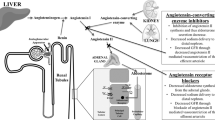
Abstract
Angiotensin II and other components of the renin–angiotensin–aldosterone system (RAAS) have a central role in the pathogenesis and progression of diabetic renal disease. A study in patients with type 1 diabetes and overt nephropathy found that RAAS inhibition with angiotensin-converting-enzyme (ACE) inhibitors was associated with a reduced risk of progression to end-stage renal disease and mortality compared with non-RAAS-inhibiting drugs. Blood-pressure control was similar between groups and proteinuria reduction was responsible for a large part of the renoprotective and cardioprotective effect. ACE inhibitors can also prevent microalbuminuria in patients with type 2 diabetes who are hypertensive and normoalbuminuric; in addition, ACE inhibitors are cardioprotective even in the early stages of diabetic renal disease. Angiotensin-II-receptor blockers (ARBs) are renoprotective (but not cardioprotective) in patients with type 2 diabetes and overt nephropathy or microalbuminuria. Studies have evaluated the renoprotective effect of other RAAS inhibitors, such as aldosterone antagonists and renin inhibitors, administered either alone or in combination with ACE inhibitors or ARBs. An important task for the future will be identifying which combination of agents achieves the best renoprotection (and cardioprotection) at the lowest cost. Such findings will have major implications, particularly in settings where money and facilities are limited and in settings where renal replacement therapy is not available and the prevention of kidney failure is life saving.
Key Points
-
Angiotensin II has a major role in the pathogenesis and progression of diabetic renal disease, and inhibition of angiotensin II production or activity using angiotensin-converting-enzyme (ACE) inhibitors or angiotensin-II-receptor blockers (ARBs) is renoprotective in patients with diabetes
-
ACE inhibitors have a cardioprotective effect in patients with diabetes and renal disease that is not seen with ARBs
-
Early intervention with ACE inhibitors may prevent the onset of microalbuminuria, which is an early sign of renal involvement and a marker of cardiovascular disease in individuals with diabetes.
-
Late intervention with ACE inhibitors or ARBs in patients who have type 2 diabetes, renal insufficiency and nephrotic-range proteinuria is not very effective, which highlights the importance of early intervention and the urgent need for novel treatments in this population
-
Increasing the dosage of ACE inhibitors and ARBs above the recommended antihypertensive doses and combined therapy with both classes of drug are the most effective ways of reducing albuminuria and, conceivably, of maximizing renoprotection
-
Aldosterone-receptor antagonists and direct renin inhibitors may also reduce albuminuria in patients with diabetes; long-term clinical trials are needed to assess whether these medications offer advantages over ACE inhibitors and ARBs, alone or in combination
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kaschina, E. & Unger, T. Angiotensin AT1/AT2 receptors: regulation, signalling and function. Blood Press. 12, 70–88 (2003).
Morgan, T. Renin, angiotensin, sodium and organ damage. Hypertens. Res. 26, 349–354 (2003).
Harris, R. C. & Martinez-Maldonado, M. Angiotensin II-mediated renal injury. Miner. Electrolyte Metab. 21, 328–335 (1995).
Ruggenenti, P. R. G. Introduction. Semin. Nephrol. 24, 91–92 (2004).
Ferrario, C. M. Role of angiotensin II in cardiovascular disease therapeutic implications of more than a century of research. J. Renin Angiotensin Aldosterone Syst. 7, 3–14 (2006).
Brewster, U. C. & Perazella, M. A. The renin–angiotensin–aldosterone system and the kidney: effects on kidney disease. Am. J. Med. 116, 263–272 (2004).
Cooper, M. E. The role of the renin–angiotensin–aldosterone system in diabetes and its vascular complications. Am. J. Hypertens. 17, 16S–20S (2004).
Carey, R. M. & Siragy, H. M. Newly recognized components of the renin–angiotensin system: potential roles in cardiovascular and renal regulation. Endocr. Rev. 24, 261–271 (2003).
Hilgers, K. F. & Mann, J. F. ACE inhibitors versus AT(1) receptor antagonists in patients with chronic renal disease. J. Am. Soc. Nephrol. 13, 1100–1108 (2002).
Siragy, H. M. & Carey, R. M. Protective role of the angiotensin AT2 receptor in a renal wrap hypertension model. Hypertension 33, 1237–1242 (1999).
