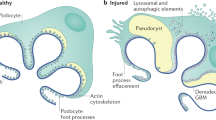
Abstract
The introduction of corticosteroids more than 50 years ago dramatically improved the prognosis of children with nephrotic syndrome. Corticosteroids remain the standard initial treatment for children with this disease, but a considerable proportion of patients do not respond and are therefore at risk of progressing to end-stage renal disease. Because of this risk, new therapeutic strategies are needed for steroid-resistant nephrotic syndrome. These strategies have historically focused on identifying effective alternative immunosuppressive agents, such as ciclosporin and tacrolimus, yet evidence now indicates that nephrotic syndrome results from podocyte dysfunction. Even conventional immunosuppressive agents, such as glucocorticoids and ciclosporin, directly affect podocyte structure and function, challenging the 'immune theory' of the pathogenesis of childhood nephrotic syndrome in which disease is caused by T cells. This Review summarizes the currently available treatments for childhood nephrotic syndrome, and discusses selected novel pathways in podocytes that could be targeted for the development of next-generation treatments for children with this syndrome.
Key Points
-
Corticosteroids have been used to treat childhood nephrotic syndrome for more than 50 years but a minority of patients, especially those with focal segmental glomerulosclerosis, are resistant to this treatment
-
Evidence now suggests that childhood nephrotic syndrome is attributable to podocyte dysfunction; many medications used to treat childhood nephrotic syndrome target the immune system, but also directly affect podocytes
-
Several currently available agents developed for other diseases, such as diabetes, are now being considered for treatment of steroid-resistant childhood nephrotic syndrome
-
Ongoing research has identified a number of pathways occuring in podocytes that may be potential targets for future therapies to treat steroid-resistant childhood nephrotic syndrome
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Arneil, G. C. The nephrotic syndrome. Pediatr. Clin. North Am. 18, 547–559 (1971).
Cameron, J. S. in The Nephrotic Syndrome (eds Cameron, J. S., Glassock, R. J. & Whelton, A.) 3–56 (Marcel Dekker, Inc., New York, 1988).
Arneil, G. C. & Lam, C. N. Long-term assessment of steroid therapy in childhood nephrosis. Lancet 2, 819–821 (1966).
Minimal change nephrotic syndrome in children: deaths during the first 5 to 15 years' observation. Report of the International Study of Kidney Disease in Children. Pediatrics 73, 497–501 (1984).
Chavers, B. M., Li, S., Collins, A. J. & Herzog, C. A. Cardiovascular disease in pediatric chronic dialysis patients. Kidney Int. 62, 648–653 (2002).
Benoit, G., Machuca, E. & Antignac, C. Hereditary nephrotic syndrome: a systematic approach for genetic testing and a review of associated podocyte gene mutations. Pediatr. Nephrol. 25, 1621–1632 (2010).
Machuca, E., Benoit, G. & Antignac, C. Genetics of nephrotic syndrome: connecting molecular genetics to podocyte physiology. Hum. Mol. Genet. 18, R185–R194 (2009).
Lowik, M. M., Groenen, P. J., Levtchenko, E. N., Monnens, L. A. & van den Heuvel, L. P. Molecular genetic analysis of podocyte genes in focal segmental glomerulosclerosis—a review. Eur. J. Pediatr. 27, 27 (2009).
Savin, V. J. et al. Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N. Engl. J. Med. 334, 878–883 (1996).
Wei, C. et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat. Med. 17, 952–960 (2011).
Hinkes, B. et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 38, 1397–1405 (2006).
Ozaltin, F. et al. Disruption of PTPRO causes childhood-onset nephrotic syndrome. Am. J. Hum. Genet. 89, 139–147 (2011).
Wei, C. & Reiser, J. Minimal change disease as a modifiable podocyte paracrine disorder. Nephrol. Dial. Transplant. 26, 1776–1777 (2011).
Schonenberger, E., Ehrich, J. H., Haller, H. & Schiffer, M. The podocyte as a direct target of immunosuppressive agents. Nephrol. Dial. Transplant. 26, 18–24 (2011).
Churg, J., Habib, R. & White, R. H. Pathology of the nephrotic syndrome in children: a report for the International Study of Kidney Disease in Children. Lancet 760, 1299–1302 (1970).
The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J. Pediatr. 98, 561–564 (1981).
Primary nephrotic syndrome in children: clinical significance of histopathologic variants of minimal change and of diffuse mesangial hypercellularity. A Report of the International Study of Kidney Disease in Children. Kidney Int. 20, 765–771 (1981).
Eddy, A. A. & Symons, J. M. Nephrotic syndrome in childhood. Lancet 362, 629–639 (2003).
Rydel, J. J., Korbet, S. M., Borok, R. Z. & Sxhwartz, M. M. Focal segmental glomerular sclerosis in adults: presentation, course, and response to treatment. Am. J. Kidney Dis. 25, 534–542 (1995).
Chun, M. J., Korbet, S. M., Schwartz, M. M. & Lewis, E. J. Focal segmental glomerulosclerosis in nephrotic adults: presentation, prognosis, and response to therapy of the histologic variants. J. Am. Soc. Nephrol. 15, 2169–2177 (2004).
