Abstract
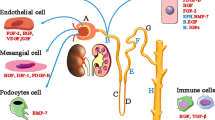
Klotho is a single-pass transmembrane protein that is highly expressed in the kidney and is known to act as a coreceptor for fibroblast growth factor 23. The extracellular domain can be produced independently or shed from membrane-bound Klotho and functions as an endocrine substance with multiple functions including antioxidation, modulation of ion transport, suppression of fibrosis, and preservation of stem cells. Emerging evidence has revealed that Klotho deficiency is an early event in acute kidney injury (AKI), and a pathogenic factor that exacerbates acute kidney damage and contributes to long-term consequences. Restoration by exogenous supplementation or stimulation of endogenous Klotho might prevent and ameliorate injury, promote recovery, and suppress fibrosis to mitigate development of chronic kidney disease. Although data are still emerging, in this Perspectives article we discuss why this renal-derived protein is a highly promising candidate as both an early biomarker and therapeutic agent for AKI.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Fonseca Ruiz, N. J., Castro, D. P., Guerra, A. M., Saldarriaga, F. M. & Hernandez, J. D. Renal injury study in critical ill patients in accordance with the new definition given by the Acute Kidney Injury Network. J. Crit. Care 26, 206–212 (2011).
Akcan-Arikan, A. et al. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 71, 1028–1035 (2007).
Waikar, S. S., Curhan, G. C., Ayanian, J. Z. & Chertow, G. M. Race and mortality after acute renal failure. J. Am. Soc. Nephrol. 18, 2740–2748 (2007).
Wald, R. et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302, 1179–1185 (2009).
Hsu, C. Y. et al. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin. J. Am. Soc. Nephrol. 4, 891–898 (2009).
Kuro-o, M. et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390, 45–51 (1997).
Ito, S. et al. Molecular cloning and expression analyses of mouse βklotho, which encodes a novel Klotho family protein. Mech. Dev. 98, 115–119 (2000).
Tomiyama, K. et al. Relevant use of Klotho in FGF19 subfamily signaling system in vivo. Proc. Natl Acad. Sci. USA 107, 1666–1671 (2010).
Kurosu, H. et al. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 281, 6120–6123 (2006).
Kurosu, H. et al. Tissue-specific expression of βKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 282, 26687–26695 (2007).
Goetz, R. et al. Molecular insights into the klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol. Cell. Biol. 27, 3417–3428 (2007).
Matsumura, Y. et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem. Biophys. Res. Commun. 242, 626–630 (1998).
Shiraki-Iida, T. et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 424, 6–10 (1998).
Imura, A. et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 565, 143–147 (2004).
Hu, M. C. et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 24, 3438–3450 (2010).
Chen, C. D., Podvin, S., Gillespie, E., Leeman, S. E. & Abraham, C. R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl Acad. Sci. USA 104, 19796–19801 (2007).
Bloch, L. et al. Klotho is a substrate for α-, β- and γ-secretase. FEBS Lett. 583, 3221–3224 (2009).
Liu, H. et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science 317, 803–806 (2007).
Kurosu, H. et al. Suppression of aging in mice by the hormone Klotho. Science 309, 1829–1833 (2005).
Liu, F., Wu, S., Ren, H. & Gu, J. Klotho suppresses RIG-I-mediated senescence-associated inflammation. Nat. Cell Biol. 13, 254–262 (2011).
German, D. C., Khobahy, I., Pastor, J., Kuro, O. M. & Liu, X. Nuclear localization of Klotho in brain: an anti-aging protein. Neurobiol. Aging http://dx.doi.org/10.1016/j.neurobiolaging.2011.12.018.
Hu, M. C. et al. Klotho deficiency is an early biomarker of renal ischemia-reperfusion injury and its replacement is protective. Kidney Int. 78, 1240–1251 (2010).
Sugiura, H. et al. Klotho reduces apoptosis in experimental ischaemic acute kidney injury via HSP-70. Nephrol. Dial. Transplant. 25, 60–68 (2010).
Mitobe, M. et al. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp. Nephrol. 101, e67–e74 (2005).
Maekawa, Y. et al. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr. Gerontol. Int. 11, 510–516 (2011).
Rakugi, H. et al. Anti-oxidative effect of Klotho on endothelial cells through cAMP activation. Endocrine 31, 82–87 (2007).
Hu, M. C., Kuro-o, M. & Moe, O. W. Secreted klotho and chronic kidney disease. Adv. Exp. Med. Biol. 728, 126–157 (2012).
Doi, S. et al. Klotho inhibits transforming growth factor-β1 (TGF-β1) signaling and suppresses renal fibrosis and cancer metastasis in mice. J. Biol. Chem. 286, 8655–8665 (2011).
Ohyama, Y. et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem. Biophys. Res. Commun. 251, 920–925 (1998).
Tang, C. et al. Downregulation of Klotho expression by dehydration. Am. J. Physiol. Renal Physiol. 301, F745–F750 (2011).