Reudelhuber, T. L. The continuing saga of the AT2 receptor: a case of the good, the bad, and the innocuous. Hypertension 46, 1261–1262 (2005).
Silvestre, J. S. et al. Antiangiogenic effect of angiotensin II type 2 receptor in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 90, 1072–1079 (2002).
Ondetti, M. A., Rubin, B. & Cushman, D. W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science 196, 441–444 (1977).
Zatz, R. et al. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J. Clin. Invest. 77, 1925–1930 (1986).
Perico, N., Benigni, A. & Remuzzi, G. Present and future drug treatments for chronic kidney diseases: evolving targets in renoprotection. Nat. Rev. Drug Discov. 7, 936–953 (2008).
[No authors listed] Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. The GISEN group. Lancet 349, 1857–1863 (1997).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Taguma, Y. et al. Effect of captopril on heavy proteinuria in azotemic diabetics. N. Engl. J. Med. 313, 1617–1620 (1985).
Bjorck, S., Mulec, H., Johnsen, S. A., Nordén, G. & Aurell, M. Renal protective effect of enalapril in diabetic nephropathy. BMJ 304, 339–343 (1992).
Parving, H. H., Hommel, E. & Smidt, U. M. Protection of kidney function and decrease in albuminuria by captopril in insulin dependent diabetics with nephropathy. BMJ 297, 1086–1091 (1988).
Brenner, B. M. et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 345, 861–869 (2001).
Lewis, E. J. et al. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 345, 851–860 (2001).
de Zeeuw, D. et al. Albuminuria, a therapeutic target for cardiovascular protection in type 2 diabetic patients with nephropathy. Circulation 110, 921–927 (2004).
Casas, J. P. et al. Effect of inhibitors of the renin-angiotensin system and other antihypertensive drugs on renal outcomes: systematic review and meta-analysis. Lancet 366, 2026–2033 (2005).
Remuzzi, G. & Ruggenenti, P. Overview of randomised trials of ACE inhibitors. Lancet 368, 555–556 (2006).
Zatz, R. et al. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J. Clin. Invest. 77, 1925 (1986).
Perico, N. et al. Evidence that an angiotensin-converting enzyme inhibitor has a different effect on glomerular injury according to the different phase of the disease at which the treatment is started. J. Am. Soc. Nephrol. 5, 1139–1146 (1994).
Viberti, G., Mogensen, C. E., Groop, L. C. & Pauls, J. F. Effect of captopril on progression to clinical proteinuria in patients with insulin-dependent diabetes mellitus and microalbuminuria. European Microalbuminuria Captopril Study Group. JAMA 271, 275–279 (1994).
ACE Inhibitors in Diabetic Nephropathy Trialist Group. Should all patients with type 1 diabetes mellitus and microalbuminuria receive angiotensin-converting enzyme inhibitors? A meta-analysis of individual patient data. Ann. Intern. Med. 134, 370–379 (2001).
Parving, H. H. & Hovind, P. Microalbuminuria in type 1 and type 2 diabetes mellitus: evidence with angiotensin converting enzyme inhibitors and angiotensin II receptor blockers for treating early and preventing clinical nephropathy. Curr. Hypertens. Rep. 4, 387–393 (2002).
Yusuf, S. et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 342, 145–153 (2000).
[No authors listed] Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 355, 253–259 (2000).
Parving, H. H. et al. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N. Engl. J. Med. 345, 870–878 (2001).
Gall, M.-A., Hougaard, P., Borch-Johnsen, K. & Parving, H.-H. Risk factors for development of incipient and overt diabetic nephropathy in patients with non-insulin dependent diabetes mellitus: prospective, observational study. BMJ 314, 783–788 (1997).
Brenner, B. M. (Ed.) Brenner & Rector's The Kidney (W. B. Saunders, Philadelphia, 2008).
Eurich, D. T., Majumdar, S. R., Tsuyuki, R. T. & Johnson, J. A. Reduced mortality associated with the use of ACE inhibitors in patients with type 2 diabetes. Diabetes Care 27, 1330–1334 (2004).
Adler, A. I. et al. Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int. 63, 225–232 (2003).
[No authors listed] Randomised placebo-controlled trial of lisinopril in normotensive patients with insulin-dependent diabetes and normoalbuminuria or microalbuminuria. The EUCLID Study Group. Lancet 349, 1787–1792 (1997).