Hodson, E. M., Willis, N. S. & Craig, J. C. Corticosteroid therapy for nephrotic syndrome in children. Cochrane Database of Systematic Reviews, Issue 4. Article No.: CD001533.doi: 10.1002/14651858.CD001533.pub4 (2007).
Saadeh, S. A. et al. Weight or body surface area dosing of steroids in nephrotic syndrome: is there an outcome difference? Pediatr. Nephrol. 26, 2167–2171 (2011).
Ransom, R. F., Lam, N. G., Hallett, M. A., Atkinson, S. J. & Smoyer, W. E. Glucocorticoids protect and enhance recovery of cultured murine podocytes via actin filament stabilization. Kidney Int. 68, 2473–2483 (2005).
Murnaghan, K., Vasmant, D. & Bensman, A. Pulse methylprednisolone therapy in severe idiopathic childhood nephrotic syndrome. Acta Paediatr. Scand. 73, 733–739 (1984).
Hari, P., Bagga, A. & Mantan, M. Short term efficacy of intravenous dexamethasone and methylprednisolone therapy in steroid resistant nephrotic syndrome. Indian Pediatr. 41, 993–1000 (2004).
Mori, K., Honda, M. & Ikeda, M. Efficacy of methylprednisolone pulse therapy in steroid-resistant nephrotic syndrome. Pediatr. Nephrol. 19, 1232–1236 (2004).
Shenoy, M. et al. Intravenous methylprednisolone in idiopathic childhood nephrotic syndrome. Pediatr. Nephrol. 25, 899–903 (2010).
Tune, B. M. et al. Intravenous methylprednisolone and oral alkylating agent therapy of prednisone-resistant pediatric focal segmental glomerulosclerosis: a long-term follow-up. Clin. Nephrol. 43, 84–88 (1995).
Tune, B. M. & Mendoza, S. A. Treatment of the idiopathic nephrotic syndrome: regimens and outcomes in children and adults. J. Am. Soc. Nephrol. 8, 824–832 (1997).
Mendoza, S. A. et al. Treatment of steroid-resistant focal segmental glomerulosclerosis with pulse methylprednisolone and alkylating agents. Pediatr. Nephrol. 4, 303–307 (1990).
Ehrich, J. H. et al. Steroid-resistant idiopathic childhood nephrosis: overdiagnosed and undertreated. Nephrol. Dial. Transplant. 22, 2183–2193 (2007).
Vester, U., Kranz, B., Zimmermann, S. & Hoyer, P. F. Cyclophosphamide in steroid-sensitive nephrotic syndrome: outcome and outlook. Pediatr. Nephrol. 18, 661–664 (2003).
Plank, C. et al. Cyclosporin A is superior to cyclophosphamide in children with steroid-resistant nephrotic syndrome—a randomized controlled multicentre trial by the Arbeitsgemeinschaft für Pädiatrische Nephrologie. Pediatr. Nephrol. 23, 1483–1493 (2008).
Sharda, S. V. et al. Do glutathione-S-transferase polymorphisms influence response to intravenous cyclophosphamide therapy in idiopathic nephrotic syndrome? Pediatr. Nephrol. 23, 2001–2006 (2008).
Vester, U., Kranz, B., Zimmermann, S., Buscher, R. & Hoyer, P. F. The response to cyclophosphamide in steroid-sensitive nephrotic syndrome is influenced by polymorphic expression of glutathion-S-transferases-M1 and -P1. Pediatr. Nephrol. 20, 478–481 (2005).
Latta, K., von Schnakenburg, C. & Ehrich, J. H. A meta-analysis of cytotoxic treatment for frequently relapsing nephrotic syndrome in children. Pediatr. Nephrol. 16, 271–282 (2001).
Dooley, M. A. et al. Mycophenolate versus azathioprine as maintenance therapy for lupus nephritis. N. Engl. J. Med. 365, 1886–1895 (2011).
Tang, S. C. et al. Long-term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney Int. 77, 543–549 (2010).
Eugui, E. M., Almquist, S. J., Muller, C. D. & Allison, A. C. Lymphocyte-selective cytostatic and immunosuppressive effects of mycophenolic acid in vitro: role of deoxyguanosine nucleotide depletion. Scand. J. Immunol. 33, 161–173 (1991).
Ishikawa, H. Mizoribine and mycophenolate mofetil. Curr. Med. Chem. 6, 575–597 (1999).
Senthil Nayagam, L. et al. Mycophenolate mofetil or standard therapy for membranous nephropathy and focal segmental glomerulosclerosis: a pilot study. Nephrol. Dial. Transplant. 23, 1926–1930 (2008).
Cattran, D. C., Wang, M. M., Appel, G., Matalon, A. & Briggs, W. Mycophenolate mofetil in the treatment of focal segmental glomerulosclerosis. Clin. Nephrol. 62, 405–411 (2004).
Moudgil, A., Bagga, A. & Jordan, S. C. Mycophenolate mofetil therapy in frequently relapsing steroid-dependent and steroid-resistant nephrotic syndrome of childhood: current status and future directions. Pediatr. Nephrol. 20, 1376–1381 (2005).
Fujinaga, S. et al. Mycophenolate mofetil therapy for childhood-onset steroid dependent nephrotic syndrome after long-term cyclosporine: extended experience in a single center. Clin. Nephrol. 72, 268–273 (2009).
de Mello, V. R. et al. Mycophenolate mofetil in children with steroid/cyclophosphamide-resistant nephrotic syndrome. Pediatr. Nephrol. 25, 453–460 (2010).
Gargah, T. T. & Lakhoua, M. R. Mycophenolate mofetil in treatment of childhood steroid-resistant nephrotic syndrome. J. Nephrol. 24, 203–207 (2011).
Gipson, D. S. et al. Clinical trial of focal segmental glomerulosclerosis in children and young adults. Kidney Int. 80, 868–878 (2011).
Fujinaga, S. et al. Single daily high-dose mizoribine therapy for children with steroid-dependent nephrotic syndrome prior to cyclosporine administration. Pediatr. Nephrol. 26, 479–483 (2011).
Tanaka, H. et al. Mizoribine pulse therapy for a pediatric patient with steroid-resistant nephrotic syndrome. Tohoku J. Exp. Med. 205, 87–91 (2005).
Borel, J. F. Mechanism of action of cyclosporin A and rationale for use in nephrotic syndrome. Clin. Nephrol. 35 (Suppl. 1), S23–S30 (1991).
Faul, C. et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat. Med. 14, 931–938 (2008).
Lieberman, K. V. & Tejani, A. A randomized double-blind placebo-controlled trial of cyclosporine in steroid-resistant idiopathic focal segmental glomerulosclerosis in children. J. Am. Soc. Nephrol. 7, 56–63 (1996).
Cattran, D. C. et al. A randomized trial of cyclosporine in patients with steroid-resistant focal segmental glomerulosclerosis. North America Nephrotic Syndrome Study Group. Kidney Int. 56, 2220–2226 (1999).
Niaudet, P. Treatment of childhood steroid-resistant idiopathic nephrosis with a combination of cyclosporine and prednisone. French Society of Pediatric Nephrology. J. Pediatr. 125, 981–986 (1994).
Iijima, K. et al. Risk factors for cyclosporine-induced tubulointerstitial lesions in children with minimal change nephrotic syndrome. Kidney Int. 61, 1801–1805 (2002).
Bowman, L. J. & Brennan, D. C. The role of tacrolimus in renal transplantation. Expert Opin. Pharmacother. 9, 635–643 (2008).
McCauley, J. et al. Pilot trial of FK 506 in the management of steroid-resistant nephrotic syndrome. Nephrol. Dial. Transplant. 8, 1286–1290 (1993).
Gulati, S. et al. Tacrolimus: a new therapy for steroid-resistant nephrotic syndrome in children. Nephrol. Dial. Transplant. 23, 910–913 (2008).
Loeffler, K., Gowrishankar, M. & Yiu, V. Tacrolimus therapy in pediatric patients with treatment-resistant nephrotic syndrome. Pediatr. Nephrol. 19, 281–287 (2004).
Westhoff, T. H., Schmidt, S., Zidek, W., Beige, J. & van der Giet, M. Tacrolimus in steroid-resistant and steroid-dependent nephrotic syndrome. Clin. Nephrol. 65, 393–400 (2006).
Choudhry, S. et al. Efficacy and safety of tacrolimus versus cyclosporine in children with steroid-resistant nephrotic syndrome: a randomized controlled trial. Am. J. Kidney Dis. 53, 760–769 (2009).
Bhimma, R., Adhikari, M., Asharam, K. & Connolly, C. Management of steroid-resistant focal segmental glomerulosclerosis in children using tacrolimus. Am. J. Nephrol. 26, 544–551 (2006).
Garcia, C. D. et al. Plasmapheresis for recurrent posttransplant focal segmental glomerulosclerosis. Transplant. Proc. 38, 1904–1905 (2006).
Bosch, T. & Wendler, T. Extracorporeal plasma treatment in primary and recurrent focal segmental glomerular sclerosis: a review. Ther. Apher. 5, 155–160 (2001).
Pradhan, M., Petro, J., Palmer, J., Meyers, K. & Baluarte, H. J. Early use of plasmapheresis for recurrent post-transplant FSGS. Pediatr. Nephrol. 18, 934–938 (2003).
Feld, S. M. et al. Plasmapheresis in the treatment of steroid-resistant focal segmental glomerulosclerosis in native kidneys. Am. J. Kidney Dis. 32, 230–237 (1998).
Ginsburg, D. S. & Dau, P. Plasmapheresis in the treatment of steroid-resistant focal segmental glomerulosclerosis. Clin. Nephrol. 48, 282–287 (1997).
Oliveira, L., Wang, D. & McCormick, B. B. A case report of plasmapheresis and cyclophosphamide for steroid-resistant focal segmental glomerulosclerosis: recovery of renal function after five months on dialysis. Ther. Apher. Dial. 11, 227–231 (2007).
Jayne, D. Role of rituximab therapy in glomerulonephritis. J. Am. Soc. Nephrol. 21, 14–17 (2010).
Ruggenenti, P. et al. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin. J. Am. Soc. Nephrol. 1, 738–748 (2006).
Fervenza, F. C. et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 73, 117–125 (2008).
Fornoni, A. et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci. Transl. Med. 3, 85ra46 (2011).
Guigonis, V. et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr. Nephrol. 23, 1269–1279 (2008).
Prytula, A. et al. Rituximab in refractory nephrotic syndrome. Pediatr. Nephrol. 25, 461–468 (2010).
Gulati, A. et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin. J. Am. Soc. Nephrol. 5, 2207–2212 (2010).
Bagga, A., Sinha, A. & Moudgil, A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N. Engl. J. Med. 356, 2751–2752 (2007).
Kari, J. A. et al. Rituximab for refractory cases of childhood nephrotic syndrome. Pediatr. Nephrol. 26, 733–737 (2011).
Suri, M., Tran, K., Sharma, A. P., Filler, G. & Grimmer, J. Remission of steroid-resistant nephrotic syndrome due to focal and segmental glomerulosclerosis using rituximab. Int. Urol. Nephrol. 40, 807–810 (2008).
Kurosu, N. et al. Successful use of single-dose rituximab for the maintenance of remission in a patient with steroid-resistant nephrotic syndrome. Intern. Med. 48, 1901–1904 (2009).
Peters, H. P., van de Kar, N. C. & Wetzels, J. F. Rituximab in minimal change nephropathy and focal segmental glomerulosclerosis: report of four cases and review of the literature. Neth. J. Med. 66, 408–415 (2008).
Smith, G. C. Is there a role for rituximab in the treatment of idiopathic childhood nephrotic syndrome? Pediatr. Nephrol. 22, 893–898 (2007).
Nakayama, M. et al. Rituximab for refractory focal segmental glomerulosclerosis. Pediatr. Nephrol. 23, 481–485 (2008).
Sharma, A. P. & Filler, G. Role of mycophenolate mofetil in remission maintenance after a successful response to rituximab. Pediatr. Nephrol. 24, 423–424 (2009).
Ravani, P. et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin. J. Am. Soc. Nephrol. 6, 1308–1315 (2011).
Sinha, A., Bagga, A., Gulati, A. & Hari, P. Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephrotic syndrome. Pediatr. Nephrol. 27, 235–241 (2011).
Sellier-Leclerc, A. L. et al. Rituximab in steroid-dependent idiopathic nephrotic syndrome in childhood—follow-up after CD19 recovery. Nephrol. Dial. Transplant. 27, 1083–1089 (2012).
Kemper, M. J. et al. Long-term follow-up after rituximab for steroid-dependent idiopathic nephrotic syndrome. Nephrol. Dial. Transplant. http://dx.doi:10.1093/ndt/gfr548.
Iijima, K. Rituximab for childhood refractory nephrotic syndrome. Pediatr. Int. 53, 617–621 (2011).
Magnasco, A. Rituximab in children with resistant idiopathic nephrotic syndrome. J. Am. Soc. Nephrol. http://dx.doi:10.1681/ASN.2011080775.
Sellier-Leclerc, A.-L. et al. Rituximab efficiency in children with steroid-dependent nephrotic syndrome. Pediatr. Nephrol. 25, 1109–1115 (2010).
Kamei, K. et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr. Nephrol. 24, 1321–1328 (2009).
Ardelean, D. S. et al. Severe ulcerative colitis after rituximab therapy. Pediatrics 126, e243–e246 (2010).
Chan, A. C. Rituximab's new therapeutic target: the podocyte actin cytoskeleton. Sci. Transl. Med. 3, 85ps21 (2011).
Savin, V. J., McCarthy, E. T., Sharma, R., Charba, D. & Sharma, M. Galactose binds to focal segmental glomerulosclerosis permeability factor and inhibits its activity. Transl. Res. 151, 288–292 (2008).
De Smet, E., Rioux, J. P., Ammann, H., Deziel, C. & Querin, S. FSGS permeability factor-associated nephrotic syndrome: remission after oral galactose therapy. Nephrol. Dial. Transplant. 24, 2938–2940 (2009).
Kopac, M., Meglic, A. & Rus, R. R. Partial remission of resistant nephrotic syndrome after oral galactose therapy. Ther. Apher. Dial. 15, 269–272 (2011).
Novel therapies in resistant focal segmental glomerulosclerosis: www.fonttrial.org (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Suranyi, M. G., Guasch, A., Hall, B. M. & Myers, B. D. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. Am. J. Kidney Dis. 21, 251–259 (1993).
Bustos, C., Gonzalez, E., Muley, R., Alonso, J. L. & Egido, J. Increase of tumour necrosis factor alpha synthesis and gene expression in peripheral blood mononuclear cells of children with idiopathic nephrotic syndrome. Eur. J. Clin. Invest. 24, 799–805 (1994).
Gupta, A., Pendyala, P., Arora, P. & Sitrin, M. D. Development of the nephrotic syndrome during treatment of Crohn's disease with adalimumab. J. Clin. Gastroenterol. 45, e30–e33 (2011).