Panesso, M. C. et al. Klotho ameliorates cisplatin nephrotoxicity. J. Am. Soc. Nephrol. 22 (Suppl.), TH-PO027 (2011).
Moreno, J. A. et al. The inflammatory cytokines TWEAK and TNFα reduce renal Klotho expression through NFκB. J. Am. Soc. Nephrol. 22, 1315–1325 (2011).
Sugiura, H. et al. Klotho reduces apoptosis in experimental ischaemic acute renal failure. Nephrol. Dial. Transplant. 20, 2636–2645 (2005).
Ohata, Y. et al. Circulating levels of soluble α-Klotho are markedly elevated in human umbilical cord blood. J. Clin. Endocrinol. Metab. 96, E943–E947 (2011).
Semba, R. D. et al. Plasma klotho and mortality risk in older community-dwelling adults. J. Gerontol. A Biol. Sci. Med. Sci. 66, 794–800 (2011).
Semba, R. D. et al. Relationship of low plasma klotho with poor grip strength in older community-dwelling adults: the InCHIANTI study. Eur. J. Appl. Physiol. 112, 1215–1220 (2012).
Devaraj, S., Syed, B., Chien, A. & Jialal, I. Validation of an immunoassay for soluble klotho protein: decreased levels in diabetes and increased levels in chronic kidney disease. Am. J. Clin. Pathol. 137, 479–485 (2012).
Semba, R. D. et al. Plasma Klotho and cardiovascular disease in adults. J. Am. Geriatr. Soc. 59, 1596–1601 (2011).
Pavik, I. et al. Soluble klotho and autosomal dominant polycystic kidney disease. Clin. J. Am. Soc. Nephrol. 7, 248–257 (2012).
Yamazaki, Y. et al. Establishment of sandwich ELISA for soluble α-Klotho measurement: age-dependent change of soluble α-Klotho levels in healthy subjects. Biochem. Biophys. Res. Commun. 398, 513–518 (2010).
Huang, C. L. & Moe, O. W. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflugers Arch. 462, 185–193 (2011).
Schrier, R. W. Diagnostic value of urinary sodium, chloride, urea, and flow. J. Am. Soc. Nephrol. 22, 1610–1613 (2011).
Parikh, C. R. et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J. Am. Soc. Nephrol. 22, 1748–1757 (2011).
Parikh, C. R. et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after pediatric cardiac surgery. J. Am. Soc. Nephrol. 22, 1737–1747 (2011).
Perco, P. & Oberbauer, R. Kidney injury molecule-1 as a biomarker of acute kidney injury in renal transplant recipients. Nat. Clin. Pract. Nephrol. 4, 362–363 (2008).
Vaidya, V. S. et al. A rapid urine test for early detection of kidney injury. Kidney Int. 76, 108–114 (2009).
Devarajan, P. Biomarkers for the early detection of acute kidney injury. Curr. Opin. Pediatr. 23, 194–200 (2011).
Liang, X. L. et al. Combination of urinary kidney injury molecule-1 and interleukin-18 as early biomarker for the diagnosis and progressive assessment of acute kidney injury following cardiopulmonary bypass surgery: a prospective nested case-control study. Biomarkers 15, 332–339 (2010).
King, G. D., Rosene, D. L. & Abraham, C. R. Promoter methylation and age-related downregulation of Klotho in rhesus monkey. Age (Dordr.) http://dx.doi.10.1007/s11357-011-9315-4.
Thurston, R. D. et al. Tumor necrosis factor and interferon-γ down-regulate Klotho in mice with colitis. Gastroenterology 138, 1384–1394 (2010).
Zarjou, A., Yang, S., Abraham, E., Agarwal, A. & Liu, G. Identification of a microRNA signature in renal fibrosis: role of miR-21. Am. J. Physiol. Renal Physiol. 301, F793–F801 (2011).
Bhatt, K., Mi, Q. S. & Dong, Z. microRNAs in kidneys: biogenesis, regulation, and pathophysiological roles. Am. J. Physiol. Renal Physiol. 300, F602–F610 (2011).
Madias, N. E. & Harrington, J. T. Platinum nephrotoxicity. Am. J. Med. 65, 307–314 (1978).
Haruna, Y. et al. Amelioration of progressive renal injury by genetic manipulation of Klotho gene. Proc. Natl Acad. Sci. USA 104, 2331–2336 (2007).
de Oliveira, R. M. Klotho RNAi induces premature senescence of human cells via a p53/p21 dependent pathway. FEBS Lett. 580, 5753–5758 (2006).
Ortiz, A., Gonzalez Cuadrado, S., Lorz, C. & Egido, J. Apoptosis in renal diseases. Front. Biosci. 1, d30–d47 (1996).
Yamamoto, M. et al. Regulation of oxidative stress by the anti-aging hormone Klotho. J. Biol. Chem. 280, 38029–38034 (2005).
