Abstract
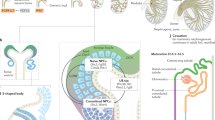
Impaired intrauterine nephrogenesis—most clearly illustrated by low nephron number—is frequently associated with low birthweight and has been recognized as a powerful risk factor for renal disease; it increases the risks of low glomerular filtration rate, of more rapid progression of primary kidney disease, and of increased incidence of chronic kidney disease or end-stage renal disease. Another important consequence of impaired nephrogenesis is hypertension, which further amplifies the risk of onset and progression of kidney disease. Hypertension is associated with low nephron numbers in white individuals, but the association is not universal and is not seen in individuals of African origin. The derangement of intrauterine kidney development is an example of a more general principle that illustrates the paradigm of plasticity during development—that is, that transcription of the genetic code is modified by epigenetic factors (as has increasingly been documented). This Review outlines the concept of prenatal programming and, in particular, describes its role in kidney disease and hypertension.
Key Points
-
Intrauterine growth restriction with reduced renal organogenesis is a powerful predictor of adult renal disease (such as albuminuria, reduced glomerular filtration rate and progressive kidney disease) and cardiovascular disease (such as hypertension and coronary heart disease)
-
Impaired kidney development in an adverse intrauterine environment results in a low nephron number, which predisposes individuals to hypertension and kidney disease in adulthood
-
The main factors that predispose individuals to reduced renal development are protein and calorie malnutrition, placental malfunction and maternal hyperglycemia
-
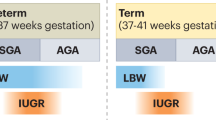
Low birthweight can be used as a surrogate marker of some, but not all, disturbances of intrauterine growth, thereby enabling the identification of at-risk children who might benefit from regular surveillance
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Vehaskari, V. M. Prenatal programming of kidney disease. Curr. Opin. Pediatr. 22, 176–182 (2010).
Barker, D. J., Osmond, C., Golding, J., Kuh, D. & Wadsworth, M. E. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. BMJ 298, 564–567 (1989).
Law, C. M. et al. Initiation of hypertension in utero and its amplification throughout life. BMJ 306, 24–27 (1993).
Barker, D. J. et al. The early origins of chronic heart failure: impaired placental growth and initiation of insulin resistance in childhood. Eur. J. Heart Fail. 12, 819–825 (2010).
Barker, D. J., Bagby, S. P. & Hanson, M. A. Mechanisms of disease: in utero programming in the pathogenesis of hypertension. Nat. Clin. Pract. Nephrol. 2, 700–707 (2006).
Widdowson, E. M. & McCance, R. A. The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc. R. Soc. Lond. B. Biol. Sci. 158, 329–342 (1963).
West-Eberhard, M. J. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102 (Suppl. 1), 6543–6549 (2005).
Dover, G. J. The Barker hypothesis: how pediatricans will diagnose and prevent common adult-onset diseases. Trans. Am. Clin. Climatol. Assoc. 120, 199–207 (2009).
Moritz, K. M. et al. Uteroplacental insufficiency causes a nephron deficit, modest renal insufficiency but no hypertension with ageing in female rats. J. Physiol. 587, 2635–2646 (2009).
Baum, M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am. J. Physiol. Renal Physiol. 298, F235–F247 (2010).
Kuure, S., Vuolteenaho, R. & Vainio, S. Kidney morphogenesis: cellular and molecular regulation. Mech. Dev. 92, 31–45 (2000).
Dötsch, J., Plank, C., Amann, K. & Ingelfinger, J. The implications of fetal programming of glomerular number and renal function. J. Mol. Med. 87, 841–848 (2009).
Nyengaard, J. R. & Bendtsen, T. F. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat. Rec. 232, 194–201 (1992).
Ingelfinger, J. R. Pathogenesis of perinatal programming. Curr. Opin. Nephrol. Hypertens. 13, 459–464 (2004).
Ojeda, N. B., Grigore, D. & Alexander, B. T. Intrauterine growth restriction: fetal programming of hypertension and kidney disease. Adv. Chronic Kidney Dis. 15, 101–106 (2008).
Woods, L. L., Ingelfinger, J. R., Nyengaard, J. R. & Rasch, R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr. Res. 49, 460–467 (2001).
