Abstract
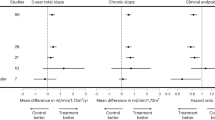
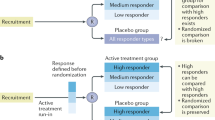
Surrogate end points have the potential to facilitate drug development because effects on surrogate end points can sometimes be demonstrated more rapidly and in smaller studies than can effects on clinical outcomes of interest. Proteinuria has been repeatedly proposed as a surrogate end point for renal outcomes in drug development; however, the FDA has generally not accepted effects on proteinuria as evidence of a drug's effectiveness. Proteinuria is an early marker of some kidney diseases and increases in proteinuria can predict risk of disease progression. Whether or not treatment effects on proteinuria can reliably predict treatment effects on renal outcomes is not known. Nevertheless, it may be reasonable to use effects on proteinuria as a basis for accelerated approval of a drug if certain conditions are met. Approval under this pathway carries with it a requirement to complete a study after drug approval that verifies the anticipated treatment benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Levey, A. S. et al. Proteinuria as a surrogate outcome in CKD: report of a scientific workshop sponsored by the National Kidney Foundation and the US Food and Drug Administration. Am. J. Kidney Dis. 54, 205–226 (2009).
Temple, R. Are surrogate markers adequate to assess cardiovascular disease drugs? JAMA 282, 790–795 (1999).
Fleming T. R. & DeMets, D. L. Surrogate end points in clinical trials: are we being misled? Ann. Intern. Med. 125, 605–613 (1996).
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8, 431–440 (1989).
Desai, M., Stockbridge, N. & Temple, R. Blood pressure as an example of a biomarker that functions as a surrogate. AAPS J. 8, E146–E152 (2006).
Foley, R. N. et al. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am. J. Kidney Dis. 28, 53–61 (1996).
Locatelli, F. et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transplant. 19, 121–132 (2004).
Besarab, A. et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N. Engl. J. Med. 339, 584–590 (1998).
Besarab, A., Goodkin, D. A. & Nissenson, A. R. The normal hematocrit study—follow-up. N. Engl. J. Med. 358, 433–434 (2008).
Pfeffer, M. A. et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N. Engl. J. Med. 361, 2019–2032 (2009).
Singh, A. K. et al. Correction of anemia with epoetin alfa in chronic kidney disease. N. Engl. J. Med. 355, 2085–2098 (2006).
Drugs@FDA: FDA Approved Drug Products. Drug label approved on 24 June 2011 for Aranesp (darbepoetin alpha) [online], (2011).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Bakris, G. L. et al. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet 375, 1173–1181 (2010).
Temple, R. Enrichment of clinical study populations. Clin. Pharmacol. Ther. 88, 774–778 (2010).
Acknowledgements
The author thanks Drs Norman Stockbridge and Robert Temple from the FDA's Center for Drug Evaluation and Research for their valuable comments on this manuscript.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Thompson, A. Proteinuria as a surrogate end point—more data are needed. Nat Rev Nephrol 8, 306–309 (2012). https://doi.org/10.1038/nrneph.2012.43
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.43
This article is cited by
-
Biomarkers and surrogate endpoints in kidney disease
Pediatric Nephrology (2016)
-
The glycocalyx—linking albuminuria with renal and cardiovascular disease
Nature Reviews Nephrology (2015)
-
Microalbuminuria: target for renoprotective therapy PRO
Kidney International (2014)
-
Biomarkers in kidney fibrosis: are they useful?
Kidney International Supplements (2014)
-
Therapeutic approaches to diabetic nephropathy—beyond the RAS
Nature Reviews Nephrology (2014)