Abstract
Shiga toxin-producing Escherichia coli-associated haemolytic uraemic syndrome (STEC-HUS) is one of the most important causes of acute kidney injury in patients of all ages, especially in children. It can occur sporadically or in outbreaks. STEC-HUS is a systemic illness caused by toxin-mediated injury to the vascular endothelium and a generalized inflammatory response. The kidney and the brain are the two primary target organs. Nearly 40% of patients with STEC-HUS require at least temporary renal replacement therapy and up to 20% will have permanent residual kidney dysfunction. Neurological injury can be sudden and severe and is the most frequent cause of acute mortality in patients with STEC-HUS. Over the past 30 years, a wide range of inflammatory mediators have been linked to the pathogenesis of STEC-HUS and associated renal and neurological complications. Recently, evidence has accumulated that abnormal activation of the alternative pathway of complement occurs in patients with STEC-HUS. In the large outbreak of STEC-HUS caused by E. coli O104:H4 that occurred in Germany in May 2011, a large number of patients received eculizumab, a monoclonal antibody directed against C5, in an open-label manner. We describe the experience with eculizumab under these emergent circumstances at one large centre.
Key Points
-
The kidney and the brain are the two principal target organs in patients with Shiga toxin-producing Escherichia coli-associated haemolytic uraemic syndrome (STEC-HUS)
-
Outbreaks of enteritis and STEC-HUS caused by novel bacterial strains continue to occur and might be associated with unique clinical features
-
The pathophysiology of organ injury in the kidney and the brain might differ and could influence the response to treatment and long-term outcomes
-
Nearly 40% of patients with STEC-HUS require renal replacement therapy and brain involvement is the most frequent cause of fatalities
-
Activation of the alternative complement pathway may contribute to the pathogenesis of STEC-HUS and further clinical research is needed to determine if blockade of this pathway attenuates the disease
Similar content being viewed by others
Introduction
Haemolytic uraemic syndrome (HUS) is defined as the triad of anaemia, thrombocytopaenia, and acute kidney injury (AKI). Shiga toxin (Stx)-producing Escherichia coli (STEC) that cause a prodromal haemorrhagic enteritis remain the most common aetiology of this illness. Although sporadic cases and outbreaks linked to unusual E. coli strains or enteric bacteria and other micro-organisms such as pneumococci continue to emerge, the predominant serotype associated with HUS around the world is still O157:H7.1 All causative enteric bacteria share the common property of producing Stx. As a consequence, the phrase diarrhoea-associated HUS or 'D+HUS' has generally been replaced by the more accurate term STEC-HUS to highlight the pivotal role of Stx in the pathogenesis of the disorder. The two principal target organs of Stx-mediated damage are the kidney and the central nervous system (CNS). Renal involvement in STEC-HUS is clinically relevant because dysfunction can be treated with temporary renal replacement therapy (haemodialysis or peritoneal dialysis). Neurological involvement is less common, usually not amenable to treatment, often the acute cause of mortality, and can result in serious long-term disability in those who survive the episode of illness.
In this Review, we focus on the clinical presentation and treatment of renal and neurological involvement in patients with STEC-HUS. We address the potential role of complement activation in the pathogenesis and eculizumab in the treatment of these complications. We present preclinical data in experimental models when available, followed by a description of the clinical manifestations of STEC-HUS. An in-depth profile is given of the experience at a single centre that was affected by the German outbreak of STEC-HUS caused by E. coli O104:H4 to highlight the experimental treatments that were prescribed to affected patients under these emergent conditions.
Definition
STEC-HUS is defined as the triad of haemolytic anaemia with erythrocyte fragmentation, thrombocytopaenia, and AKI that occurs after a prodromal infection by a Stx-producing strain of bacteria. The criterion for anaemia is age-dependent and sex-dependent and the confirmation of the microangiopathic character is based on microscopic review of the peripheral smear and detection of schistocytes. The platelet count that is diagnostic of STEC-HUS is <150 × 109/l. The definition of renal dysfunction varies between reports and ranges from abnormalities in the urinalysis to an elevation in the serum creatinine concentration above the 95th percentile for age and sex. Variations in the stringency of the definition of kidney disease may account for some of the variation in the reported incidence and severity of HUS.2 At the tissue level, HUS is characterized by the presence of thrombotic microangiopathy. This histopathological lesion is associated with endothelial damage in arterioles and capillaries with detachment of cells from the underlying basement membrane, deposition of amorphous material in the subendothelial space, and the presence of platelet–fibrin thrombi in the vascular lumen.3
Epidemiology
The incidence of STEC-HUS ranges from 6 in 100,000 children aged <5 years, to 2 in 100,000 in the overall population including adults.1,3 E. coli O157:H7 remains the most common strain that causes STEC-HUS with a minority of cases caused by other serotypes such as O111 and O26.1,4 STEC-HUS is a disease primarily seen in children except in epidemics when it may occur in patients with a wider range of ages. For example, from May 2011 until July 2011, several European countries, particularly northern Germany, experienced one of the largest STEC-HUS outbreaks ever reported. The E. coli strain O104:H4 caused a unique multinational epidemic with 3,816 patients who suffered from enterohaemorrhagic E. coli infection, 845 cases of HUS, and 54 deaths.5 The incidence of STEC-HUS has been fairly steady over the past decade despite increased public awareness and efforts by governments and the food industry to reduce the risk of food and waterborne transmission of STEC.
Toxic effects of Stx
Stx1 and Stx2, which are molecularly similar to Shigella toxin, cause endothelial injury and thrombotic microangiopathy.1 Stx is a multi-subunit AB5 protein complex in which the A subunit mediates its toxic effects and the B component promotes binding to certain eukaryotic cell types. The initial step in the pathogenesis of the disease is translocation of the intact toxin across the gastrointestinal epithelium via a transcellular pathway; this process is increased by transmigration of leukocytes across the endothelium.6 Controversy exists regarding the mode of transport of Stx from the gut to target organs. Free Stx has not been detected in the serum and it is rapidly cleared from the circulation following intravenous administration.7 Instead, it has been suggested that Stx is transported to the periphery by leukocytes that possess a surface receptor with an affinity for the toxin that is lower than the affinity for the glycolipid receptor globotriaosylceramide (Gb3) on the endothelial cell surface.8,9,10 However, a follow-up study failed to confirm this mechanism and it remains unknown how Stx transits from the gut lumen to reach the microcirculation of the kidney and brain.11 Regardless of how Stx reaches the tissue, the initial step in the pathogenesis of STEC-HUS is binding of the B subunit to Gb3 on the cell membrane. This step is followed by retrograde transport of the A subunit to the Golgi apparatus and inhibition of protein synthesis in the ribosome.3,12,13 A 2012 study indicated that manganese can interfere with the retrograde movement of Stx to the Golgi, leading to increased degradation of the toxin in lysosomes.14 Pretreatment of mice with this metal reduced the lethal effects of Stx.
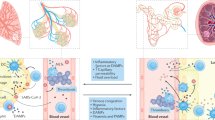
STEC-HUS is a systemic process that affects the vasculature of every organ. In addition to its direct effects on the endothelium that promote cell injury, Stx induces a broad inflammatory response that is triggered by much lower levels of Stx than the amount needed to inhibit protein synthesis. This process involves a ribotoxic stress response, upregulation of adhesion molecules for leukocytes, and promotion of a prothrombotic state in blood vessels.3 Recent findings indicate that Stx increases endothelial cell expression of the chemokine receptor CXCR4 and its ligand stromal cell-derived factor 1 (SDF-1). Specific blockade of this ligand–receptor system ameliorated STEC-HUS in mice.15 Interestingly, plasma levels of SDF-1 are nearly fourfold higher in children with STEC enteritis who progress to HUS than in children who do not progress to HUS.15 The pathophysiological pathway and signalling molecules that are triggered by Stx and that cause organ injury and dysfunction are summarized in Figure 1.
Stx is released by disease-causing strains of bacteria, primarily Stx-producing Escherichia coli, in the GI tract and absorbed into the systemic circulation where it binds to globotriaosylceramide on the surface of vascular endothelial cells. Stx causes direct endothelial injury by increasing inflammation, inducing expression of cytokines and chemokines, and triggering a ribotoxic stress response. Injury to endothelial cells leads to the formation of microthrombi, activation of the alternative complement pathway and damage to target organs, especially the kidney and the brain. Abbreviations: GI, gastrointestinal; Stx, Shiga toxin.