Langley-Evans, S. C. et al. Intrauterine programming of hypertension: the role of the renin-angiotensin system. Biochem. Soc. Trans. 27, 88–93 (1999).
Wlodek, M. E., Westcott, K., Siebel, A. L., Owens, J. A. & Moritz, K. M. Growth restriction before or after birth reduces nephron number and increases blood pressure in male rats. Kidney Int. 74, 187–195 (2008).
Ergaz, Z., Avgil, M. & Ornoy, A. Intrauterine growth restriction-etiology and consequences: what do we know about the human situation and experimental animal models? Reprod. Toxicol. 20, 301–322 (2005).
Amri, K., Freund, N., Van Huyen, J. P., Merlet-Bénichou, C. & Lelièvre-Pégorier, M. Altered nephrogenesis due to maternal diabetes is associated with increased expression of IGF-II/mannose-6-phosphate receptor in the fetal kidney. Diabetes 50, 1069–1075 (2001).
Bursztyn, M. & Ariel, I. Maternal-fetal deprivation and the cardiometabolic syndrome. J. Cardiometab. Syndr. 1, 141–145 (2006).
Tran, S. et al. Maternal diabetes modulates renal morphogenesis in offspring. J. Am. Soc. Nephrol. 19, 943–952 (2008).
Bursztyn, M. et al. Adult hypertension in intrauterine growth-restricted offspring of hyperinsulinemic rats: evidence of subtle renal damage. Hypertension 48, 717–723 (2006).
Doublier, S. et al. Overexpression of human insulin-like growth factor binding protein-1 in the mouse leads to nephron deficit. Pediatr. Res. 49, 660–666 (2001).
Balbi, A. P., Costa, R. S. & Coimbra, T. M. Postnatal renal development of rats from mothers that received increased sodium intake. Pediatr. Nephrol. 19, 1212–1218 (2004).
Wintour, E. M. et al. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J. Physiol. 549, 929–935 (2003).
Gilbert, T., Gaonach, S., Moreau, E. & Merlet-Bénichou, C. Defect of nephrogenesis induced by gentamicin in rat metanephric organ culture. Lab. Invest. 70, 656–666 (1994).
Schwedler, S. B. et al. Nephrotoxin exposure in utero reduces glomerular number in sclerosis-prone but not sclerosis-resistant mice. Kidney Int. 56, 1683–1690 (1999).
Tendron-Franzin, A. et al. Long-term effects of in utero exposure to cyclosporin A on renal function in the rabbit. J. Am. Soc. Nephrol. 15, 2687–2693 (2004).
Bhat, P. V. & Manolescu, D. C. Role of vitamin A in determining nephron mass and possible relationship to hypertension. J. Nutr. 138, 1407–1410 (2008).
Blake, K. V. et al. Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum. Dev. 57, 137–147 (2000).
Kwong, W. Y., Wild, A. E., Roberts, P., Willis, A. C. & Fleming, T. P. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 127, 4195–4202 (2000).
Lillycrop, K. A., Phillips, E. S., Jackson, A. A., Hanson, M. A. & Burdge, G. C. Dietary protein restriction of pregnant rats induces and folic acid supplementation prevents epigenetic modification of hepatic gene expression in the offspring. J. Nutr. 135, 1382–1386 (2005).
Cox, L. A. et al. Effect of 30 per cent maternal nutrient restriction from 0.16 to 0.5 gestation on fetal baboon kidney gene expression. J. Physiol. 572, 67–85 (2006).
Buffat, C. et al. Kidney gene expression analysis in a rat model of intrauterine growth restriction reveals massive alterations of coagulation genes. Endocrinology 148, 5549–5557 (2007).
Pham, T. D. et al. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am. J. Physiol. Regul. Integr. Comp. Physiol. 285, R962–R970 (2003).
Hershkovitz, D., Burbea, Z., Skorecki, K. & Brenner, B. M. Fetal programming of adult kidney disease: cellular and molecular mechanisms. Clin. J. Am. Soc. Nephrol. 2, 334–342 (2007).