Chaturvedi, N. et al. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised, placebo-controlled trials. Lancet 372, 1394–1402 (2008).
Mauer, M. et al. Renal and retinal effects of enalapril and losartan in type 1 diabetes. N. Engl. J. Med. 361, 40–51 (2009).
Baba, S. Nifedipine and enalapril equally reduce the progression of nephropathy in hypertensive type 2 diabetics. Diabetes Res. Clin. Pract. 54, 191–201 (2001).
Ruggenenti, P. et al. Preventing microalbuminuria in type 2 diabetes. N. Engl. J. Med. 351, 1941–1951 (2004).
Ruggenenti, P., Perna, A., Ganeva, M., Ene-Iordache, B. & Remuzzi, G. Impact of blood pressure control and angiotensin-converting enzyme inhibitor therapy on new-onset microalbuminuria in type 2 diabetes: a post hoc analysis of the BENEDICT trial. J. Am. Soc. Nephrol. 17, 3472–3481 (2006).
Haller, H. et al. Preventing microalbuminuria in patients with diabetes: rationale and design of the Randomised Olmesartan and Diabetes Microalbuminuria Prevention (ROADMAP) study. J. Hypertens. 24, 403–408 (2006).
Patel, A. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 358, 2560–2572 (2008).
Estacio, R. O. et al. The effect of nisoldipine as compared with enalapril on cardiovascular outcomes in patients with non-insulin-dependent diabetes and hypertension. N. Engl. J. Med. 338, 645–652 (1998).
Lindholm, L. H. et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet 359, 1004–1010 (2002).
Bloom, J. M. Losartan for cardiovascular disease in patient's with and without diabetes in the LIFE study. Lancet 359, 2201 (2002).
Williams, B. et al. Differential impact of blood pressure-lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation 113, 1213–1225 (2006).
Barnett, A. H. et al. Angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N. Engl. J. Med. 351, 1952–1961 (2004).
Abuissa, H., Jones, P. G., Marso, S. P. & O'Keefe, J. H. Jr. Angiotensin-converting enzyme inhibitors or angiotensin receptor blockers for prevention of type 2 diabetes: a meta-analysis of randomized clinical trials. J. Am. Coll. Cardiol. 46, 821–826 (2005).
Investigators, T. D. T. Effect of ramipril on the incidence of diabetes. N. Engl. J. Med. 355, 1551–1562 (2006).
Ruggenenti, P., Bettinaglio, P., Pinares, F. & Remuzzi, G. Angiotensin converting enzyme insertion/deletion polymorphism and renoprotection in diabetic and nondiabetic nephropathies. Clin. J. Am. Soc. Nephrol. 3, 1511–1525 (2008).
Marre, M. Genetics and the prediction of complications in type 1 diabetes. Diabetes Care 22 (Suppl. 2), B53–B58 (1999).
Allen, T. J., Waldron, M. J., Casley, D., Jerums, G. & Cooper, M. E. Salt restriction reduces hyperfiltration, renal enlargement, and albuminuria in experimental diabetes. Diabetes 46, 19–24 (1997).
Fabris, B., Jackson, B. & Johnston, C. I. Salt blocks the renal benefits of ramipril in diabetic hypertensive rats. Hypertension 17, 497–503 (1991).
Vogt, L., Waanders, F., Boomsma, F., de Zeeuw, D. & Navis, G. Effects of dietary sodium and hydrochlorothiazide on the antiproteinuric efficacy of losartan. J. Am. Soc. Nephrol. 19, 999–1007 (2008).
Ekinci, E. I. et al. Effects of salt supplementation on the albuminuric response to telmisartan with or without hydrochlorothiazide therapy in hypertensive patients with type 2 diabetes are modulated by habitual dietary salt intake. Diabetes Care 32, 1398–1403 (2009).
Cianciaruso, B. et al. Salt intake and renal outcome in patients with progressive renal disease. Miner. Electrolyte Metab. 24, 296–301 (1998).
O'Hare, J. A. et al. Blood pressure may be sodium-dependent in diabetic patients without overt nephropathy. Ir. J. Med. Sci. 154, 455–460 (1985).
Weir, M. R. Impact of salt intake on blood pressure and proteinuria in diabetes: importance of the renin-angiotensin system. Miner. Electrolyte Metab. 24, 438–445 (1998).
De'Oliveira, J. M. et al. Autonomy of the renin system in type II diabetes mellitus: dietary sodium and renal hemodynamic responses to ACE inhibition. Kidney Int. 52, 771–777 (1997).
Jerums, G., Allen, T. J., Tsalamandris, C. & Cooper, M. E. Angiotensin converting enzyme inhibition and calcium channel blockade in incipient diabetic nephropathy. The Melbourne Diabetic Nephropathy Study Group. Kidney Int. 41, 904–911 (1992).
Ritchie, S. A. & Connell, J. M. The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutr. Metab. Cardiovasc. Dis. 17, 319–326 (2007).
Reisin, E. & Jack, A. V. Obesity and hypertension: mechanisms, cardio-renal consequences, and therapeutic approaches. Med. Clin. North Am. 93, 733–751 (2009).
Sharma, A. M. Is there a rationale for angiotensin blockade in the management of obesity hypertension? Hypertension 44, 12–19 (2004).
Burgess, E. et al. Supramaximal dose of candesartan in proteinuric renal disease. J. Am. Soc. Nephrol. 20, 893–900 (2009).
Hollenberg, N. K. et al. Albuminuria response to very high-dose valsartan in type 2 diabetes mellitus. J. Hypertens. 25, 1921–1926 (2007).
Weir, M. R. et al. Antihypertensive effects of double the maximum dose of valsartan in African-American patients with type 2 diabetes mellitus and albuminuria. J. Hypertens. 28, 186–193 (2010).
Hou, F. F. et al. Renoprotection of Optimal Antiproteinuric Doses (ROAD) Study: a randomized controlled study of benazepril and losartan in chronic renal insufficiency. J. Am. Soc. Nephrol. 18, 1889–1898 (2007).
Ruggenenti, P., Cravedi, P. & Remuzzi, G. Proteinuria: increased angiotensin-receptor blocking is not the first option. Nat. Rev. Nephrol. 5, 367–368 (2009).
Jacobsen, P., Andersen, S., Rossing, K., Jensen, B. R. & Parving, H. H. Dual blockade of the renin-angiotensin system versus maximal recommended dose of ACE inhibition in diabetic nephropathy. Kidney Int. 63, 1874–1880 (2003).
Jacobsen, P., Andersen, S., Rossing, K., Hansen, B. V. & Parving, H. H. Dual blockade of the renin-angiotensin system in type 1 patients with diabetic nephropathy. Nephrol. Dial. Transplant. 17, 1019–1024 (2002).
Jacobsen, P., Andersen, S., Jensen, B. R. & Parving, H. H. Additive effect of ACE inhibition and angiotensin II receptor blockade in type I diabetic patients with diabetic nephropathy. J. Am. Soc. Nephrol. 14, 992–999 (2003).
Mogensen, C. E. et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 321, 1440–1444 (2000).
Campbell, R. et al. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 63, 1094–1103 (2003).
Ruggenenti, P. & Remuzzi, G. Proteinuria: is the ONTARGET renal substudy actually off target? Nat. Rev. Nephrol. 5, 436–437 (2009).
Maschio, G. et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N. Engl. J. Med. 334, 939–945 (1996).
Locatelli, F. et al. Long-term progression of chronic renal insufficiency in the AIPRI Extension Study. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Kidney Int. Suppl. 63, S63–S66 (1997).
Takaichi, K., Takemoto, F., Ubara, Y. & Mori, Y. Analysis of factors causing hyperkalemia. Intern. Med. 46, 823–829 (2007).
Mann, J. F. et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 372, 547–553 (2008).
Epstein, M. Re-examining RAS-blocking treatment regimens for abrogating progression of chronic kidney disease. Nat. Clin. Pract. Nephrol. 5, 12–13 (2009).
Lindeman, R. D., Tobin, J. & Shock, N. W. Longitudinal studies on the rate of decline in renal function with age. J. Am. Geriatr. Soc. 33, 278–285 (1985).
Ruggenenti, P. et al. Role of remission clinics in the longitudinal treatment of CKD. J. Am. Soc. Nephrol. 19, 1213–1224 (2008).
Os, I., Gudmundsdottir, H., Kjeldsen, S. E. & Oparil, S. Treatment of isolated systolic hypertension in diabetes mellitus type 2. Diabetes Obes. Metab. 8, 381–387 (2006).
Ruggenenti, P. et al. