Abstract
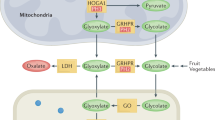
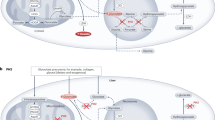
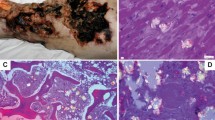
The autosomal recessive inherited primary hyperoxalurias types I, II and III are caused by defects in glyoxylate metabolism that lead to the endogenous overproduction of oxalate. Type III primary hyperoxaluria was first described in 2010 and further types are likely to exist. In all forms, urinary excretion of oxalate is strongly elevated (>1 mmol/1.73 m2 body surface area per day; normal <0.5 mmol/1.73 m2 body surface area per day), which results in recurrent urolithiasis and/or progressive nephrocalcinosis. All entities can induce kidney damage, which is followed by reduced oxalate elimination and consequent systemic deposition of calcium oxalate crystals. Systemic oxalosis should be prevented, but diagnosis is all too often missed or delayed until end-stage renal disease (ESRD) occurs; this outcome occurs in >30% of patients with primary hyperoxaluria type I. The fact that such a large proportion of patients have such poor outcomes is particularly unfortunate as ESRD can be delayed or even prevented by early intervention. Treatment options for primary hyperoxaluria include alkaline citrate, orthophosphate, or magnesium. In addition, pyridoxine treatment can be used to normalize or reduce oxalate excretion in about 30% of patients with primary hyperoxaluria type I. Time on dialysis should be short to avoid overt systemic oxalosis. Transplantation methods depend on the type of primary hyperoxaluria and on the particular patient, but combined liver and kidney transplantation is the method of choice in patients with primary hyperoxaluria type I and isolated kidney transplantation is the preferred method in those with primary hyperoxaluria type II. To the best of our knowledge, progression to ESRD has not yet been reported in any patient with primary hyperoxaluria type III.
Key Points
-
The primary hyperoxalurias are rare genetic diseases caused by deficiencies in glyoxylate metabolism
-
The main first symptoms of primary hyperoxaluria are recurrent urolithiasis and/or progressive nephrocalcinosis, or early end-stage renal disease in the case of infantile oxalosis
-
Every child with a first kidney stone and all adults who have recurrent calcium oxalate kidney stones should be screened for primary hyperoxaluria
-
Primary hyperoxaluria type I in particular is a devastating disease that all too often leads to early end-stage renal disease
-
If the diagnosis of primary hyperoxaluria is made early, disease progression can be slowed
-
The transplantation procedure of choice is isolated kidney transplantation in patients with type II primary hyperoxaluria, and usually combined liver–kidney transplantation in type I disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Van Woerden, C. S., Groothoff, J. W., Wanders, R. J., Davin, J. C. & Wijburg, F. A. Primary hyperoxaluria type 1 in The Netherlands: prevalence and outcome. Nephrol. Dial. Transplant. 18, 273–279 (2003).
Hoppe, B. & Langman, C. A United States survey on diagnosis, treatment and outcome of primary hyperoxaluria. Pediatr. Nephrol. 18, 986–991 (2003).
Cochat, P. et al. Primary hyperoxaluria type 1: still challenging! Pediatr. Nephrol. 21, 1075–1081 (2006).
Kopp, N. & Leumann, E. Changing pattern of primary hyperoxaluria in Switzerland. Nephrol. Dial. Transplant. 10, 2224–2227 (1995).
Hoppe, B., Beck, B. B. & Milliner, D. S. The primary hyperoxalurias. Kidney Int. 75, 1264–1271 (2009).
Leumann, E. & Hoppe, B. The primary hyperoxalurias. J. Am. Soc. Nephrol. 12, 1986–1993 (2001).
Hoppe, B., Latta, K., von Schnakenburg, C. & Kemper, M. J. Primary hyperoxaluria—the German experience. Am. J. Nephrol. 25, 276–281 (2005).
Latta, K. & Brodehl, J. Primary hyperoxaluria type I. Eur. J. Pediatr. 149, 518–522 (1990).
Akhan, O. et al. Systemic oxalosis: pathognomonic renal and specific extrarenal findings on US and CT. Pediatr. Radiol. 25, 15–16 (1995).
Hoppe, B. et al. Plasma calcium-oxalate supersaturation in children with primary hyperoxaluria and end-stage renal failure. Kidney Int. 56, 268–274 (1999).
Herrmann, G., Krieg, T., Weber, M., Sidhu, H. & Hoppe, B. Unusual painful sclerotic like plaques on the legs of a patient with late diagnosis of primary hyperoxaluria type I. Br. J. Dermatol. 151, 1104–1107 (2004).
Milliner, D. S. The primary hyperoxalurias: an algorithm for diagnosis. Am. J. Nephrol. 25, 154–160 (2005).
