas, V. et al. Mutations in the human laminin beta2 (LAMB2) gene and the associated phenotypic spectrum. Hum. Mutat. 31, 992–1002 (2010).
Jefferson, J. A., Shankland, S. J. & Pichler, R. H. Proteinuria in diabetic kidney disease: a mechanistic viewpoint. Kidney Int. 74, 22–36 (2008).
de Boer, I. H. et al. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 305, 2532–2539 (2011).
Farquhar, M. G. The glomerular basement membrane: not gone, just forgotten. J. Clin. Invest. 116, 2090–2093 (2006).
Miner, J. H. The glomerular basement membrane. Exp. Cell Res. 318, 973–978 (2012).
St John, P. L. & Abrahamson, D. R. Glomerular endothelial cells and podocytes jointly synthesize laminin-1 and -11 chains. Kidney Int. 60, 1037–1046 (2001).
Eremina, V. et al. Glomerular-specific alterations of VEGF-A expression lead to distinct congenital and acquired renal diseases. J. Clin. Invest. 111, 707–716 (2003).
Farquhar, M. G. Editorial: The primary glomerular filtration barrier—basement membrane or epithelial slits? Kidney Int. 8, 197–211 (1975).
Farquhar, M. G. & Palade, G. E. Glomerular permeability. II. Ferritin transfer across the glomerular capillary wall in nephrotic rats. J. Exp. Med. 114, 699–716 (1961).
Farquhar, M. G., Wissig, S. L. & Palade, G. E. Glomerular permeability. I. Ferritin transfer across the normal glomerular capillary wall. J. Exp. Med. 113, 47–66 (1961).
Brenner, B. M., Hostetter, T. H. & Humes, H. D. Molecular basis of proteinuria of glomerular origin. N. Engl. J. Med. 298, 826–833 (1978).
Miner, J. H. & Yurchenco, P. D. Laminin functions in tissue morphogenesis. Annu. Rev. Cell. Dev. Biol. 20, 255–284 (2004).
Paulsson, M. Basement membrane proteins: structure, assembly, and cellular interactions. Crit. Rev. Biochem. Molec. Biol. 27, 93–127 (1992).
Ekblom, P. & Timpl, R. Cell-to-cell contact and extracellular matrix. A multifaceted approach emerging. Curr. Opin. Cell Biol. 8, 599–601 (1996).
Yurchenco, P. D. & Cheng, Y. S. Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J. Biol. Chem. 268, 17286–17299 (1993).
Cheng, Y. S., Champliaud, M. F., Burgeson, R. E., Marinkovich, M. P. & Yurchenco, P. D. Self-assembly of laminin isoforms. J. Biol. Chem. 272, 31525–31532 (1997).
Timpl, R. et al. Structure and function of laminin LG modules. Matrix Biol. 19, 309–317 (2000).
Colognato, H. & Yurchenco, P. D. Form and function: the laminin family of heterotrimers. Dev. Dyn. 218, 213–234 (2000).
Henry, M. D. & Campbell, K. P. Dystroglycan inside and out. Curr. Opin. Cell Biol. 11, 602–607 (1999).
Hynes, R. O. Integrins: bidirectional, allosteric signaling machines. Cell 110, 673–687 (2002).
Kreidberg, J. A. et al. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development 122, 3537–3547 (1996).
Kikkawa, Y., Sanzen, N. & Sekiguchi, K. Isolation and characterization of laminin-10/11 secreted by human lung carcinoma cells. laminin-10/11 mediates cell adhesion through integrin alpha3 beta1. J. Biol. Chem. 273, 15854–15859 (1998).
Kikkawa, Y., Virtanen, I. & Miner, J. H. Mesangial cells organize the glomerular capillaries by adhering to the G domain of laminin alpha5 in the glomerular basement membrane. J. Cell Biol. 161, 187–196 (2003).
Wizemann, H. et al. Distinct requirements for heparin and alpha-dystroglycan binding revealed by structure-based mutagenesis of the laminin alpha2 LG4-LG5 domain pair. J. Mol. Biol. 332, 635–642 (2003).
Jarad, G., Pippin, J. W., Shankland, S. J., Kreidberg, J. A. & Miner, J. H. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am. J. Physiol. Renal Physiol. 300, F811–F820 (2011).
Chen, Y. M. & Miner, J. H. Glomerular basement membrane and related glomerular disease. Transl. Res. 160, 291–297 (2012).
Colognato, H., Winkelmann, D. A. & Yurchenco, P. D. Laminin polymerization induces a receptor-cytoskeleton network. J. Cell Biol. 145, 619–631 (1999).
Poschl, E. et al. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 131, 1619–1628 (2004).
Smyth, N. et al. Absence of basement membranes after targeting the LAMC1 gene results in embryonic lethality due to failure of endoderm differentiation. J. Cell Biol. 144, 151–160 (1999).
Vanacore, R. et al. A sulfilimine bond identified in collagen IV. Science 325, 1230–1234 (2009).
Hudson, B. G. The molecular basis of Goodpasture and Alport syndromes: beacons for the discovery of the collagen IV family. J. Am. Soc. Nephrol. 15, 2514–2527 (2004).
Miner, J. H. Developmental biology of glomerular basement membrane components. Curr. Opin. Nephrol. Hypertens. 7, 13–19 (1998).
Gunwar, S. et al. Glomerular basement membrane. Identification of a novel disulfide-cross-linked network of alpha3, alpha4, and alpha5 chains of type IV collagen and its implications for the pathogenesis of Alport syndrome. J. Biol. Chem. 273, 8767–8775 (1998).
Kohfeldt, E., Sasaki, T., Gohring, W. & Timpl, R. Nidogen-2: a new basement membrane protein with diverse binding properties. J. Mol. Biol. 282, 99–109 (1998).
Timpl, R. Structure and biological activity of basement membrane proteins. Eur. J. Biochem. 180, 487–502 (1989).
Miosge, N., Sasaki, T. & Timpl, R. Evidence of nidogen-2 compensation for nidogen-1 deficiency in transgenic mice. Matrix Biol. 21, 611–621 (2002).
Miosge, N. et al. Ultrastructural colocalization of nidogen-1 and nidogen-2 with laminin-1 in murine kidney basement membranes. Histochem. Cell Biol. 113, 115–124 (2000).
Schymeinsky, J. et al. Gene structure and functional analysis of the mouse nidogen-2 gene: nidogen-2 is not essential for basement membrane formation in mice. Mol. Cell. Biol. 22, 6820–6830 (2002).
Murshed, M. et al. The absence of nidogen 1 does not affect murine basement membrane formation. Mol. Cell. Biol. 20, 7007–7012 (2000).
Bader, B. L. et al. Compound genetic ablation of nidogen 1 and 2 causes basement membrane defects and perinatal lethality in mice. Mol. Cell. Biol. 25, 6846–6856 (2005).
Groffen, A. J. et al. Agrin is a major heparan sulfate proteoglycan in the human glomerular basement membrane. J. Histochem. Cytochem. 46, 19–27 (1998).
Harvey, S. J. et al. Disruption of glomerular basement membrane charge through podocyte-specific mutation of agrin does not alter glomerular permselectivity. Am. J. Pathol. 171, 139–152 (2007).
Rennke, H. G., Cotran, R. S. & Venkatachalam, M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J. Cell Biol. 67, 638–646 (1975).
Bezakova, G. & Ruegg, M. A. New insights into the roles of agrin. Nat. Rev. Mol. Cell Biol. 4, 295–308 (2003).
Park, J. E., Keller, G. A. & Ferrara, N. The vascular endothelial growth factor (VEGF) isoforms: differential deposition into the subepithelial extracellular matrix and bioactivity of extracellular matrix-bound VEGF. Mol. Biol. Cell 4, 1317–1326 (1993).
Goldberg, S., Harvey, S. J., Cunningham, J., Tryggvason, K. & Miner, J. H. Glomerular filtration is normal in the absence of both agrin and perlecan-heparan sulfate from the glomerular basement membrane. Nephrol. Dial. Transplant. 24, 2044–2051 (2009).
Morita, H. et al. Heparan sulfate of perlecan is involved in glomerular filtration. J. Am. Soc. Nephrol. 16, 1703–1710 (2005).
Pierson, M., Cordier, J., Hervouuet, F. & Rauber, G. An unusual congenital and familial congenital malformative combination involving the eye and kidney. J. Genet. Hum. 12, 184–213 (1963).
Zenker, M. et al. Human laminin beta2 deficiency causes congenital nephrosis with mesangial sclerosis and distinct eye abnormalities. Hum. Mol. Genet. 13, 2625–2632 (2004).
Zenker, M., Pierson, M., Jonveaux, P. & Reis, A. Demonstration of two novel LAMB2 mutations in the original Pierson syndrome family reported 42 years ago. Am. J. Med. Genet. A 138, 73–74 (2005).
Lehnhardt, A. et al. Pierson syndrome in an adolescent girl with nephrotic range proteinuria but a normal GFR. Pediatr. Nephrol. 27, 865–868 (2012).
Kagan, M., Cohen, A. H., Matejas, V., Vlangos, C. & Zenker, M. A milder variant of Pierson syndrome. Pediatr. Nephrol. 23, 323–327 (2008).
Hasselbacher, K. et al. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 70, 1008–1012 (2006).
Chen, Y. M., Kikkawa, Y. & Miner, J. H. A missense LAMB2 mutation causes congenital nephrotic syndrome by impairing laminin secretion. J. Am. Soc. Nephrol. 22, 849–858 (2011).
Purvis, A. & Hohenester, E. Laminin network formation studied by reconstitution of ternary nodes in solution. J. Biol. Chem. 287, 44270–44277 (2012).
Noakes, P. G. et al. The renal glomerulus of mice lacking s-laminin/laminin beta 2: nephrosis despite molecular compensation by laminin beta 1. Nat. Genet. 10, 400–406 (1995).
Noakes, P. G., Gautam, M., Mudd, J., Sanes, J. R. & Merlie, J. P. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin beta 2. Nature 374, 258–262 (1995).
Jarad, G., Cunningham, J., Shaw, A. S. & Miner, J. H. Proteinuria precedes podocyte abnormalities in Lamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J. Clin. Invest. 116, 2272–2279 (2006).
Ryan, G. B. & Karnovsky, M. J. Distribution of endogenous albumin in the rat glomerulus: role of hemodynamic factors in glomerular barrier function. Kidney Int. 9, 36–45 (1976).
Wartiovaara, J. et al. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J. Clin. Invest. 114, 1475–1483 (2004).
Noone, D. & Licht, C. An update on the pathomechanisms and future therapies of Alport syndrome. Pediatr. Nephrol. 28, 1025–1036 (2013).
Abrahamson, D. R., Hudson, B. G., Stroganova, L., Borza, D. B. & St John, P. L. Cellular origins of type IV collagen networks in developing glomeruli. J. Am. Soc. Nephrol. 20, 1471–1479 (2009).
Abrahamson, D. R. et al. Laminin compensation in collagen alpha3(IV) knockout (Alport) glomeruli contributes to permeability defects. J. Am. Soc. Nephrol. 18, 2465–2472 (2007).
Gross, O. et al. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 63, 438–446 (2003).
Gross, O. et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 81, 494–501 (2012).
Wyss, H. M. et al. Biophysical properties of normal and diseased renal glomeruli. Am. J. Physiol. Cell Physiol. 300, C397–C405 (2011).
Meehan, D. T. et al. Biomechanical strain causes maladaptive gene regulation, contributing to Alport glomerular disease. Kidney Int. 76, 968–976 (2009).
Smithies, O. Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc. Natl Acad. Sci. USA 100, 4108–4113 (2003).
Suh, J. H., Jarad, G., Vandevoorde, R. G. & Miner, J. H. Forced expression of laminin beta1 in podocytes prevents nephrotic syndrome in mice lacking laminin beta2, a model for Pierson syndrome. Proc. Natl Acad. Sci. USA 108, 15348–15353 (2011).
Chen, Y. M. et al. Laminin β2 gene missense mutation produces endoplasmic reticulum stress in podocytes. J. Am. Soc. Nephrol. http://dx.doi.org/10.1681/ASN.2012121149.
Kikkawa, Y. & Miner, J. H. Molecular dissection of laminin alpha 5 in vivo reveals separable domain-specific roles in embryonic development and kidney function. Dev. Biol. 296, 265–277 (2006).
Brinkkoetter, P. T., Ising, C. & Benzing, T. The role of the podocyte in albumin filtration. Nat. Rev. Nephrol. http://dx.doi.org/10.1038/nrneph.2013.78.
Faul, C., Asanuma, K., Yanagida-Asanuma, E., Kim, K. & Mundel, P. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol. 17, 428–437 (2007).
Acknowledgements
The authors are supported by NIH grants R01DK078314, R21DK095419, and P30DK079333 and by a grant from the Alport Syndrome Foundation. J. H. Suh is also supported by NIH training grant T32DK007126.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Suh, J., Miner, J. The glomerular basement membrane as a barrier to albumin. Nat Rev Nephrol 9, 470–477 (2013). https://doi.org/10.1038/nrneph.2013.109
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2013.109
This article is cited by
-
Complexities of the glomerular basement membrane
Nature Reviews Nephrology (2021)
-
The Potential of Albuminuria as a Biomarker of Diabetic Complications
Cardiovascular Drugs and Therapy (2021)
-
Type IV collagen and diabetic kidney disease
Nature Reviews Nephrology (2020)
-
Isotopic Nitrogen-15 Labeling of Mice Identified Long-lived Proteins of the Renal Basement Membranes
Scientific Reports (2020)
-
Prostaglandin E1 attenuates high glucose-induced apoptosis in proximal renal tubular cells by inhibiting the JNK/Bim pathway
Acta Pharmacologica Sinica (2020)