Burdge, G. C., Hanson, M. A., Slater-Jefferies, J. L. & Lillycrop, K. A. Epigenetic regulation of transcription: a mechanism for inducing variations in phenotype (fetal programming) by differences in nutrition during early life? Br. J. Nutr. 97, 1036–1046 (2007).
Kremenskoy, M. et al. Genome-wide analysis of DNA methylation status of CpG islands in embryoid bodies, teratomas, and fetuses. Biochem. Biophys. Res. Commun. 311, 884–890 (2003).
Akkad, A. et al. Telomere length in small-for-gestational-age babies. BJOG 113, 318–323 (2006).
Barker, D. J., Forsén, T., Eriksson, J. G. & Osmond, C. Growth and living conditions in childhood and hypertension in adult life: a longitudinal study. J. Hypertens. 20, 1951–1956 (2002).
Ingelfinger, J. R. Is microanatomy destiny? N. Engl. J. Med. 348, 99–100 (2003).
Cullen-McEwen, L. A., Kett, M. M., Dowling, J., Anderson, W. P. & Bertram, J. F. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension 41, 335–340 (2003).
Ingelfinger, J. R. Disparities in renal endowment: causes and consequences. Adv. Chronic Kidney Dis. 15, 107–114 (2008).
Weber, S. et al. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J. Am. Soc. Nephrol. 17, 2864–2870 (2006).
Godley, L. A. et al. Wild-type p53 transgenic mice exhibit altered differentiation of the ureteric bud and possess small kidneys. Genes Dev. 10, 836–850 (1996).
Haas, C. S. et al. Glomerular and renal vascular structural changes in α8 integrin-deficient mice. J. Am. Soc. Nephrol. 14, 2288–2296 (2003).
Jena, N., Martin-Seisdedos, C., McCue, P. & Croce, C. M. BMP7 null mutation in mice: developmental defects in skeleton, kidney, and eye. Exp. Cell Res. 230, 28–37 (1997).
He, C. et al. Dissociation of glomerular hypertrophy, cell proliferation, and glomerulosclerosis in mouse strains heterozygous for a mutation (Os) which induces a 50% reduction in nephron number. J. Clin. Invest. 97, 1242–1249 (1996).
Gassler, N., Elger, M., Inoue, D., Kriz, W. & Amling, M. Oligonephronia, not exuberant apoptosis, accounts for the development of glomerulosclerosis in the bcl-2 knockout mouse. Nephrol. Dial. Transplant. 13, 2509–2518 (1998).
Lumbers, E. R. Functions of the renin-angiotensin system during development. Clin. Exp. Pharmacol. Physiol. 22, 499–505 (1995).
Kingdom, J. C., Hayes, M., McQueen, J., Howatson, A. G. & Lindop, G. B. Intrauterine growth restriction is associated with persistent juxtamedullary expression of renin in the fetal kidney. Kidney Int. 55, 424–429 (1999).
Peers, A., Campbell, D. J., Wintour, E. M. & Dodic, M. The peripheral renin–angiotensin system is not involved in the hypertension of sheep exposed to prenatal dexamethasone. Clin. Exp. Pharmacol. Physiol. 28, 306–311 (2001).
Moreau, E., Vilar, J., Lelièvre-Pégorier, M., Merlet-Bénichou, C. & Gilbert, T. Regulation of c-ret expression by retinoic acid in rat metanephros: implication in nephron mass control. Am. J. Physiol. 275, F938–F945 (1998).
Quaia, M. et al. Glucocorticoids promote the proliferation and antagonize the retinoic acid-mediated growth suppression of Epstein–Barr virus-immortalized B lymphocytes. Blood 96, 711–718 (2000).
Bertram, C., Trowern, A. R., Copin, N., Jackson, A. A. & Whorwood, C. B. The maternal diet during pregnancy programs altered expression of the glucocorticoid receptor and type 2 11β-hydroxysteroid dehydrogenase: potential molecular mechanisms underlying the programming of hypertension in utero. Endocrinology 142, 2841–2853 (2001).
Dagan, A., Gattineni, J., Cook, V. & Baum, M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1230–R1235 (2007).
Ahokas, R. A., Anderson, G. D. & Lipshitz, J. Cardiac output and uteroplacental blood flow in diet-restricted and diet-repleted pregnant rats. Am. J. Obstet. Gynecol. 146, 6–13 (1983).