Lieske, J. C. et al. International Registry for primary hyperoxaluria. Am. J. Nephrol. 25, 290–296 (2005).
van Woerden, C. et al. The collaborative European cohort of primary hyperoxalurias: clinical and genetic characterization with prediction of outcome [abstract]. Pediatr. Nephrol. 25, 1911 (2010).
Danpure, C. J. Molecular aetiology of primary hyperoxaluria type 1. Nephron Exp. Nephrol. 98, e39–e44 (2004).
Danpure, C. J., Lumb, M. J., Birdsey, G. M. & Zhang, X. Alanine:glyoxylate aminotransferase peroxisome-to-mitochondrion mistargeting in human hereditary kidney stone disease. Biochim. Biophys. Acta 1647, 70–75 (2003).
Hoppe, B., Dittlich, K., Fehrenbach, H., Plum, G. & Beck, B. B. Reduction of plasma oxalate levels by oral application of Oxalobacter formigenes in 2 patients with infantile oxalosis. Am. J. Kidney Dis. 58, 453–455 (2011).
Hoppe, B. et al. A vertical (pseudodominant) pattern of inheritance in the autosomal recessive disease primary hyperoxaluria type I: lack of relationship between genotype, enzymic phenotype and disease severity. Am. J. Kidney Dis. 29, 36–44 (1997).
Hoppe, B. Evidence of true genotype-phenotype correlation in primary hyperoxaluria type 1. Kidney Int. 77, 383–385 (2010).
Harambat, J. et al. Genotype–phenotype correlation in primary hyperoxaluria type 1: the p.Gly170Arg AGXT mutation is associated with a better outcome. Kidney Int. 77, 443–449 (2010).
Lorenzo, V. et al. Presentation and role of transplantation in adult patients with type 1 primary hyperoxaluria and the I244T AGXT mutation: single-center experience. Kidney Int. 70, 1115–1119 (2006).
Coulter-Mackie, M. B. & Rumsby, G. Genetic heterogeneity in primary hyperoxaluria type 1: impact on diagnosis. Mol. Genet. Metab. 83, 38–46 (2004).
Takayama, T., Nagata, M., Ichiyama, A. & Ozono, S. Primary hyperoxaluria type 1 in Japan. Am. J. Nephrol. 25, 297–302 (2005).
Cregeen, D. P., Williams, E. L., Hulton, S. & Rumsby, G. Molecular analysis of the glyoxylate reductase (GRHPR) gene and description of mutations underlying primary hyperoxaluria type 2. Hum. Mutat. 22, 497–506 (2003).
Kemper, M. J., Conrad, S. & Müller-Wiefel, D. E. Primary hyperoxaluria type 2. Eur. J. Pediatr. 156, 509–512 (1997).
Milliner, D. S., Wilson, D. M. & Smith, L. H. Phenotypic expression of primary hyperoxaluria: comparative features of types I and II. Kidney Int. 59, 31–36 (2001).
Rumsby, G., Sharma, A., Cregeen, D. P. & Solomon, L. R. Primary hyperoxaluria type 2 without L-glycericaciduria: is the disease under-diagnosed? Nephrol. Dial. Transplant. 16, 1697–1699 (2001).
Belostotsky, R. et al. Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am. J. Hum. Gen. 87, 392–399 (2010).
Monico, C. G. et al. Primary hyperoxaluria type III gene HOGA1 (formerly DHDPSL) as a possible risk factor for idiopathic calcium oxalate urolithiasis. Clin. J. Am. Soc. Nephrol. 6, 2289–2295 (2011).
Williams, E. L. et al. The enzyme 4-hydroxy-2-oxoglutarate aldolase is deficient in primary hyperoxaluria type 3. Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfs039.
Riedel, T. J. et al. Structural and biochemical studies of human 4-hydroxy-2-oxoglutarate aldolase: implications for hydroxyproline metabolism in primary hyperoxaluria. PLoS ONE 6, e26021 (2011).
Habbig, S., Beck, B. B. & Hoppe, B. Nephrocalcinosis and urolithiasis in children. Kidney Int. 80, 1278–1291 (2011).
Daudon, M. et al. Examination of whewellite kidney stones by scanning electron microscopy and powder neutron diffraction techniques. J. Appl. Cryst. 42, 109–115 (2009).
Daudon, M., Jungers, P. & Bazin, D. Peculiar morphology of stones in primary hyperoxaluria. N. Engl. J. Med. 359, 100–102 (2008).
Hoppe, B. & Leumann, E. In Physician's Guide to the Treatment and Follow-up of Metabolic Diseases (eds Blau, N., Hoffmann, G., Leonard, J. & Clarke, J.) 279–285 (Springer Verlag, Heidelberg, 2005).