Nowak, B., Jeka, S., Wiland, P. & Szechinski, J. Rapid and complete resolution of ascites and hydrothorax due to nephrotic syndrome caused by renal amyloidosis in a patient with juvenile chronic arthritis treated with adalimumab. Joint Bone Spine 76, 217–219 (2009).
Joy, M. S. et al. Phase 1 trial of adalimumab in Focal Segmental Glomerulosclerosis (FSGS): II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am. J. Kidney Dis. 55, 50–60 (2010).
Michalik, L. et al. International Union of Pharmacology. LXI. Peroxisome proliferator-activated receptors. Pharmacol. Rev. 58, 726–741 (2006).
Ma, L. J., Marcantoni, C., Linton, M. F., Fazio, S. & Fogo, A. B. Peroxisome proliferator-activated receptor-gamma agonist troglitazone protects against nondiabetic glomerulosclerosis in rats. Kidney Int. 59, 1899–1910 (2001).
Yang, H. C., Ma, L. J., Ma, J. & Fogo, A. B. Peroxisome proliferator-activated receptor-gamma agonist is protective in podocyte injury-associated sclerosis. Kidney Int. 69, 1756–1764 (2006).
Cha, D. R. et al. Peroxisome proliferator activated receptor alpha/gamma dual agonist tesaglitazar attenuates diabetic nephropathy in db/db mice. Diabetes 56, 2036–2045 (2007).
Sarafidis, P. A., Stafylas, P. C., Georgianos, P. I., Saratzis, A. N. & Lasaridis, A. N. Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am. J. Kidney Dis. 55, 835–847 (2010).
Mao, Z. & Ong, A. C. Peroxisome proliferator-activated receptor gamma agonists in kidney disease--future promise, present fears. Nephron Clin. Pract. 112, c230–c241 (2009).
Kanjanabuch, T. et al. PPAR-gamma agonist protects podocytes from injury. Kidney Int. 71, 1232–1239 (2007).
Liu, H. F. et al. Thiazolidinedione attenuate proteinuria and glomerulosclerosis in Adriamycin-induced nephropathy rats via slit diaphragm protection. Nephrology (Carlton) 15, 75–83 (2010).
Agrawal, S., Guess, A. J., Benndorf, R. & Smoyer, W. E. Comparison of direct action of thiazolidinediones and glucocorticoids on renal podocytes: protection from injury and molecular effects. Mol. Pharmacol. 80, 389–399 (2011).
Kawai, T. et al. PPAR-gamma agonist attenuates renal interstitial fibrosis and inflammation through reduction of TGF-β. Lab. Invest. 89, 47–58 (2009).
Coulthard, L. R., White, D. E., Jones, D. L., McDermott, M. F. & Burchill, S. A. p38(MAPK): stress responses from molecular mechanisms to therapeutics. Trends Mol. Med. 15, 369–379 (2009).
Grande, M. T. & Lopez-Novoa, J. M. Therapeutical relevance of MAP-kinase inhibitors in renal diseases: current knowledge and future clinical perspectives. Curr. Med. Chem. 15, 2054–2070 (2008).
Gaestel, M. Specificity of signaling from MAPKs to MAPKAPKs: kinases' tango nuevo. Front. Biosci. 13, 6050–6059 (2008).
Stambe, C., Atkins, R. C., Hill, P. A. & Nikolic-Paterson, D. J. Activation and cellular localization of the p38 and JNK MAPK pathways in rat crescentic glomerulonephritis. Kidney Int. 64, 2121–2132 (2003).
Sheryanna, A. et al. Inhibition of p38 mitogen-activated protein kinase is effective in the treatment of experimental crescentic glomerulonephritis and suppresses monocyte chemoattractant protein-1 but not IL-1β or IL-6. J. Am. Soc. Nephrol. 18, 1167–1179 (2007).
Koshikawa, M. et al. Role of p38 mitogen-activated protein kinase activation in podocyte injury and proteinuria in experimental nephrotic syndrome. J. Am. Soc. Nephrol. 16, 2690–2701 (2005).
Stambe, C., Nikolic-Paterson, D. J., Hill, P. A., Dowling, J. & Atkins, R. C. p38 Mitogen-activated protein kinase activation and cell localization in human glomerulonephritis: correlation with renal injury. J. Am. Soc. Nephrol. 15, 326–336 (2004).
Polzer, K. et al. Selective p38MAPK isoform expression and activation in antineutrophil cytoplasmatic antibody-associated crescentic glomerulonephritis: role of p38MAPKα. Ann. Rheum. Dis. 67, 602–608 (2008).
Pengal, R. et al. Inhibition of the protein kinase MK-2 protects podocytes from nephrotic syndrome-related injury. Am. J. Physiol. Renal Physiol. 301, F509–F519 (2011).
Yoshida, S., Nagase, M., Shibata, S. & Fujita, T. Podocyte injury induced by albumin overload in vivo and in vitro: involvement of TGF-β and p38 MAPK. Nephron Exp. Nephrol. 108, e57–e68 (2008).