Itoh, S. et al. Vasodilation to vascular endothelial growth factor in the uterine artery of the pregnant rat is blunted by low dietary protein intake. Pediatr. Res. 51, 485–491 (2002).
Oliver, J. (ed.) Nephrons and Kidneys (Haper and Row, San Francisco, 1968).
Barker, D. J. & Bagby, S. P. Developmental antecedents of cardiovascular disease: a historical perspective. J. Am. Soc. Nephrol. 16, 2537–2544 (2005).
Brenner, B. M., Garcia, D. L. & Anderson, S. Glomeruli and blood pressure. Less of one, more the other? Am. J. Hypertens. 1, 335–347 (1988).
Horster, M., Kemler, B. J. & Valtin, H. Intracortical distribution of number and volume of glomeruli during postnatal maturation in the dog. J. Clin. Invest. 50, 796–800 (1971).
Larsson, L., Aperia, A. & Wilton, P. Effect of normal development on compensatory renal growth. Kidney Int. 18, 29–35 (1980).
Gubhaju, L. et al. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am. J. Physiol. Renal Physiol. 297, F1668–F1677 (2009).
Keller, G., Zimmer, G., Mall, G., Ritz, E. & Amann, K. Nephron number in patients with primary hypertension. N. Engl. J. Med. 348, 101–108 (2003).
Hoy, W. E., Hughson, M. D., Bertram, J. F., Douglas-Denton, R. & Amann, K. Nephron number, hypertension, renal disease, and renal failure. J. Am. Soc. Nephrol. 16, 2557–2564 (2005).
Hughson, M. D., Douglas-Denton, R., Bertram, J. F. & Hoy, W. E. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int. 69, 671–678 (2006).
Rostand, S. G., Cliver, S. P. & Goldenberg, R. L. Racial disparities in the association of foetal growth retardation to childhood blood pressure. Nephrol. Dial. Transplant. 20, 1592–1597 (2005).
Skov, K., Nyengaard, J. R., Korsgaard, N. & Mulvany, M. J. Number and size of renal glomeruli in spontaneously hypertensive rats. J. Hypertens. 12, 1373–1376 (1994).
Rettig, R. et al. Role of the kidney in primary hypertension: a renal transplantation study in rats. Am. J. Physiol. 258, F606–F611 (1990).
Curtis, J. J. et al. Remission of essential hypertension after renal transplantation. N. Engl. J. Med. 309, 1009–1015 (1983).
Rettig, R. & Grisk, O. The kidney as a determinant of genetic hypertension: evidence from renal transplantation studies. Hypertension 46, 463–468 (2005).
Mañalich, R., Reyes, L., Herrera, M., Melendi, C. & Fundora, I. Relationship between weight at birth and the number and size of renal glomeruli in humans: a histomorphometric study. Kidney Int. 58, 770–773 (2000).
Hardy, R., Kuh, D., Langenberg, C. & Wadsworth, M. E. Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. Lancet 362, 1178–1183 (2003).
Silver, L. E., Decamps, P. J., Korst, L. M., Platt, L. D. & Castro, L. C. Intrauterine growth restriction is accompanied by decreased renal volume in the human fetus. Am. J. Obstet. Gynecol. 188, 1320–1325 (2003).
Konje, J. C., Okaro, C. I., Bell, S. C., de Chazal, R. & Taylor, D. J. A cross-sectional study of changes in fetal renal size with gestation in appropriate- and small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 10, 22–26 (1997).
Woods, L. L., Weeks, D. A. & Rasch, R. Hypertension after neonatal uninephrectomy in rats precedes glomerular damage. Hypertension 38, 337–342 (2001).
Mei-Zahav, M. et al. Ambulatory blood pressure monitoring in children with a solitary kidney—a comparison between unilateral renal agenesis and uninephrectomy. Blood Press. Monit. 6, 263–267 (2001).
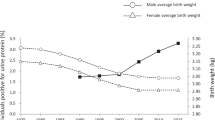
Schmidt, I. M. et al. Impaired kidney growth in low-birth-weight children: distinct effects of maturity and weight for gestational age. Kidney Int. 68, 731–740 (2005).