Pathophysiology of STEC-HUS
Involvement of the kidney
Stx binds to both the glomerulus and proximal tubules in patients with STEC-HUS, although glomerular binding is lower in elderly patients than in paediatric patients with the disease.1 Within the glomerulus, Stx binds to podocytes, mesangial cells, and glomerular endothelial cells in human tissue.16 In the kidney of three children who died during an episode of STEC-HUS, Stx1 and Stx2 were detected in these cellular locations by immunohistochemical staining.17 These results are based on a limited sample and caution is warranted before generalizing findings. The toxin has also been localized to the distal tubule in clinical specimens, which supports preclinical data that Stx has direct effects on this nephron segment.17
A number of animal models of STEC-HUS have been developed to study the associated kidney disease; however, none of these models fully recapitulates all of the features of the human illness. These experimental systems include intravenous administration of Stx alone or in combination with lipopolysaccharide (LPS) or tumour necrosis factor (TNF) to rodents,18 oral administration of STEC to various inbred strains of mice,19,20 oral administration of STEC to germ-free (gnotobiotic) piglets,21 and intravenous administration of Stx to baboons.22 These animals display a spectrum of renal injury that includes tubular cell necrosis and apoptosis, inflammatory infiltrates in the renal interstitium, and mild thrombotic microangiopathic lesions in the glomerular microcirculation.18,19,20,21 In general, rodent models are characterized by less glomerular involvement and more profound tubular damage than that observed in human disease. For example, infusion of Stx1 directly into the kidney of rats via the renal artery led to diffuse kidney injury with cortical infarcts, platelet aggregates in vessels, and infiltration of monocytes in glomeruli and the tubulointerstitium.23 The tubular injury is associated with marked upregulation of a number of cytokine and chemokine genes including TNF, PDGF, and IL8.23,24
Because of shortcomings of the in vivo models of STEC-HUS, much work has been done with specific cells using in vitro methods. Localization of Gb3 within distinct structures in the kidney is predictive of the cellular sites of Stx action.25 Both glomerular endothelial and proximal tubular epithelial cells express Gb3 on their cell surface and are therefore susceptible to toxin-mediated injury. In vitro, tubular epithelial and mesangial cells are as susceptible to the cytotoxic effects of Stx1 and Stx2 as endothelial cells and their sensitivity parallels Gb3 expression and toxin binding.26 In mesangial cells, Stx inhibits protein synthesis and proliferation without altering cell viability.27 However, Stx is not cytotoxic at the same dose in all kidney cell types that express Gb3 and susceptibility in the same cell type can vary between species.25,26 Stx also causes apoptosis and can generate an inflammatory and/or ribotoxic stress response in glomerular endothelial and tubular cells in the cortical regions of the kidney in children with STEC-HUS.28 Together, these observations suggest that an array of biological responses are implicated in the development of renal disease in STEC-HUS.
It is well established that Stx2 causes direct damage to human glomerular endothelial cells, which results in thrombotic microangiopathic lesions in the afferent arteriole.1,12,25 Stx injury to glomeruli is augmented by concomitant exposure to LPS and may involve induction of IFN-γ within the kidney.29 The endothelial injury after administration of Stx leads to a reduction in glomerular filtration rate and renal plasma flow. These functional disturbances modify renal handling of drugs such as the antibiotic levofloxacin, without any alteration in the expression of drug transport systems, such as the multidrug resistance-associated protein 2.30
In human proximal and distal tubular epithelial cells, specific Stx binding to Gb3 has been confirmed, with subsequent inhibition of protein synthesis and apoptosis in a dose-dependent and time-dependent manner.31,32 Distal tubule cells also express Gb3 and, as targets of the toxin, undergo similar perturbations to that of proximal tubular cells. In tubular cells, Stx causes inhibition of protein synthesis at concentrations as low as 0.001 ng/ml and within 1 h of incubation with intact Stx2.33 Mouse cortical and medullary collecting duct epithelial cells express Gb3, bind Stx, and undergo apoptosis after exposure to the toxin.34 Human renal tubular epithelial cells demonstrate dose-dependent and time-dependent cytotoxicity following exposure to Stx2 and its B subunit, which is potentiated by IL-1, LPS, and butyrate but not by TNF, IL-6 or IL-8.35 Using HK-2 cells, an immortalized human proximal tubular epithelial cell line, Lentz et al.36 documented increased susceptibility to Stx1 compared with Stx2. This interesting finding conflicts with the clinical observation that Stx2-mediated disease is generally more severe than cases caused by Stx1-producing strains of STEC. In the study by Lentz et al.,36 Stx1 was detected in the lysosomal and endoplasmic reticulum compartments, which might explain the heightened cytotoxicity observed in this study. Both toxins increase poly [ADP-ribose] polymerase cleavage without any change in procaspase 3 expression, which indicates that Stx causes proximal tubular cell apoptosis in a caspase 3-independent manner.36
The tubular damage caused by Stx can lead to a reduction in the renal water handling capacity. Stx2 and its B subunit inhibit water absorption across human renal tubular epithelial cell (HRTEC) monolayers without altering the short circuit current and the permeability of [3H]mannitol. Quantitative evaluation of [14C]inulin transport across HRTEC monolayers showed a similar transport rate before and after HRTEC treatment with Stx2, which confirms that the integrity of the paracellular pathway is maintained.33 Stx administration to rats led to a reduction in aquaporin-2 levels in the kidney and a marked elevation in aquaporin-2 excretion in the urine.37 These data indicate that whereas Stx2 acts primarily by inhibiting protein synthesis mediated by the A subunit, binding of the Stx2 B subunit to Gb3 may directly affect the membrane mechanisms related to water absorption. Impaired water absorption by proximal tubular cells in vivo during episodes of STEC-HUS might contribute to early events in the pathogenesis of renal injury.
The inflammatory response of neutrophils and monocytes triggered by Stx is independent of receptor-mediated actions of the toxin molecule on the endothelial cell and contributes directly to the renal injury. Chemokine receptors participate in the pathogenesis of a variety of glomerular diseases by modulating renal infiltration by myeloid cells. In CCR1-knockout mice, administration of Stx2 was associated with improved survival, diminished leukocytosis and monocytosis, and reduced numbers of neutrophils and monocytes within the kidney compared with that of wild-type animals.38 In addition, the peak circulating levels of TNF and IL-6 were reduced and delayed in onset. A similar role for SDF-1/CXCR4 in the pathogenesis of STEC-HUS in mice has also been described.15 These findings underscore the importance of the inflammatory response in the development of STEC-HUS and the potential impact of treatments that interfere with this cascade, such as blockade of CCR1 and CXCR4.
Involvement of the CNS
Although neurological complications are not as common as renal involvement, disturbances in CNS function are the main cause of acute mortality in patients with STEC-HUS.39 This finding is presumed to reflect the sudden occurrence of microvascular injury in vital cerebral regions such as the brain stem. Similar to the renal manifestations of STEC-HUS, animal models do not fully recapitulate all aspects of CNS injury in human disease and are unable to sufficiently explain the entire spectrum and course of CNS involvement in patients with STEC-HUS. Thus, in vitro systems are needed to clarify the pathogenesis of the CNS injury in this disease.
Although Gb3 is present in the cerebral vasculature, as a single stressor, Stx has been shown in vitro to have no major effect on human brain microvascular endothelial cells (HBECs) in concentrations up to 10−5 g/l.40 The localization of Gb3 on various neurons, such as those of the cerebrum, cerebellum, brain stem, and spinal cord within the mouse CNS and the co-localization of Gb3 and Stx2 in the motor neurons of the spinal cord of mice injected intraperitoneally with Stx2 mice has been described.40,41 In human cadaver tissue, Gb3 was detected in neuronal cell walls and at the luminal side of vascular endothelial cells.41
The typical thrombotic microangiopathic lesion of HUS that is found in renal vessels is not observed in brain endothelium.42,43 However, early work describes Stx-induced ischaemic brain lesions in rabbits44 and brain arteriolar necrosis and endothelial cell injury in piglets.21 These findings indicate that different types of endothelium react in distinctive ways to Stx. For example, transformed human intestinal microvascular endothelial cells are more susceptible to the cytotoxic effects of Stx than are macrovascular endothelial cells from human saphenous veins.45
Inflammatory cytokines have a pivotal role modulating the susceptibility of HBECs to the adverse effects of Stx. Shiraishi et al.46 suggest that various inflammatory proteins predict CNS involvement in patients with STEC-HUS. sTNFR1 and TIMP-1 reach considerably higher serum concentrations in patients with HUS and encephalopathy than in those with HUS without encephalopathy, those with acute colitis and no HUS, and healthy controls. Of note, serum levels of sTNFR1 and TIMP-1 are significantly higher in patients with HUS without encephalopathy and in those with acute colitis without HUS than in healthy controls. Preincubation of HBECs with TNF prior to the exposure to Stx elicits a decrease in protein synthesis and in cell viability47 as well as an approximately 103-fold reduction in the cytotoxic dose of Stx.48 Furthermore, Eisenhauer et al.47 demonstrated a threefold upregulation of Gb3 covalently linked to fatty acids after treatment with TNF. Stricklett et al.49 reported similar findings, namely that TNF and IL-1 individually and even more so in combination increased the cytotoxicity and inhibitory effect of Stx1 on protein synthesis in HBECs. In addition, the investigators showed that after treatment with a combination of the two cytokines, the concentration of Gb3 and the activity of the three enzymes involved in Gb3 synthesis were higher than after treatment with either cytokine alone. These results are supported by Ergonul et al.40 who describe increased activity of the proapoptotic enzyme caspase 3 and DNA fragmentation in endothelial cells after preincubation with TNF before the addition of Stx. The DNA fragmentation in the brain endothelial cells was reversed by the addition of a specific caspase 3 inhibitor. The contribution of caspase 3 in brain endothelial cell apoptosis contrasts with observations made in proximal tubule cells.36 These results indicate that apoptosis is exacerbated by the inflammatory response in STEC-HUS and has an important role in brain damage during the illness.
Activation of the complement system
The role of complement activation in atypical HUS is well established; however, its contribution to the pathogenesis of STEC-HUS is still a subject of controversy.50 In vitro studies of endothelial cells have demonstrated that exposure to Stx increases expression of P-selectin, which binds and activates C3 via the alternative pathway.51 The C3a that is produced following the cleavage of C3 potentiates the activation of the alternative pathway of complement, reduces the expression of thrombomodulin, and promotes thrombus formation. Similar findings were made in a murine model of HUS induced by combined injection of Stx and LPS.51 The process was attenuated by administration of a P-selectin antibody, an antagonist to the C3a receptor, and in factor B-knockout mice. This observation links activation of the alternative pathway of complement to several of the key clinical features of STEC-HUS. Stx is able to bind factor H and reduce its cell surface cofactor activity in vitro; moreover, it induces the formation of the membrane attack complex.52 However, there are serine proteases that are produced by STEC that may downregulate the complement pathway.53 Thus, the net effect of STEC infection on the alternative pathway of complement in vivo is likely to represent a complex interaction between stimulatory and inhibitory processes.
Studies carried out in the 1980s involving small series of patients with STEC-HUS documented increased serum levels of complement degradation products C3 and factor B. In a study of 17 patients enrolled in the SYNSORB Pk® (Synsorb Biotech, Calgary, AB, Canada) trial (a randomized clinical trial to assess the efficacy of an oral Stx-binding agent), plasma levels of Bb and sC5b–9 were significantly elevated at baseline compared with in healthy controls. The abnormal concentrations of the complement byproducts returned to normal by day 28 after discharge from the hospital following the acute episode.54 Ståhl et al.55 examined whether complement was activated on leukocyte–platelet complexes and microparticles in patients with STEC-HUS, and whether Stx and LPS (O157LPS) induced complement activation on leukocyte–platelet complexes and microparticles. Using flow cytometry, whole blood from a child with HUS had surface-bound C3 on 30% of platelet–monocyte complexes compared with 14% after recovery and 12% in paediatric controls. Acute-phase samples from 12 children with STEC-HUS exhibited high levels of platelet microparticles and, to a lesser extent, monocyte microparticles, both bearing C3 and C9. Levels decreased significantly at recovery. Both Stx and O157LPS induced the release of C3-bearing and C9-bearing microparticles from platelets and monocytes.55 These findings indicate that the complement system contributes to the abnormal vascular function during HUS. A more comprehensive discussion of the contribution of the alternative pathway of complement to STEC-HUS and its potential to serve as a target for treatment is reviewed elsewhere.56
Clinical aspects of STEC-HUS
Effects on the kidney
By definition, all patients with STEC-HUS manifest some degree of renal insufficiency. Approximately 30–40% of cases will require dialysis therapy for an average duration of approximately 10 days, whereas the remainder will have milder renal involvement without the need for dialysis therapy.12 In most published series, STEC enteritis progresses to HUS in 5–15% of cases. Fever and a high white blood cell count may indicate patients at increased risk of developing HUS.1 In an epidemic of HUS linked to E. coli O103:H25 disease that occurred in Norway, 10 patients (median age 4.3 years) of 16 infected with enterohaemorrhagic E. coli developed STEC-HUS.57 Eight of these patients had persistent oligoanuria and required dialysis for a median duration of 15 days. The STEC-HUS resulted in irreversible AKI in one girl who subsequently received a kidney transplant. This outbreak of STEC was characterized by a high incidence of HUS among the infected children and more severe renal disease than is usually encountered in routine cases. A possible explanation is that the O103:H25 (Eae [adhesion molecule] and Stx2-positive) strain is highly pathogenic. A similar spectrum of severe AKI was reported in the May 2011 outbreak centred in Hamburg, Germany and linked to another uniquely virulent strain of E. coli, namely O104:H4 (discussed below).58 Although the data from these outbreaks are somewhat atypical, the findings with these distinctive E. coli serotypes highlight the magnitude of renal injury that can occur in STEC-HUS.
In a series of 22 cases of HUS treated in the Paediatric Nephrology Unit at the Azienda Ospedaliero-Universitaria Meyer in Florence, Italy between January 1997 and December 2008, 60% of the patients had STEC-HUS and 40% had atypical HUS.59 The latter category represents a disease entity that is not linked to an antecedent gastrointestinal infection and that probably occurs secondary to genetic mutations in complement regulatory genes.56 This distribution may reflect the tertiary nature of care and the referral pattern to this centre. The age distribution of patients ranged from 12 days to 13 years. 20 patients (90%) had oligoanuria, lasting 6.4 ± 4 days for patients with STEC-HUS versus 11.8 ± 4 days for patients with atypical HUS.59 This finding indicates that the renal disease at the time of diagnosis is more severe in children with atypical HUS than in those with STEC-HUS, which is consistent with a report of all cases of HUS identified during the SYNSORB Pk® clinical trial.60 The risk of developing chronic kidney disease correlated with the blood pressure level at presentation, the length of oligoanuria, and the duration of hospitalization.
The need for acute renal replacement therapy may be adversely affected by depletion of the extracellular fluid volume during the antecedent diarrhoeal illness. A retrospective study61 and a prospective observational study62 demonstrated that the intravenous administration of adequate volumes of fluid and sodium during the prodromal phase of STEC enteritis may markedly reduce the risk of developing oligoanuria and the requirement for temporary dialysis support. Neither study entailed randomization to different intravenous fluid regimens and the reported benefit may therefore represent selection bias whereby children received parenteral hydration because of unidentified confounding factors. STEC has been reported to cause upper urinary tract infections in patients with or without concurrent HUS,63 which indicates that the bacteria can cause renal inflammation and disease without triggering HUS.
With regard to the long-term effects of STEC-HUS on renal function, Dobiliene et al.64 evaluated the kidney functional outcome of HUS in 20 children (eight boys, aged 3 months to 12 years) who were hospitalized and treated at the Clinic of Children's Diseases at Kaunas University of Medicine Hospital in Lithuania between 1995 and 2006. The course of acute disease and the health status were evaluated at discharge from hospital and after 6, 12, and 36 months of follow-up. Children were divided into two groups based on the severity of the acute illness. Group A (severe course) consisted of 15 patients with leukocytosis (white blood cell count >20 × 109/l) and signs of CNS involvement, who required renal replacement therapy. By contrast, Group B (mild course) was made up of five children who did not have such symptoms. 12 (60%) children underwent dialysis during the acute illness and two patients died (10%). Eight (61.5%) patients from Group A had renal insufficiency, nine (69.2%) had proteinuria, and two (15.4%) had arterial hypertension at discharge from hospital. 3 years after onset of the disease, two (20%) patients still had arterial hypertension, proteinuria was detected in two (20%) patients, and renal insufficiency persisted in six (60%) children. In Group B, one (20%) patient had proteinuria, four (80%) had renal insufficiency, and three (60%) had arterial hypertension at discharge from hospital. Subsequently, these abnormalities resolved and 3 years later arterial hypertension was detected in only one (20%) patient.64 These data indicate that STEC-HUS can have serious long-term ramifications for kidney function that are strongly influenced by the severity of the acute phase of the illness. In a meta-analysis, Garg et al.65 reported that 20–25% of patients have evidence of permanent kidney injury (proteinuria, hypertension, and/or reduced glomerular filtration rate) after recovery from STEC-HUS. However, these results may be overestimates that reflect a reporting bias for adverse outcomes because of selective follow-up of patients with persistent abnormalities in kidney function.
Effects on the CNS
Neurological involvement is the most life-threatening acute complication of STEC-HUS and can cause sudden death. The complication occurs in 20–25% of patients with STEC-HUS.66 The disease is associated with a wide range of neurological disturbances, including lethargy, apnoea, coma, seizures, cortical blindness, and haemiparesis.1,39 Some evidence indicates that the frequency and severity of neurological complications might be related to the intensity of the antecedent enteritis, evidenced by the degree of bloody diarrhoea and gastrointestinal symptoms.39 The neurological manifestations represent a combined effect of Stx-induced vascular injury, endothelial dysfunction, hypertension, and electrolyte disorders. This concurrence of multiple abnormalities simultaneously in the same patient may account for the severity of the neurological disease in patients with STEC-HUS. For example, in a recent report, a 4-year-old child with HUS caused by E. coli O157:H7 developed posterior reversible leukoencephalopathy syndrome, which was diagnosed based on the presence of areas of high signal intensity in the bilateral occipital lobes on T2-weighted MRI testing. Despite adequate control of blood pressure, the syndrome did not resolve but progressed to multiple subcortical cavitations.67
The largest recent cohort study of CNS disease in STEC-HUS consisted of data from 52 children collected over 33 years who had severe initial neurological involvement that occurred during the acute phase of the disease. Their clinical findings ranged from seizures (71%) to alteration of consciousness (85%), and paresis (40%).68 In this retrospective multicentre series, 24 patients had confirmed STEC infection. All but two patients had AKI that required temporary renal replacement therapy, indicating the presence of severe disease. One group of eight patients maintained normal consciousness, five of whom had protracted seizures. A second group of 23 patients had stuporous coma; five of these children had prolonged seizure activity, and 18 had an evident neurological defect such as pyramidal syndrome, haemiplegia or haemiparesis, and extrapyramidal findings. A third group of 21 patients had severe coma. Plasma exchange was undertaken in 25 patients, 11 of whom were treated within 24 h after the first neurological sign. Of these patients, four died, two survived with severe permanent disability, and five survived without neurological defect. MRI was done in 29 patients, revealing involvement of virtually every structure in the CNS. The overall prognosis for the children in this case series was severe, characterized by the death of nine patients and permanent sequelae in 13 patients. No correlation was found between a specific pattern of localization of injury in the early MRI and the final prognosis. In the outbreak of O103:H25 in Norway described above, four patients had seizures and/or reduced consciousness. Cerebral oedema and herniation caused the death of a 4-year-old boy.57
In addition to obvious morbidity, such as stroke, haemiplegia, cortical blindness, and psychomotor retardation at the time of discharge after an acute episode of STEC-HUS, there may be milder, more subtle neuropsychological effects in children after they recover from the illness. In a multicentre case–control study of patients treated at six Canadian hospitals who survived an acute episode of STEC-HUS without recognizable neurological dysfunction at discharge (n = 91), no overall differences between patients with HUS and paired controls were evident on tests of IQ, behaviour, verbal abilities, or academic achievement, nor was there an increased risk of attention deficit disorder among patients with STEC-HUS.69 However, the percentage of patients with abnormal test findings in select domains of the neurocognitive profile was higher in patients with STEC-HUS than in the matched controls.69 Scores on some verbal ability tests were lower in those with the highest serum creatinine concentrations during illness. These findings substantiate the requirement for long-term follow-up of patients after an episode of STEC-HUS to identify those children with subtle neurocognitive defects that may interfere with learning and school performance. The acute and long-term renal and neurological consequences of STEC-HUS are summarized in Figure 2.
Acute renal events include acute kidney injury and hypertension. Long-term renal outcomes include hypertension, proteinuria, and reduced GFR. Acute neurological events include seizures, loss of consciousness, thrombotic strokes, and cortical blindness. Long-term neurological outcomes include haemiparesis, blindness, seizures, and learning disabilities. Abbreviations: GFR, glomerular filtration rate; STEC-HUS, Shiga toxin-producing Escherichia coli-associated haemolytic uraemic syndrome.
STEC-HUS O104:H4 outbreak in 2011
The experience at one large referral centre in Germany that was involved in the unique outbreak of STEC-HUS O104:H4 in 2011 is described below in order to provide an up-to-date picture of the clinical manifestations and consequences of STEC-HUS. This description highlights the approach to the care of patients with a life-threatening illness under emergent circumstances and provides a useful comparison to randomized clinical trials performed under more standard conditions. Although STEC-HUS is not as rare an entity as atypical HUS and the size of the case series is generally larger, STEC-HUS is still an uncommon illness and caution is advised in applying any of the opinions and recommendations made in this Review. The rarity of the disease and small sample sizes in most case series underscores the importance of creating international registries and biorepositories to share clinical data and biosamples in support of research and to perform clinical trials of promising therapies.
Epidemiology and microbiology
From May to July 2011, northern Germany experienced a massive outbreak of enterohaemorrhagic E. coli that had several distinctive features compared with previous epidemics. In earlier European epidemic outbreaks, 63–97% of STEC-HUS cases were attributed to O157 strains.70 The 2011 epidemic was caused by an uncommon serotype, namely O104:H4,71,72 which had previously been reported to be responsible for only two cases of STEC-HUS, one each in Korea73 and Germany.74 The genetic code was rapidly sequenced and showed a distinct virulence profile combining characteristics of Stx-producing and enteroaggregative E. coli. This strain produced β-D-glucuronidase and fermented sorbitol and was resistant to tellurite. Furthermore, the bacterium incorporated virulence genes coding for Stx2, adhesins, an iron uptake system, adherence fimbriae, the enteroaggregative E. coli global regulator AggR, dispersin, Shigella enterotoxin 1, an autotransporter serine protease and both an extended-spectrum β-lactamase (ESBL phenotype) and resistance to trimethoprim–sulfamethoxazole.71,72 Contaminated sprouts were considered to be the vector of the STEC based on epidemiological data; however, bacteria were not detected on any of the plants.75
During the outbreak, 3,816 infected patients were reported to the authorities. The incidence grew to a maximum of 30 per 100,000, peaking at 63 reported HUS cases per day in the beginning of May 2011. An unusually high percentage of patients (n = 845, 22%) developed STEC-HUS of whom 36 died (4.3%). The overall mortality was 1.4%. In contrast to former epidemics, most affected patients were adults (88%) with a median age of 42 years, and were predominantly women (68%).75
Clinical features
The following clinical description is based on 130 patients with STEC-HUS who were treated in the University Hospital in Hamburg–Eppendorf, Hamburg, Germany. The presentation might be biased because, as a referral centre, patients with severe illness may have preferentially been sent there for management. Reports from other hospitals, such as the one by Loos et al.76 that focuses on paediatric cases, may display a different distribution of the clinical patterns. Prior to the onset of HUS, most patients did not have a fever and leukocyte counts were not strikingly elevated. Abdominal pain and bloody diarrhoea were very common. The diagnosis of STEC-HUS was made when the patients experienced platelet counts of <150 × 109/l, haemolytic anaemia with red blood cell fragmentation, and AKI. In some centres, urinalysis findings were considered diagnostic of renal disease whereas others required an abnormal serum creatinine concentration for diagnosis.2,76 The spectrum of the disease among patients was remarkably broad. About 50% of patients developed mild to moderate disease with lactate dehydrogenase (LDH) levels around 500 U/l, mildly elevated serum creatinine levels (1.3–2 mg/dl [99–152.5 μmol/l]) and no neurological symptoms. Other patients developed severe AKI with oligoanuria, LDH levels up to 1,500 U/l, and neurological symptoms (Figure 3). Care in the intensive care unit was required by 42% of patients and almost 50% of those patients required mechanical or noninvasive ventilator support (R. A. K. Stahl, unpublished work).
a | Platelet levels decreased and LDH levels increased during the early stages of disease in both the mild and severe cases of STEC-HUS. The case with mild STEC-HUS had no neurological symptoms whereas the patient with severe disease experienced neurological complications. b | Creatinine levels were only very slightly elevated in the case with mild STEC-HUS whereas the case with severe STEC-HUS had high levels of serum creatinine and required renal replacement therapy. Abbreviations: LDH, lactate dehydrogenase; STEC-HUS, Shiga toxin-producing Escherichia coli-associated haemolytic uraemic syndrome.
A total of 54% of the patients required acute renal replacement therapy. The remaining patients had an average serum creatinine concentration of 2.2 mg/dl (167.8 μmol/l) at the time of STEC-HUS diagnosis. Renal biopsies in select patients revealed the typical findings of thrombotic microangiopathy. A severe tubulointerstitial cell infiltrate was observed in addition to the glomerular and preglomerular vascular lesions. In this cohort and in patients with STEC-HUS at other hospitals in Hamburg, nearly half of the patients (104 of 217, 48%) presented with neurological symptoms. In five cases, these symptoms occurred prior to identification of HUS. The median age of the STEC patients with neurological findings was 39 years (range: 18–84 years). The most prominent initial neurological symptom was altered mental status consisting of disorientation (49%). Other symptoms included headache (5.8%), myoclonus (5.8%), oculomotor disturbances (9.6%), paresis of extremities (18.3%), apraxia (22.8%), aphasia (46.2%), and epileptic seizures (34.6%). An abnormal electroencephalogram was recorded in 78% of the patients. MRI scans performed in 70 patients showed mainly symmetrical hyperintensity signals in the nucleus of the sixth cranial nerve (23%) and the lateral thalamus (30%). 36% of patients had no abnormal MRI findings despite severe clinical disease. Neuropathological examinations performed in five patients who died revealed astrogliosis and microgliosis; there were no signs of thrombosis or microhaemorrhage.43
Treatment and outcome
In general, plasma exchange is not considered to be an effective treatment for STEC-HUS.2 Based on weak evidence from an outbreak in Scotland in which treatment with plasma exchange was associated with a survival benefit,77 however, patients were treated with plasma exchange at the beginning of the 2011 outbreak. This therapy was implemented within 3 weeks of diagnosis and hospital admission in 108 patients who received on average 4.5 treatment sessions. Platelet counts were used as a clinical parameter to assess the efficiency of plasma exchange. In about 25% of patients, platelet counts increased to normal values within 7 days of treatment. However, there was no improvement in the remaining 75% of patients. Furthermore, renal function deteriorated in most patients during plasma exchange, the number of patients who needed dialysis increased, and the frequency of neurological symptoms increased.78 Thus, there was no short-term benefit of plasma exchange on any clinical index of STEC-HUS disease severity. These findings confirmed the prevailing consensus that plasma exchange is not an effective therapy in adult patients with STEC-HUS. Our experience contrasts with a published case series of five patients in the 2011 O104:H4 outbreak who were treated in Denmark and in whom there was an inverse relationship between the time from onset of bloody diarrhoea to initiation of plasma exchange and reduction of LDH levels.79 These single-centre findings are at variance not only with our observations, but with the findings of a recently published prospective study in 619 children with HUS in which an association was found between the use of plasma exchange and poor long-term outcome.80
During the peak of the outbreak (25 May to 28 May 2011) when the number of STEC-HUS cases in our institution was still rising and neurological symptoms continued to worsen in most patients, a Letter to the Editor of the New England Journal of Medicine was published describing three children, all 3 years of age, with severe STEC-HUS who were treated with the monoclonal antibody eculizumab.81 The patients had required dialysis and two of them had been unresponsive to plasma exchange. The neurological status as well as the platelet count and LDH levels improved dramatically after the first administration of eculizumab. Dialysis could be discontinued within 16 days and all three children were discharged without neurological findings.81 Thus, like many other physicians in Germany, we approached the manufacturer of eculizumab (Alexion Pharmaceuticals, Cheshire, CT, USA) and requested a supply of the monoclonal antibody for off-label compassionate use in STEC-HUS patients with the most severe clinical symptoms. Eculizumab is a humanized anti-C5 monoclonal antibody that inhibits complement activation and prevents the formation of the membrane attack complex. The biological agent is approved for the treatment of paroxysmal nocturnal haemoglobinuria and was approved by the FDA for the therapy of atypical HUS in September 2011. Data about the efficacy of eculizumab in the treatment of STEC-HUS during the German outbreak were obtained based on open-label use of the antibody. The etiologic bacteria had novel features that were not characteristic of prevalent O157 strains. Thus, the effects of eculizumab may not be representative of its use to treat patients with episodes of sporadic disease linked to standard strains of E coli such as O157:H7. In addition, the outcomes after eculizumab treatment may vary from centre to centre,76 which will mandate careful analysis of aggregate outcomes in all patients regardless of how they were managed during the acute episode.
By early June 2011, together with Alexion Pharmaceuticals, the Paul–Ehrlich–Institut, and the local ethics committee in Hamburg, we initiated a multicentre, single-arm, open-label 28-week clinical study to test the safety and efficacy of eculizumab on clinical markers of thrombotic microangiopathy and serious organ complications in patients with STEC-HUS. The protocol involved intravenous administration of the antibody, 900 mg weekly for 4 weeks, then 1,200 mg biweekly for a total of seven doses, based on prior experience in treating atypical HUS (R. A. K. Stahl, unpublished work). Treatment could be extended for an additional 8 weeks based on clinical discretion in patients with persistent disease activity or abnormal laboratory tests. A total of 328 patients with STEC-HUS received eculizumab during the outbreak and 198 patients from 25 centres are included in the study.82 In this cohort, 137 patients (72%) needed dialysis, 43 patients (25%) had seizures and 158 (80%) had brain and kidney involvement. The study will assess outcomes at 8 weeks and 28 weeks. The data analysis is still in progress but will be presented at ASN Kidney Week in San Diego 2012.83 It is important to acknowledge the heroic efforts of all the clinicians who contributed to the care of the massive influx of patients with STEC-HUS during this outbreak and who confronted urgent pressure to intervene under these trying circumstances.84 However, regardless of the findings of the effect of eculizumab, it will be important to evaluate this agent in more controlled conditions. It is hoped that there will be extended follow-up of all patients to document long-term renal and neurological sequelae comparable to the effort that was made after the outbreak in Walkerton, ON, Canada.85
During the German outbreak, other nonstandard treatments were offered to adult patients with STEC-HUS who had severe neurological impairment. 12 patients were enrolled in a prospective noncontrolled trial involving two centres to assess the effectiveness of IgG immunoadsorption.86 The justification for this intervention is that the delayed onset of neurological symptoms indicates an immune-mediated mechanism for this complication. The neurological symptoms in this subgroup included delirium, myoclonus, aphasia, and altered consciousness. Six patients had seizures and nine patients required mechanical ventilation because of the neurological findings. 10 patients developed new-onset or worsening of the neurological symptoms despite repeated plasma exchange. Eight patients received eculizumab of whom five showed worsening, two had no change, and one had improvement of neurological findings. After immunoadsorption, there was neurological improvement in all patients. Six patients required a second course of immunoadsorption after a median of 7 days, one patient needed three courses, and two patients required four courses of immunoadsorption.86 The investigators did not test the specificity of the removed antibodies toward any potential CNS antigens and thus it is not possible to explain how this intervention acted in this clinical circumstance.
The report of Loos et al.76 focuses on the clinical disease in 90 children affected by the epidemic and who were treated at centres that participated in the German HUS Registry. The median age, 11.5 years, was older than in previous outbreaks. 71% of the patients (64 of 90) required at least temporary renal replacement therapy and 23 of 90 patients (26%) had neurological symptoms. Most of the patients (67 of 90, 74%) received supportive care only, 17 received plasma exchange, and 13 were given eculizumab (together with plasma exchange in seven cases). Most of the patients recovered and only one child died. After a median follow-up of 4 months, kidney function in all but four patients had returned to normal and only five patients had residual neurological deficits that were slowly improving in all cases. Based on their findings, Loos and colleagues concluded that the clinical profile of O104:H4 STEC is comparable to the disease caused by the O157 serotype and that there is no need for novel treatments besides intensive supportive care. In addition, Menne et al.87 have examined their experience treating 298 patients with STEC-HUS at 23 hospitals in northern Germany during the massive O104:H4 outbreak. In this cohort, 160 (54%) of the patients required dialysis, 37 (12%) had seizures, 54 (18%) required mechanical ventilation, and there were 12 (4%) fatalities. 251 (84%) of the patients were treated with plasmapheresis (with or without concomitant glucocorticoids) and 67 (23%) received eculizumab; however, there was no demonstrable benefit of either intervention on the course of the disease or the outcome at the time of discharge from the hospital. It is very difficult to draw definitive conclusions about the efficacy of eculizumab treatment for STEC-HUS from any of the published reports based on its emergent use on a compassionate basis during the outbreak because of potential biases in patient selection and because of the impact of concomitant therapy. This situation underscores the need for a controlled clinical trial to address this important question.
One major uncertainty during the German outbreak was whether or not to treat patients with bloody diarrhoea with antibiotics. The current recommendation is not to treat gastroenteritis associated with enterohaemorrhagic E. coli with antibiotics because they increase the risk of developing HUS.1 This finding has been confirmed in a large prospective study of 259 children with STEC enteritis treated in the Pacific Northwest region of the USA.88 However, data show that unlike the O157:H7 serotype, the O104:H4 strain does not release Stx after incubation with therapeutic concentrations of ciprofloxacin, meropenem, fosfomycin or chloramphenicol.89 During the German epidemic, patients who received eculizumab required antibiotic prophylaxis for the prevention of meningococcal infections until vaccination was effective and azithromycin was prescribed for this purpose. Patients who had STEC in their stool and who were given azithromycin eliminated the bacteria from their stool faster than did patients in the comparison group who did not receive antibiotics. After 35 days of observation, no STEC carrier could be found in the treated cohort whereas 58% of the untreated cohort were carriers.90 No signs of exacerbation or relapse of the HUS in the azithromycin-treated cohort were observed. The effect of antibiotics on the shedding of the O104:H4 strain has been studied extensively; however, the role of late-in-illness treatment or gut decolonization after recovery from STEC-HUS is still uncertain.91
Conclusions
STEC-HUS continues to represent an important cause of AKI and severe neurological disease in children and adults. The outbreak in Germany in 2011 underscores the potential for the emergence of novel STEC serotypes with increased virulence and greater risk of causing HUS. General paediatricians, internists, and subspecialists need to be fully aware of the clinical manifestations and complications of STEC-HUS. Current therapy includes adequate hydration during the prodromal enteritis and intensive medical supportive care in those who progress to HUS. Timely intervention with renal replacement therapy and at the earliest signs of neurological dysfunction may improve outcomes. Recent data indicate that activation of the alternative pathway of complement contributes to the pathogenesis of the disease and inhibition of the cascade with eculizumab can ameliorate the illness. Preliminary experience with eculizumab in a large outbreak of STEC-HUS indicates the feasibility of this treatment and although some of the early reports are encouraging, a comprehensive evaluation of efficacy is ongoing. There are many unanswered questions such as which patients to treat, whether children and adults should be considered separately, when to initiate therapy, and the proper dose and duration of treatment. It is therefore advisable that the role of eculizumab in the routine treatment of STEC-HUS is evaluated in an international, multicentre, randomized clinical trial to validate the benefit of this costly novel therapy.
Review criteria
PubMed was searched for publications appearing in the year 2000 and later using the terms “haemolytic uraemic syndrome”, “Shiga toxin”, “diarrhoea-associated HUS”, “brain”, “neurological”, “cerebral”, “kidney”, “acute kidney injury”, “renal replacement therapy”, “complement”, “globotriaosylceramide”, and “outcomes”. Articles and abstracts were included with a focus on reports that were written in English. The reference lists of select articles published in the past 2 years were retrieved and systematically searched to identify further leads.
References
Tarr, P. I., Gordon, C. A. & Chandler, W. L. Shiga-toxin-producing Escherichia coli and haemolytic uraemic syndrome. Lancet 365, 1073–1086 (2005).
Tarr, P. I. & Karpman, D. Editorial commentary: Escherichia coli O104:H4 and the hemolytic uremic syndrome: the analysis begins. Clin. Infect. Dis. 55, 760–763 (2012).
Petruzziello-Pellegrini, T. N. & Marsden, P. A. Shiga toxin-associated hemolytic uremic syndrome: advances in pathogenesis and therapeutics. Curr. Opin. Nephrol. Hypertens. 21, 433–440 (2012).
Ruggenenti, P., Noris, M. & Remuzzi, G. Thrombotic microangiopathy, hemolytic uremic syndrome, and thrombotic thrombocytopenic purpura. Kidney Int. 60, 831–846 (2001).
Robert Koch Institut. Final presentation and evaluation of epidemiological findings in the EHEC O104:H4 outbreak Germany 2011 [online], (2011).
Hurley, B. P., Thorpe, C. M. & Acheson, D. W. Shiga toxin translocation across intestinal epithelial cells is enhanced by neutrophil transmigration. Infect. Immun. 69, 6148–6155 (2001).
Bielaszewska, M., Clarke, I., Karmali, M. A. & Petric, M. Localization of intravenously administered verocytotoxins (Shiga-like toxins) 1 and 2 in rabbits immunized with homologous and heterologous toxoids and toxin subunits. Infect. Immun. 65, 2509–2516 (1997).
te Loo, D. M. et al. Binding and transfer of verocytotoxin by polymorphonuclear leukocytes in hemolytic uremic syndrome. Blood 95, 3396–3402 (2000).
Tazzari, P. L. et al. Flow cytometry detection of Shiga toxins in the blood from children with hemolytic uremic syndrome. Cytometry B Clin. Cytom. 61, 40–44 (2004).
Brigotti, M. et al. Shiga toxins present in the gut and in the polymorphonuclear leukocytes circulating in the blood of children with hemolytic-uremic syndrome. J. Clin. Microbiol. 44, 313–317 (2006).
Geelen, J. M. et al. Lack of specific binding of Shiga-like toxin (verocytotoxin) and non-specific interaction of Shiga-like toxin 2 antibody with human polymorphonuclear leucocytes. Nephrol. Dial. Transplant. 22, 749–755 (2007).
Scheiring, J., Rosales, A. & Zimmerhackl, L. B. Clinical practice. Today's understanding of the haemolytic uraemic syndrome. Eur. J. Pediatr. 169, 7–13 (2010).
Noris, M. & Remuzzi, G. Hemolytic uremic syndrome. J. Am. Soc. Nephrol. 16, 1035–1050 (2005).
Mukhopadhyay, S. & Linstedt, A. D. Manganese blocks intracellular trafficking of Shiga toxin and protects against Shiga toxicosis. Science 335, 332–335 (2012).
Petruzziello-Pellegrini, T. N. et al. The CXCR4/CXCR7/SDF-1 pathway contributes to the pathogenesis of Shiga toxin-associated hemolytic uremic syndrome in humans and mice. J. Clin. Invest. 122, 759–776 (2012).
Chaisri, U. et al. Localization of Shiga toxins of enterohaemorrhagic Escherichia coli in kidneys of paediatric and geriatric patients with fatal haemolytic uraemic syndrome. Microb. Pathog. 31, 59–67 (2001).
Uchida, H., Kiyokawa, N., Horie, H., Fujimoto, J. & Takeda, T. The detection of Shiga toxins in the kidney of a patient with hemolytic uremic syndrome. Pediatr. Res. 45, 133–137 (1999).
Keepers, T. R., Psotka, M. A., Gross, L. K. & Obrig, T. G. A murine model of HUS: Shiga toxin with lipopolysaccharide mimics the renal damage and physiologic response of human disease. J. Am. Soc. Nephrol. 17, 3404–3414 (2006).
Sauter, K. A. et al. Mouse model of hemolytic-uremic syndrome caused by endotoxin-free Shiga toxin 2 (Stx2) and protection from lethal outcome by anti-Stx2 antibody. Infect. Immun. 76, 4469–4478 (2008).
Mohawk, K. L. & O'Brien, A. D. Mouse models of Escherichia coli O157:H7 infection and Shiga toxin injection. J. Biomed. Biotechnol. 2011, 258185 (2011).
Tzipori, S., Chow, C. W. & Powell, H. R. Cerebral infection with Escherichia coli O157:H7 in humans and gnotobiotic piglets. J. Clin. Pathol. 41, 1099–1103 (1988).
Siegler, R. L., Pysher, T. J., Lou, R., Tesh, V. L. & Taylor, F. B. Jr. Response to Shiga toxin-1, with and without lipopolysaccharide, in a primate model of hemolytic uremic syndrome. Am. J. Nephrol. 21, 420–425 (2001).
Yamamoto, E. T. et al. Shiga toxin 1 causes direct renal injury in rats. Infect. Immun. 73, 7099–7106 (2005).
Lee, J. E. et al. Cytokine expression in the renal tubular epithelial cells stimulated by Shiga toxin 2 of Escherichia coli O157:H7. Ren. Fail. 24, 567–575 (2002).
Obrig, T. G. Escherichia coli Shiga toxin mechanisms of action in renal disease. Toxins (Basel) 2, 2769–2794 (2010).
Williams, J. M. et al. A comparison of the effects of verocytotoxin-1 on primary human renal cell cultures. Toxicol. Lett. 105, 47–57 (1999).
Van Setten, P. A. et al. Verocytotoxin inhibits mitogenesis and protein synthesis in purified human glomerular mesangial cells without affecting cell viability: evidence for two distinct mechanisms. J. Am. Soc. Nephrol. 8, 1877–1888 (1997).
Karpman, D. et al. Apoptosis of renal cortical cells in the hemolytic-uremic syndrome: in vivo and in vitro studies. Infect. Immun. 66, 638–644 (1998).
Shimizu, K., Tanaka, K., Akatsuka, A., Endoh, M. & Koga, Y. Induction of glomerular lesions in the kidneys of mice infected with vero toxin-producing Escherichia coli by lipopolysaccharide injection. J. Infect. Dis. 180, 1374–1377 (1999).
Zhao, Y. L. et al. Shiga-like toxin II derived from Escherichia coli O157:H7 modifies renal handling of levofloxacin in rats. Antimicrob. Agents Chemother. 46, 1522–1528 (2002).
Kodama, T. et al. Induction of apoptosis in human renal proximal tubular epithelial cells by Escherichia coli verocytotoxin 1 in vitro. Med. Microbiol. Immunol. 188, 73–78 (1999).
Kiyokawa, N. et al. Induction of apoptosis in normal human renal tubular epithelial cells by Escherichia coli Shiga toxins 1 and 2. J. Infect. Dis. 178, 178–184 (1998).
Silberstein, C., Pistone Creydt, V., Gerhardt, E., Núñez, P. & Ibarra, C. Inhibition of water absorption in human proximal tubular epithelial cells in response to Shiga toxin-2. Pediatr. Nephrol. 23, 1981–1990 (2008).
Psotka, M. A. et al. Shiga toxin 2 targets the murine renal collecting duct epithelium. Infect. Immun. 77, 959–969 (2009).
Creydt, V. P., Silberstein, C., Zotta, E. & Ibarra, C. Cytotoxic effect of Shiga toxin-2 holotoxin and its B subunit on human renal tubular epithelial cells. Microbes Infect. 8, 410–419 (2006).
Lentz, E. K., Leyva-Illades, D., Lee, M. S., Cherla, R. P. & Tesh, V. L. Differential response of the human renal proximal tubular epithelial cell line HK-2 to Shiga toxin types 1 and 2. Infect. Immun. 79, 3527–3540 (2011).
Sugatani, J. et al. Urinary concentrating defect in rats given Shiga toxin: elevation in urinary AQP2 level associated with polyuria. Life Sci. 71, 171–189 (2002).
Ramos, M. V. et al. Chemokine receptor CCR1 disruption limits renal damage in a murine model of hemolytic uremic syndrome. Am. J. Pathol. 180, 1040–1048 (2012).
Siegler, R. L. The hemolytic uremic syndrome. Pediatr. Clin. North Am. 42, 1505–1529 (1995).
Ergonul, Z., Hughes, A. K. & Kohan, D. E. Induction of apoptosis of human brain microvascular endothelial cells by Shiga toxin 1. J. Infect. Dis. 187, 154–158 (2003).
Obata, F. et al. Shiga toxin 2 affects the central nervous system through receptor globotriaosylceramide localized to neurons. J. Infect. Dis. 198, 1398–1406 (2008).
Ohlmann, D., Hamann, G. F., Hassler, M. & Schimrigk, K. Involvement of the central nervous system in hemolytic uremic syndrome/thrombotic thrombocytopenic purpura [German]. Nervenarzt 67, 880–882 (1996).
Magnus, T. et al. The neurological syndrome in adults during the 2011 northern German E. coli serotype O104:H4 outbreak. Brain 135, 1850–1859 (2012).
Fujii, J. et al. Magnetic resonance imaging and histopathological study of brain lesions in rabbits given intravenous verotoxin 2. Infect. Immun. 64, 5053–5060 (1996).
Jacewicz, M. S. et al. Responses of human intestinal microvascular endothelial cells to Shiga toxins 1 and 2 and pathogenesis of hemorrhagic colitis. Infect. Immun. 67, 1439–1444 (1999).
Shiraishi, M. et al. Soluble tumor necrosis factor receptor 1 and tissue inhibitor of metalloproteinase-1 in hemolytic uremic syndrome with encephalopathy. J. Neuroimmunol. 196, 147–152 (2008).
Eisenhauer, P. B. et al. Tumor necrosis factor alpha increases human cerebral endothelial cell Gb3 and sensitivity to Shiga toxin. Infect. Immun. 69, 1889–1894 (2001).
Ramegowda, B., Samuel, J. E. & Tesh, V. L. Interaction of Shiga toxins with human brain microvascular endothelial cells: cytokines as sensitizing agents. J. Infect. Dis. 180, 1205–1213 (1999).
Stricklett, P. K., Hughes, A. K., Ergonul, Z. & Kohan, D. E. Molecular basis for up-regulation by inflammatory cytokines of Shiga toxin 1 cytotoxicity and globotriaosylceramide expression. J. Infect. Dis. 186, 976–982 (2002).
Orth, D. & Würzner, R. Complement in typical hemolytic uremic syndrome. Semin. Thromb. Hemost. 36, 620–624 (2010).
Morigi, M. et al. Alternative pathway activation of complement by Shiga toxin promotes exuberant C3a formation that triggers microvascular thrombosis. J. Immunol. 187, 172–180 (2011).
Orth, D. et al. Shiga toxin activates complement and binds factor H: evidence for an active role of complement in hemolytic uremic syndrome. J. Immunol. 182, 6394–6400 (2009).
Orth, D. et al. EspP, a serine protease of enterohemorrhagic Escherichia coli, impairs complement activation by cleaving complement factors C3/C3b and C5. Infect. Immun. 78, 4294–4301 (2010).
Thurman, J. M. et al. Alternative pathway of complement in children with diarrhea-associated hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 4, 1920–1924 (2009).
Ståhl, A. L., Sartz, L. & Karpman, D. Complement activation on platelet-leukocyte complexes and microparticles in enterohemorrhagic Escherichia coli-induced hemolytic uremic syndrome. Blood 117, 5503–5513 (2011).
Noris, M., Mescia, F. & Remuzzi, G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat. Rev. Nephrol. http://dx.doi.org/10.1038/nrneph.2012.195.
Krogvold, L. et al. Clinical aspects of a nationwide epidemic of severe haemolytic uremic syndrome (HUS) in children. Scand. J. Trauma Resusc. Emerg. Med. 19, 44 (2011).
Frank, C. et al. for the HUS Investigation Team. Epidemic profile of Shiga-toxin-producing Escherichia coli O104:H4 outbreak in Germany. N. Engl. J. Med. 365, 1771–1780 (2011).
Micheletti, M. V., Lavoratti, G., Materassi, M. & Pela, I. Hemolytic uremic syndrome: epidemiological and clinical features of a pediatric population in Tuscany. Kidney Blood Press. Res. 33, 399–404 (2010).
Constantinescu, A. R. et al. Non-enteropathic hemolytic uremic syndrome: causes and short-term course. Am. J. Kidney Dis. 43, 976–982 (2004).
Ake, J. A. et al. Relative nephroprotection during Escherichia coli O157:H7 infections: association with intravenous volume expansion. Pediatrics 115, e673–e680 (2005).
Hickey, C. A. et al. Early volume expansion during diarrhea and relative nephroprotection during subsequent hemolytic uremic syndrome. Arch. Pediatr. Adolesc. Med. 165, 884–889 (2011).
Miedouge, M., Hacini, J., Grimont, F. & Watine, J. Shiga toxin-producing Escherichia coli urinary tract infection associated with hemolytic-uremic syndrome in an adult and possible adverse effect of ofloxacin therapy. Clin. Infect. Dis. 30, 395–396 (2000).
Dobiliene, D., Pundziene, B. & Mitkiene, R. The clinical course and long-term outcome of hemolytic-uremic syndrome in children [Lithuanian]. Medicina (Kaunas) 43 (Suppl. 1), 23–27 (2007).
Garg, A. X. et al. Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 290, 1360–1370 (2003).
Gerber, A., Karch, H., Allerberger, F., Verweyen, H. M. & Zimmerhackl, L. B. Clinical course and the role of Shiga toxin-producing Escherichia coli infection in the hemolytic-uremic syndrome in pediatric patients, 1997–2000, in Germany and Austria: a prospective study. J. Infect. Dis. 186, 493–500 (2002).
Fujii, K., Matsuo, K., Takatani, T., Uchikawa, H. & Kohno, Y. Multiple cavitations in posterior reversible leukoencephalopathy syndrome associated with hemolytic-uremic syndrome. Brain Dev. 34, 318–321 (2012).
Nathanson, S. et al. Acute neurological involvement in diarrhea-associated hemolytic uremic syndrome. Clin. J. Am. Soc. Nephrol. 5, 1218–1228 (2010).
Schlieper, A. et al. Neuropsychological sequelae of haemolytic uraemic syndrome. Investigators of the HUS Cognitive Study. Arch. Dis. Child. 80, 214–220 (1999).
Pomajzl, R. J., Varman, M., Holst, A. & Chen, A. Hemolytic uremic syndrome (HUS)—incidence and etiologies at a regional Children's Hospital in 2001–2006. Eur. J. Clin. Microbiol. Infect. Dis. 28, 1431–1435 (2009).
Bielaszewska, M. et al. Characterisation of the Escherichia coli strain associated with an outbreak of haemolytic uraemic syndrome in Germany, 2011: a microbiological study. Lancet Infect. Dis. 11, 671–676 (2011).
Rohde, H. et al. and the E. coli O104:H4 Genome Analysis Crowd-Sourcing Consortium. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N. Engl. J. Med. 365, 718–724 (2011).
Bae, W. K. et al. A case of hemolytic uremic syndrome caused by Escherichia coli O104:H4. Yonsei Med. J. 47, 437–439 (2006).
Mellmann, A. et al. Analysis of collection of hemolytic uremic syndrome-associated enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 14, 1287–1290 (2008).
Buchholz, U. et al. German outbreak of Escherichia coli O104:H4 associated with sprouts. N. Engl. J. Med. 365, 1763–1770 (2011).
Loos, S. et al. An outbreak of Shiga-toxin producing E. coli O104:H4 hemolytic uremic syndrome (STEC-HUS) in Germany: presentation and short-term outcome in children. Clin. Infect. Dis. 55, 753–759 (2012).
Dundas, S. et al. Effectiveness of therapeutic plasma exchange in the 1996 Lanarkshire Escherichia coli O157:H7 outbreak. Lancet 354, 1327–1330 (1999).
Lewinski, M. & Stahl, R. A. K. The number of plasma exchanges and the extended need of dialysis correlate with poor long term outcome in adult patients with Escherichia coli associated hemolytic uremic syndrome during the 2011 German Outbreak [accepted abstract]. To be presented at ASN Kidney Week 2012.
Colic, E., Dieperink, H., Titlestad, K. & Tepel, M. Management of an acute outbreak of diarrhoea-associated haemolytic uraemic syndrome with early plasma exchange in adults from southern Denmark: an observational study. Lancet 378, 1089–1093 (2011).
Rosales, A. et al. for the German-Austrian HUS Study Group. Need for long-term follow-up in enterohemorrhagic Escherichia coli-associated hemolytic uremic syndrome due to late-emerging sequela. Clin. Infect. Dis. 54, 1413–1421 (2012).
Lapeyraque, A.-L. et al. Eculizumab in severe Shiga-toxin-associated HUS. N. Engl. J. Med. 364, 2561–2563 (2011).
European Centre for Disease Prevention and Control. Shiga toxin-producing E. coli (STEC): update on outbreak in the EU [online], (2011).
Stahl, R. A. K. for the Eculizumab Study Group. Open-label, multi-center trial of eculizumab in patients with Shiga-toxin-producing E. coli hemolytic uremic syndrome (STEC-HUS). To be presented at ASN Kidney Week 2012.
Harendza, S. “HUS diary” of a German nephrologist during the current EHEC outbreak in Europe. Kidney Int. 80, 687–689 (2011).
Clark, W. F. et al. Long term risk for hypertension, renal impairment, and cardiovascular disease after gastroenteritis from drinking water contaminated with Escherichia coli O157:H7: a prospective cohort study. BMJ 341, c6020 (2010).
Greinacher, A. et al. Treatment of severe neurological deficits with IgG depletion through immunoadsorption in patients with Escherichia coli O104:H4-associated haemolytic uraemic syndrome: a prospective trial. Lancet 378, 1166–1173 (2011).
Menne, J. et al. on behalf of the EHEC-HUS consortium. Validation of treatment strategies for enterohaemorrhagic Escherichia coli O104:H4 induced haemolytic uraemic syndrome: case-control study. BMJ 345, e4565 (2012).
Wong, C. S. et al. Risk factors for the hemolytic uremic syndrome in children infected with Escherichia coli O157:H7: a multivariable analysis. Clin. Infect. Dis. 55, 33–41 (2012).
Corogeanu, D. et al. Therapeutic concentrations of antibiotics inhibit Shiga toxin release from enterohemorrhagic E. coli O104:H4 from the 2011 German outbreak. BMC Microbiol. 12, 160 (2012).
Nitschke, M. et al. Association between azithromycin therapy and duration of bacterial shedding among patients with Shiga toxin-producing enteroaggregative Escherichia coli O104:H4. JAMA 307, 1046–1052 (2012).
Seifert, M. E. & Tarr, P. I. Therapy: azithromycin and decolonization after HUS. Nat. Rev. Nephrol. 8, 317–318 (2012).
Author information
Authors and Affiliations
Contributions
All authors researched data to include in the article and reviewed and edited the manuscript before submission. H. Trachtman and R. A. K. Stahl contributed equally to discussion of content and writing the article.
Corresponding author
Ethics declarations
Competing interests
H. Trachtman has been a consultant for Alexion Pharmaceuticals. R. A. K. Stahl received eculizumab from Alexion Pharmaceuticals to treat patients at the University Medical Centre in Hamburg–Eppendorf. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Trachtman, H., Austin, C., Lewinski, M. et al. Renal and neurological involvement in typical Shiga toxin-associated HUS. Nat Rev Nephrol 8, 658–669 (2012). https://doi.org/10.1038/nrneph.2012.196
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.196
This article is cited by
-
Eculizumab in Shiga toxin-producing Escherichia coli hemolytic uremic syndrome: a systematic review
Pediatric Nephrology (2024)
-
Therapeutic plasma exchange in paediatric nephrology in Ireland
Irish Journal of Medical Science (1971 -) (2023)
-
Shiga toxin-producing Escherichia coli-associated hemolytic uremic syndrome with recurrent acute cholecystitis: a case report
CEN Case Reports (2023)
-
A unique peptide-based pharmacophore identifies an inhibitory compound against the A-subunit of Shiga toxin
Scientific Reports (2022)
-
A case of hemolytic uremic syndrome caused by Shiga toxin-producing Escherichia coli after pericardiectomy
Clinical Journal of Gastroenterology (2022)