Sekar, M. C., Yang, M., Meezan, E. & Pillion, D. J. Angiotensin II and bradykinin stimulate phosphoinositide breakdown in intact rat kidney glomeruli but not in proximal tubules: glomerular response modulated by phorbol ester. Biochem. Biophys. Res. Commun. 166, 373–379 (1990).
Nowicki, S., Kruse, M. S., Brismar, H. & Aperia, A. Dopamine-induced translocation of protein kinase C isoforms visualized in renal epithelial cells. Am. J. Physiol. Cell. Physiol. 279, C1812–C1818 (2000).
Huber, T. B. et al. Loss of podocyte aPKCλ/ι causes polarity defects and nephrotic syndrome. J. Am. Soc. Nephrol. 20, 798–806 (2009).
Menne, J. et al. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-α-deficient diabetic mice. Diabetes 53, 2101–2109 (2004).
Tossidou, I. et al. PKC-α modulates TGF-β signaling and impairs podocyte survival. Cell. Physiol. Biochem. 24, 627–634 (2009).
Schiffer, M. et al. Apoptosis in podocytes induced by TGF-β and Smad7. J. Clin. Invest. 108, 807–816 (2001).
Shen, G. X. Selective protein kinase C inhibitors and their applications. Curr. Drug Targets Cardiovasc. Haematol. Disord. 3, 301–307 (2003).
Gaestel, M., Mengel, A., Bothe, U. & Asadullah, K. Protein kinases as small molecule inhibitor targets in inflammation. Curr. Med. Chem. 14, 2214–2234 (2007).
Baier, G. & Wagner, J. PKC inhibitors: potential in T cell-dependent immune diseases. Curr. Opin. Cell. Biol. 21, 262–267 (2009).
Wagner, E. F. & Nebreda, A. R. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537–549 (2009).
Yong, H. Y., Koh, M. S. & Moon, A. The p38 MAPK inhibitors for the treatment of inflammatory diseases and cancer. Expert Opin. Investig. Drugs 18, 1893–1905 (2009).
Roffey, J. et al. Protein kinase C intervention: the state of play. Curr. Opin. Cell. Biol. 21, 268–279 (2009).
Niranjan, T., Murea, M. & Susztak, K. The pathogenic role of Notch activation in podocytes. Nephron Exp. Nephrol. 111, e73–e79 (2009).
Barisoni, L. Notch signaling: a common pathway of injury in podocytopathies? J. Am. Soc. Nephrol. 19, 1045–1046 (2008).
Niranjan, T. et al. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat. Med. 14, 290–298 (2008).
Walsh, D. W. et al. Co-regulation of Gremlin and Notch signalling in diabetic nephropathy. Biochim. Biophys. Acta 1782, 10–21 (2008).
Waters, A. M. et al. Ectopic notch activation in developing podocytes causes glomerulosclerosis. J. Am. Soc. Nephrol. 19, 1139–1157 (2008).
Lasagni, L. et al. Notch activation differentially regulates renal progenitors proliferation and differentiation toward the podocyte lineage in glomerular disorders. Stem Cells 28, 1674–1685 (2010).
Sharma, S., Sirin, Y. & Susztak, K. The story of Notch and chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 20, 56–61 (2011).
Purow, B. Notch inhibition as a promising new approach to cancer therapy. Adv. Exp. Med. Biol. 727, 305–319 (2012).
Araya, C. et al. T regulatory cell function in idiopathic minimal lesion nephrotic syndrome. Pediatr. Nephrol. 3, 3 (2009).
Assadi, F. Neonatal nephrotic syndrome associated with placental transmission of proinflammatory cytokines. Pediatr. Nephrol. 26, 469–471 (2011).
Jiang, H. K., Luo, G. & Jiang, H. Interleukin-18 expression in peripheral blood mononuclear cells in children with steroid-resistant nephrotic syndrome [Chinese]. Zhongguo Dang Dai Er Ke Za Zhi 11, 337–340 (2009).
Kanai, T. et al. Elevated serum interleukin-7 level in idiopathic steroid-sensitive nephrotic syndrome. Pediatr. Int. 53, 906–909 (2011).
Souto, M. F. et al. Immune mediators in idiopathic nephrotic syndrome: evidence for a relation between interleukin 8 and proteinuria. Pediatr. Res. 64, 637–642 (2008).
van den Berg, J. G. & Weening, J. J. Role of the immune system in the pathogenesis of idiopathic nephrotic syndrome. Clin. Sci. 107, 125–136 (2004).
Yap, H. K. et al. Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: evidence for increased IL-13 mRNA expression in relapse. J. Am. Soc. Nephrol. 10, 529–537 (1999).
Jiang, H. K., Jiang, H., Luo, G. & Sun, G. L. Interleukin-13 expression before and after pulse treatment with methylprednisolone in children with steroid-responsive nephrotic syndrome [Chinese]. Zhongguo Dang Dai Er Ke Za Zhi 9, 533–536 (2007).
Tain, Y. L., Chen, T. Y. & Yang, K. D. Implications of serum TNF-β and IL-13 in the treatment response of childhood nephrotic syndrome. Cytokine 21, 155–159 (2003).