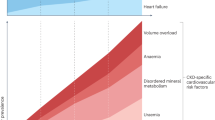
Chawla, L. S., Amdur, R. L., Amodeo, S., Kimmel, P. L. & Palant, C. E. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 79, 1361–1369 (2011).
Forbes, J. M., Hewitson, T. D., Becker, G. J. & Jones, C. L. Ischemic acute renal failure: long-term histology of cell and matrix changes in the rat. Kidney Int. 57, 2375–2385 (2000).
Basile, D. P., Donohoe, D., Roethe, K. & Osborn, J. L. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am. J. Physiol. Renal Physiol. 281, F887–F899 (2001).
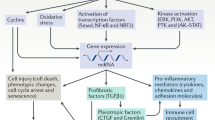
Yang, L., Besschetnova, T. Y., Brooks, C. R., Shah, J. V. & Bonventre, J. V. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat. Med. 16, 535–543 (2010).
Sugiura, H. et al. TGF-β was upregulated in renal fibrosis model of Klotho defect mouse and affected renal Klotho expression level [abstract]. J. Am. Soc. Nephrol. 21, 376A (2010).
Samarakoon, R., Overstreet, J. M., Higgins, S. P. & Higgins, P. J. TGF-β1 → SMAD/p53/USF2 → PAI-1 transcriptional axis in ureteral obstruction-induced renal fibrosis. Cell Tissue Res. 347, 117–128 (2012).
Li, X. et al. Synergistic effect of hypoxia and TNF-α on production of PAI-1 in human proximal renal tubular cells. Kidney Int. 68, 569–583 (2005).
Takeshita, K. et al. Increased expression of plasminogen activator inhibitor-1 with fibrin deposition in a murine model of aging, “Klotho” mouse. Semin. Thromb. Hemost. 28, 545–554 (2002).
Sugiura, H. et al. Reduced Klotho expression level in kidney aggravates renal interstitial fibrosis. Am. J. Physiol. Renal Physiol. http://dx.doi:10.1152/ajprenal.00294-2011.
Basile, D. P. The endothelial cell in ischemic acute kidney injury: implications for acute and chronic function. Kidney Int. 72, 151–156 (2007).
Brodsky, S. V. et al. Endothelial dysfunction in ischemic acute renal failure: rescue by transplanted endothelial cells. Am. J. Physiol. Renal Physiol. 282, F1140–F1149 (2002).
Gueler, F. et al. Postischemic acute renal failure is reduced by short-term statin treatment in a rat model. J. Am. Soc. Nephrol. 13, 2288–2298 (2002).
Chade, A. R. et al. Simvastatin promotes angiogenesis and prevents microvascular remodeling in chronic renal ischemia. FASEB J. 20, 1706–1708 (2006).
Narumiya, H. et al. HMG-CoA reductase inhibitors up-regulate anti-aging klotho mRNA via RhoA inactivation in IMCD3 cells. Cardiovasc. Res. 64, 331–336 (2004).
King, G. D. et al. Identification of novel small molecules that elevate Klotho expression. Biochem. J. 441, 453–461 (2012).
Yamagishi, T. et al. Troglitazone improves endothelial function and augments renal klotho mRNA expression in Otsuka Long-Evans Tokushima Fatty (OLETF) rats with multiple atherogenic risk factors. Hypertens. Res. 24, 705–709 (2001).
Acknowledgements
The authors were supported by the NIH (R01-DK091392 and R01-DK092461), the George M. O'Brien Kidney Research Center/University of Texas Southwestern Medical Center (P30-DK-07938), American Heart Association (0865235F), the Simmons Family Foundation and a Seed Grant from the Charles and Jane Pak Center for Mineral Metabolism and Clinical Research, University of Texas Southwestern Medical Center. The authors would like to thank M. Kuro-o for long-term valuable collaboration, and M. Shi for expert assistance with some key experiments performed in the authors' laboratories cited in this article.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
M.–C. Hu has received grant support from the American Heart Association and NIH. O. W. Moe has received grant support from the NIH and Simmons Family Foundation.
Rights and permissions
About this article
Cite this article
Hu, MC., Moe, O. Klotho as a potential biomarker and therapy for acute kidney injury. Nat Rev Nephrol 8, 423–429 (2012). https://doi.org/10.1038/nrneph.2012.92
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.92
This article is cited by
-
BMSC-derived exosomes protect against kidney injury through regulating klotho in 5/6 nephrectomy rats
European Journal of Medical Research (2022)
-
A systematic review and meta-analysis demonstrating Klotho as an emerging exerkine
Scientific Reports (2022)
-
Klotho inhibits the formation of calcium oxalate stones by regulating the Keap1-Nrf2-ARE signaling pathway
International Urology and Nephrology (2022)
-
Association of fibroblast growth factor 23 and α-klotho in hemodialysis patients during administration of ferric citrate hydrate: post hoc analysis of ASTRIO study
BMC Nephrology (2021)
-
Post-kidney transplant soluble Klotho levels are determined by pretransplant soluble Klotho levels in both living donors and recipients
Clinical and Experimental Nephrology (2021)