Leumann, E. P., Dietl, A. & Matasovic, A. Urinary oxalate and glycolate excretion in healthy infants and children. Pediatr. Nephrol. 4, 493–497 (1990).
Hoppe, B., Leumann, E. & Milliner, D. In Comprehensive Pediatric Nephrology (eds Geary, D. & Schäfer, F.) 499–525 (Elsevier/WB Saunders, New York, 2008).
Marangella, M., Petrarulo, M., Vitale, C., Cosseddu, D. & Linari, F. Plasma and urine glycolate assays for differentiating the hyperoxaluria syndromes. J. Urol. 148, 986–989 (1992).
Marangella, M. et al. Plasma profiles and dialysis kinetics of oxalate in patients receiving hemodialysis. Nephron 60, 64–70 (1992).
Williams, E. & Rumsby, G. Selected exonic sequencing of the AGXT gene provides a genetic diagnosis in 50% of patients with primary hyperoxaluria type 1. Clin. Chem. 53, 1216–1221 (2007).
Rumsby, G., Williams, E. & Coulter-Mackie, M. Evaluation of mutation screening as a first line test for the diagnosis of the primary hyperoxalurias. Kidney Int. 66, 959–963 (2004).
Monico, C. G. et al. Comprehensive mutation screening in 55 probands with type 1 primary hyperoxaluria shows feasibility of a gene-based diagnosis. J. Am. Soc. Nephrol. 18, 1905–1914 (2007).
Williams, E. L. et al. Primary hyperoxaluria type 1: update and additional mutation analysis of the AGXT gene. Hum. Mutat. 30, 910–917 (2009).
van Woerden, C. S. et al. Clinical implications of mutation analysis in primary hyperoxaluria type 1. Kidney Int. 66, 746–752 (2004).
Pirulli, D., Marangella, M. & Amoroso, A. Primary hyperoxaluria: genotype-phenotype correlation. J. Nephrol. 16, 297–309 (2003).
Monico, C. G., Rossetti, S., Olson, J. B. & Milliner, D. S. Pyridoxine effect in type I primary hyperoxaluria is associated with the most common mutant allele. Kidney Int. 67, 1704–1709 (2005).
Sikora, P. et al. [13C2] oxalate absorption in children with idiopathic calcium oxalate urolithiasis or primary hyperoxaluria. Kidney Int. 73, 1181–1186 (2008).
Hatch, M., Freel, R. W. & Vaziri, N. D. Regulatory aspects of oxalate secretion in enteric oxalate elimination. J. Am. Soc. Nephrol. 10 (Suppl. 14), S324–S328 (1999).
Hatch, M. & Freel, R. W. Intestinal transport of an obdurate anion: oxalate. Urol. Res. 33, 1–16 (2005).
Hatch, M. et al. Oxalobacter sp. reduces urinary oxalate excretion promoting enteric oxalate excretion. Kidney Int. 69, 691–698 (2006).
Allison, M. J., Dawson, K. A., Mayberry, W. R. & Foss, J. G. Oxalobacter formigenes gen. nov., sp. nov.: oxalate-degrading anaerobes that inhabit the gastrointestinal tract. Arch. Microbiol. 141, 1–7 (1985).
Hoppe, B. et al. Oxalobacter formigenes: a potential tool for the treatment of primary hyperoxaluria type I. Kidney Int. 70, 1305–1311 (2006).
Grujic, D. et al. Hyperoxaluria is reduced and nephrocalcinosis prevented with an oxalate-degrading enzyme in mice with hyperoxaluria. Am. J. Nephrol. 29, 86–93 (2009).
Hatch, M., Gjymishka, A., Salido, E. C., Allison, M. J. & Freel, R. W. Enteric oxalate elimination is induced and oxalate is normalized in a mouse model of primary hyperoxaluria following intestinal colonization with Oxalobacter. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G461–G469 (2011).
Hoppe, B. et al. Efficacy and safety of Oxalobacter formigenes to reduce urinary oxalate in primary hyperoxaluria. Nephrol. Dial. Transplant. 26, 3609–3615 (2011).
Robijn, S., Hoppe, B., Vervaet, B. A., D'Haese, P. C. & Verhulst, A. Hyperoxaluria: a gut-kidney axis? Kidney Int. 80, 1146–1158 (2011).
Leumann, E., Hoppe, B. & Neuhaus, T. Management of primary hyperoxaluria: efficacy of oral citrate administration. Pediatr. Nephrol. 7, 207–211 (1993).
Milliner, D. S., Eickholt, J. T., Bergstralh, E. J., Wilson, D. M. & Smith, L. H. Results of long-term treatment with orthophosphate and pyridoxine in patients with primary hyperoxaluria. N. Engl. J. Med. 331, 1553–1558 (1994).