Lai, K. W. et al. Overexpression of interleukin-13 induces minimal-change-like nephropathy in rats. J. Am. Soc. Nephrol. 18, 1476–1485 (2007).
Corren, J. et al. Lebrikizumab treatment in adults with asthma. N. Engl. J. Med. 365, 1088–1098 (2011).
Chakrabarti, A., Chen, A. W. & Varner, J. D. A review of the mammalian unfolded protein response. Biotechnol. Bioeng. 108, 2777–2793 (2011).
Markan, S. et al. Up regulation of the GRP-78 and GADD-153 and down regulation of Bcl-2 proteins in primary glomerular diseases: a possible involvement of the ER stress pathway in glomerulonephritis. Mol. Cell. Biochem. 324, 131–138 (2009).
Ito, N. et al. mTORC1 activation triggers the unfolded protein response in podocytes and leads to nephrotic syndrome. Lab. Invest. 91, 1584–1595 (2011).
Cybulsky, A. V. et al. Complement C5b-9 membrane attack complex increases expression of endoplasmic reticulum stress proteins in glomerular epithelial cells. J. Biol. Chem. 277, 41342–41351 (2002).
Kitamura, M. Endoplasmic reticulum stress and unfolded protein response in renal pathophysiology: Janus faces. Am. J. Physiol. Renal Physiol. 295, F323–F334 (2008).
Henderson, J. M., Alexander, M. P. & Pollak, M. R. Patients with ACTN4 mutations demonstrate distinctive features of glomerular injury. J. Am. Soc. Nephrol. 20, 961–968 (2009).
Cybulsky, A. V. & Kennedy, C. R. Podocyte injury associated with mutant α-actinin-4. J. Signal Transduct. 2011, 563128 (2011).
Liu, X. L. et al. Defective trafficking of nephrin missense mutants rescued by a chemical chaperone. J. Am. Soc. Nephrol. 15, 1731–1738 (2004).
Ehrnhoefer, D. E. et al. Green tea (-)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington's disease models. Hum. Mol. Genet. 15, 2743–2751 (2006).
Mishra, O. P. et al. Antioxidant status of children with idiopathic nephrotic syndrome. Pediatr. Nephrol. 26, 251–256 (2011).
Pavlova, E. L., Lilova, M. I. & Savov, V. M. Oxidative stress in children with kidney disease. Pediatr. Nephrol. 20, 1599–1604 (2005).
Kamireddy, R. et al. Oxidative stress in pediatric nephrotic syndrome. Clin. Chim. Acta 325, 147–150 (2002).
Ece, A. et al. Paraoxonase, total antioxidant response, and peroxide levels in children with steroid-sensitive nephrotic syndrome. Pediatr. Nephrol. 20, 1279–1284 (2005).
Ghodake, S. R. et al. Role of free radicals and antioxidant status in childhood nephrotic syndrome. Indian J. Nephrol. 21, 37–40 (2011).
Agardh, C. D., Stenram, U., Torffvit, O. & Agardh, E. Effects of inhibition of glycation and oxidative stress on the development of diabetic nephropathy in rats. J. Diabetes Complications 16, 395–400 (2002).
Sawant, S. U., Chandran, S., Almeida, A. F. & Rajan, M. G. Correlation between oxidative stress and thyroid function in patients with nephrotic syndrome. Int. J. Nephrol. 2011, 256420 (2011).
Duann, P. et al. Superoxide dismutase mimetic preserves the glomerular capillary permeability barrier to protein. J. Pharmacol. Exp. Ther. 316, 1249–1254 (2006).
Nakakura, H., Ashida, A., Hirano, K. & Tamai, H. Oxidative stress in a rat model of nephrosis can be quantified by electron spin resonance. Pediatr. Nephrol. 19, 266–270 (2004).
Marshall, C. B., Pippin, J. W., Krofft, R. D. & Shankland, S. J. Puromycin aminonucleoside induces oxidant-dependent DNA damage in podocytes in vitro and in vivo. Kidney Int. 70, 1962–1973 (2006).
Vega-Warner, V., Ransom, R. F., Vincent, A. M., Brosius, F. C. & Smoyer, W. E. Induction of antioxidant enzymes in murine podocytes precedes injury by puromycin aminonucleoside. Kidney Int. 66, 1881–1889 (2004).
Kinugasa, S. et al. Selective albuminuria via podocyte albumin transport in puromycin nephrotic rats is attenuated by an inhibitor of NADPH oxidase. Kidney Int. 80, 1328–1338 (2011).
Alvarez, B., Carballal, S., Turell, L. & Radi, R. Formation and reactions of sulfenic acid in human serum albumin. Methods Enzymol. 473, 117–136 (2010).
Candiano, G. et al. The oxido-redox potential of albumin methodological approach and relevance to human diseases. J. Proteomics 73, 188–195 (2009).
Bruschi, M. et al. Analysis of the oxido-redox status of plasma proteins. Technology advances for clinical applications. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 879, 1338–1344 (2011).