Loos, R. J., Fagard, R., Beunen, G., Derom, C. & Vlietinck, R. Birth weight and blood pressure in young adults: a prospective twin study. Circulation 104, 1633–1638 (2001).
Kasiske, B. L., Ma, J. Z., Louis, T. A. & Swan, S. K. Long-term effects of reduced renal mass in humans. Kidney Int. 48, 814–819 (1995).
Boudville, N. et al. Meta-analysis: risk for hypertension in living kidney donors. Ann. Intern. Med. 145, 185–196 (2006).
Manning, J., Beutler, K., Knepper, M. A. & Vehaskari, V. M. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am. J. Physiol. Renal Physiol. 283, F202–F206 (2002).
de Boer, M. P. et al. Birth weight relates to salt sensitivity of blood pressure in healthy adults. Hypertension 51, 928–932 (2008).
Vehaskari, V. M. et al. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am. J. Physiol. Renal Physiol. 287, F262–F267 (2004).
Houang, M., Morineau G., le Bouc, Y., Fiet, J. & Gourmelen, M. The cortisol–cortisone shuttle in children born with intrauterine growth retardation. Pediatr. Res. 46, 189–193 (1999).
Alexander, B. T., Hendon, A. E., Ferril, G. & Dwyer, T. M. Renal denervation abolishes hypertension in low-birth-weight offspring from pregnant rats with reduced uterine perfusion. Hypertension 45, 754–758 (2005).
Lurbe, E. et al. First-year blood pressure increase steepest in low birthweight newborns. J. Hypertens. 25, 81–86 (2007).
Bhargava, S. K. et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N. Engl. J. Med. 350, 865–875 (2004).
Järvelin, M. R. et al. Early life factors and blood pressure at age 31 years in the 1966 northern Finland birth cohort. Hypertension 44, 838–846 (2004).
Huxley, R. R., Shiell, A. W. & Law, C. M. The role of size at birth and postnatal catch-up growth in determining systolic blood pressure: a systematic review of the literature. J. Hypertens. 18, 815–831 (2000).
Uiterwaal, C. S. et al. Birth weight, growth, and blood pressure: an annual follow-up study of children aged 5 through 21 years. Hypertension 30, 267–271 (1997).
Horta, B. L., Barros, F. C., Victora, C. G. & Cole, T. J. Early and late growth and blood pressure in adolescence. J. Epidemiol. Community Health 57, 226–230 (2003).
Spassov, L. et al. Heart rate and heart rate variability during sleep in small-for-gestational age newborns. Pediatr. Res. 35, 500–505 (1994).
Leeson, C. P. et al. Flow-mediated dilation in 9- to 11-year-old children: the influence of intrauterine and childhood factors. Circulation 96, 2233–2238 (1997).
Ligi, I., Grandvuillemin, I., Andres, V., Dignat-George, F. & Simeoni, U. Low birth weight infants and the developmental programming of hypertension: a focus on vascular factors. Semin. Perinatol. 34, 188–192 (2010).
Varvarigou, A. A. Intrauterine growth restriction as a potential risk factor for disease onset in adulthood. J. Pediatr. Endocrinol. Metab. 23, 215–224 (2010).
Kajantie, E., Osmond, C., Barker, D. J. & Eriksson, J. G. Preterm birth—a risk factor for type 2 diabetes? The Helsinki Birth Cohort Study. Diabetes Care 33, 2623–2625 (2010).
Fagerudd, J. et al. Birth weight is inversely correlated to adult systolic blood pressure and pulse pressure in type 1 diabetes. Hypertension 44, 832–837 (2004).
Antonios, T. F., Singer, D. R., Markandu, N. D., Mortimer, P. S. & MacGregor, G. A. Structural skin capillary rarefaction in essential hypertension. Hypertension 33, 998–1001 (1999).
Antonios, T. F. et al. Rarefaction of skin capillaries in normotensive offspring of individuals with essential hypertension. Heart 89, 175–178 (2003).
Mitchell, P. et al. Evidence of arteriolar narrowing in low-birth-weight children. Circulation 118, 518–524 (2008).
Chapman, N. et al. Retinal vascular network architecture in low-birth-weight men. J. Hypertens. 15, 1449–1453 (1997).
Kajantie, E. et al. Body size at birth predicts hypothalamic-pituitary-adrenal axis response to psychosocial stress at age 60 to 70 years. J. Clin. Endocrinol. Metab. 92, 4094–4100 (2007).
Painter, R. C. et al. Microalbuminuria in adults after prenatal exposure to the Dutch famine. J. Am. Soc. Nephrol. 16, 189–194 (2005).
Brantsma, A. H., Bakker, S. J., de Zeeuw, D., de Jong, P. E. & Gansevoort, R. T. Urinary albumin excretion as a predictor of the development of hypertension in the general population. J. Am. Soc. Nephrol. 17, 331–335 (2006).
Wang, T. J. et al. Low-grade albuminuria and the risks of hypertension and blood pressure progression. Circulation 111, 1370–1376 (2005).
Hallan, S. I. et al. Combining GFR and albuminuria to classify CKD improves prediction of ESRD. J. Am. Soc. Nephrol. 20, 1069–1077 (2009).
Viazzi, F. et al. Microalbuminuria is a predictor of chronic renal insufficiency in patients without diabetes and with hypertension: the MAGIC study. Clin. J. Am. Soc. Nephrol. 5, 1099–1106 (2010).
Hallan, S. et al. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord. Trondelag Health (HUNT 2) Study. Am. J. Kidney Dis. 51, 10–20 (2008).
Li, S. et al. Low birth weight is associated with chronic kidney disease only in men. Kidney Int. 73, 637–642 (2008).
Vikse, B. E., Irgens, L. M., Leivestad, T., Hallan, S. & Iversen, B. M. Low birth weight increases risk for end-stage renal disease. J. Am. Soc. Nephrol. 19, 151–157 (2008).
Na, Y. W. et al. Effect of intrauterine growth retardation on the progression of nephrotic syndrome. Am. J. Nephrol. 22, 463–467 (2002).
Zidar, N., Cavic, M. A., Kenda, R. B., Koselj, M. & Ferluga, D. Effect of intrauterine growth retardation on the clinical course and prognosis of IgA glomerulonephritis in children. Nephron 79, 28–32 (1998).
Nenov, V. D., Taal, M. W., Sakharova, O. V. & Brenner, B. M. Multi-hit nature of chronic renal disease. Curr. Opin. Nephrol. Hypertens. 9, 85–97 (2000).
Plank, C. et al. Intrauterine growth retardation aggravates the course of acute mesangioproliferative glomerulonephritis in the rat. Kidney Int. 70, 1974–1982 (2006).
Fassi, A. et al. Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J. Am. Soc. Nephrol. 9, 1399–1406 (1998).
Argueso, L. R. et al. Prognosis of patients with unilateral renal agenesis. Pediatr. Nephrol. 6, 412–416 (1992).
Stewart, T., Jung, F. F., Manning, J. & Vehaskari, V. M. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 68, 2180–2188 (2005).
Author information
Authors and Affiliations
Contributions
N. Koleganova and K. Benz researched data for the article. E. Ritz, K. Amann and K. Benz provided substantial contributions to discussion of the content and contributed equally to writing the article. K. Amann and K. Benz contributed equally to reviewing and editing the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ritz, E., Amann, K., Koleganova, N. et al. Prenatal programming—effects on blood pressure and renal function. Nat Rev Nephrol 7, 137–144 (2011). https://doi.org/10.1038/nrneph.2011.1
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2011.1
This article is cited by
-
Associations of prematurity and low birth weight with blood pressure and kidney function in middle-aged participants of the Brazilian Longitudinal Study of Adult Health: ELSA-Brasil
Journal of Nephrology (2023)
-
Arterial hypertension and cystatin C during neonatal physiologic dehydration
Journal of Human Hypertension (2022)
-
Assessment of nephron number and single-nephron glomerular filtration rate in a clinical setting
Hypertension Research (2021)
-
Prenatal alcohol exposure affects renal function in overweight schoolchildren: birth cohort analysis
Pediatric Nephrology (2020)
-
Hyperglycemia During Pregnancy and Long-Term Offspring Outcomes
Current Diabetes Reports (2019)