Hamm, L. L. Renal handling of citrate. Kidney Int. 38, 728–735 (1990).
Monico, C. G., Olson, J. B. & Milliner, D. S. Implications of genotype and enzyme phenotype in pyridoxine response of patients with type I primary hyperoxaluria. Am. J. Nephrol. 25, 183–188 (2005).
Harambat, J. et al. Characteristics and outcomes of children with primary oxalosis requiring renal replacement therapy. Clin. J. Am. Soc. Nephrol. 7, 458–465 (2012).
Illies, F., Bonzel, K. E., Wingen, A. M., Latta, K. & Hoyer, P. F. Clearance and removal of oxalate in children on intensified dialysis for primary hyperoxaluria type 1. Kidney Int. 70, 1642–1648 (2006).
Hoppe, B. et al. Oxalate elimination via hemodialysis or peritoneal dialysis in children with chronic renal failure. Pediatr. Nephrol. 10, 488–492 (1996).
Bunchman, T. E. & Swartz, R. D. Oxalate removal in type I hyperoxaluria or acquired oxalosis using HD and equilibration PD. Perit. Dial. Int. 14, 81–84 (1994).
Bergstralh, E. J. et al. Transplantation outcomes in primary hyperoxaluria. Am. J. Transplant. 10, 2493–2501 (2010).
Brinkert, F. et al. Transplantation procedures in children with primary hyperoxaluria type 1: outcome and longitudinal growth. Transplantation 87, 1415–1421 (2009).
Jamieson, N. V. & European PHI Transplantation Study Group. A 20-year experience of combined liver/kidney transplantation for primary hyperoxaluria (PH1): the European PH1 transplant registry experience 1984–2004. Am. J. Nephrol. 25, 282–289 (2005).
Nolkemper, D. et al. Long-term results of pre-emptive liver transplantation in primary hyperoxaluria type 1. Pediatr. Transplant. 3, 177–181 (2000).
Saborio, P. & Scheinman, J. I. Transplantation for primary hyperoxaluria in the United States. Kidney Int. 56, 1094–1100 (1999).
Monico, C. G. & Milliner, D. S. Combined liver-kidney and kidney-alone transplantation in primary hyperoxaluria. Liver Transpl. 7, 954–963 (2001).
Decramer, S. et al. Urine in clinical proteomics. Mol. Cell Proteomics 7, 1850–1862 (2008).
Canales, B. K. et al. Proteome of human calcium kidney stones. Urology 76, 1017.e.13–e20 (2010).
Wu, Z., Asokan, A. & Samulski, R. J. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol. Ther. 14, 316–327 (2006).
Salido, E. et al. Phenotypic correction of a mouse model for primary hyperoxaluria with adeno-associated virus gene transfer. Mol. Ther. 19, 870–875 (2011).
Tanriover, B., Mejia, A., Foster, S. V. & Mubarak, A. Primary hyperoxaluria involving the liver and hepatic artery: images of an aggressive disease. Kidney Int. 77, 651 (2010).
Beck, B. B. et al. Liver cell transplantation in severe infantile oxalosis—a potential bridging procedure to orthotopic liver transplantation? Nephrol. Dial. Transplant. http://dx.doi.org/10.1093/ndt/gfr776.
Danpure, C. J. Primary hyperoxaluria: from gene defects to designer drugs. Nephrol. Dial. Transplant. 20, 1525–1529 (2005).
Pey, A. L., Salido, E. & Sanchez-Ruiz, J. M. Role of low native state kinetic stability and interaction of partially unfolded states with molecular chaperones in the mitochondrial protein mistargeting associated with primary hyperoxaluria. Amino Acids 41, 1233–1245 (2011).
Hopper, E. D., Pittman, A. M., Fitzgerald, M. C. & Tucker, C. L. In vivo and in vitro examination of stability of primary hyperoxaluria-associated human alanine:glyoxylate aminotransferase. J. Biol. Chem. 283, 30493–30502 (2008).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Hoppe, B. An update on primary hyperoxaluria. Nat Rev Nephrol 8, 467–475 (2012). https://doi.org/10.1038/nrneph.2012.113
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2012.113
This article is cited by
-
Primäre Hyperoxalurie Typ 1 – eine seltene hereditäre Stoffwechselstörung als Ursache einer Livedo racemosa
Die Dermatologie (2024)
-
Purslane-induced oxalate nephropathy: case report and literature review
BMC Nephrology (2023)
-
Clinical practice recommendations for primary hyperoxaluria: an expert consensus statement from ERKNet and OxalEurope
Nature Reviews Nephrology (2023)
-
Case series and literature review of primary hyperoxaluria type 1 in Chinese patients
Urolithiasis (2023)
-
Nedosiran in primary hyperoxaluria subtype 3: results from a phase I, single-dose study (PHYOX4)
Urolithiasis (2023)