Lee, H. S. et al. Dietary antioxidant inhibits lipoprotein oxidation and renal injury in experimental focal segmental glomerulosclerosis. Kidney Int. 51, 1151–1159 (1997).
Matsumura, H., Ashida, A., Hirano, K., Nakakura, H. & Tamai, H. Protective effect of radical scavenger edaravone against puromycin nephrosis. Clin. Nephrol. 66, 405–410 (2006).
Tahzib, M., Frank, R., Gauthier, B., Valderrama, E. & Trachtman, H. Vitamin E treatment of focal segmental glomerulosclerosis: results of an open-label study. Pediatr. Nephrol. 13, 649–652 (1999).
Kestilä, M. et al. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell 1, 575–582 (1998).
Chiang, C. K. & Inagi, R. Glomerular diseases: genetic causes and future therapeutics. Nat. Rev. Nephrol. 6, 539–554 (2010).
McCarthy, H. J. & Saleem, M. A. Genetics in clinical practice: nephrotic and proteinuric syndromes. Nephron Exp. Nephrol. 118, e1–e8 (2011).
Pei, Y. INF2 is another piece of the jigsaw puzzle for FSGS. J. Am. Soc. Nephrol. 22, 197–199 (2011).
Akilesh, S. et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Invest. 121, 4127–4137 (2011).
Hasselbacher, K. et al. Recessive missense mutations in LAMB2 expand the clinical spectrum of LAMB2-associated disorders. Kidney Int. 70, 1008–1012 (2006).
Santin, S. et al. Clinical utility of genetic testing in children and adults with steroid-resistant nephrotic syndrome. Clin. J. Am. Soc. Nephrol. 6, 1139–1148 (2011).
Hinkes, B. G. et al. Nephrotic syndrome in the first year of life: two thirds of cases are caused by mutations in 4 genes (NPHS1, NPHS2, WT1, and LAMB2). Pediatrics 119, e907–e919 (2007).
Schlondorff, J., Del Camino, D., Carrasquillo, R., Lacey, V. & Pollak, M. R. TRPC6 mutations associated with focal segmental glomerulosclerosis cause constitutive activation of NFAT-dependent transcription. Am. J. Physiol. Cell. Physiol. 296, C558–C569 (2009).
Dryer, S. E. & Reiser, J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am. J. Physiol. Renal Physiol. 299, F689–F701 (2010).
Bensman, A. & Niaudet, P. Non-immunologic mechanisms of calcineurin inhibitors explain its antiproteinuric effects in genetic glomerulopathies. Pediatr. Nephrol. 25, 1197–1199 (2010).
Nijenhuis, T. et al. Angiotensin II contributes to podocyte injury by increasing TRPC6 expression via an NFAT-mediated positive feedback signaling pathway. Am. J. Pathol. 179, 1719–1732 (2011).
Gipson, D. S. et al. Differential risk of remission and ESRD in childhood FSGS. Pediatr. Nephrol. 21, 344–349 (2006).
Redmond, E. M., Guha, S., Walls, D. & Cahill, P. A. Investigational Notch and Hedgehog inhibitors—therapies for cardiovascular disease. Expert Opin. Investig. Drugs 20, 1649–1664 (2011).
Woolf, A. S. & Pitera, J. E. in Pediatric Nephrology (eds Avner, E. D., Harmon, W. E., Niaudet, P. & Yoshikawa, N.) 3–3 (Lippincott Williams & Wilkins, Philadelphia, 2009).
Kopan, R., Cheng, H. T. & Surendran, K. Molecular insights into segmentation along the proximal-distal axis of the nephron. J. Am. Soc. Nephrol. 18, 2014–2020 (2007).
Chen, L. & Al-Awqati, Q. Segmental expression of Notch and Hairy genes in nephrogenesis. Am. J. Physiol. Renal Physiol. 288, F939–F952 (2005).
McCright, B. et al. Defects in development of the kidney, heart and eye vasculature in mice homozygous for a hypomorphic Notch2 mutation. Development 128, 491–502 (2001).
Cheng, H. T. et al. Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134, 801–811 (2007).
Cheng, H. T. & Kopan, R. The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 68, 1951–1952 (2005).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Greenbaum, L., Benndorf, R. & Smoyer, W. Childhood nephrotic syndrome—current and future therapies. Nat Rev Nephrol 8, 445–458 (2012). https://doi.org/10.1038/nrneph.2012.115
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.115
This article is cited by
-
The immunopathogenesis of idiopathic nephrotic syndrome: a narrative review of the literature
European Journal of Pediatrics (2022)
-
Alteration of N6-methyladenosine epitranscriptome profile in lipopolysaccharide-induced mouse mesangial cells
Naunyn-Schmiedeberg's Archives of Pharmacology (2022)
-
The role of the immune system in idiopathic nephrotic syndrome
Molecular and Cellular Pediatrics (2021)
-
Serum and urine ANGPTL8 expression levels are associated with hyperlipidemia and proteinuria in primary nephrotic syndrome
BMC Nephrology (2021)
-
Impact of steroids and steroid-sparing agents on quality of life in children with nephrotic syndrome
Pediatric Nephrology (2021)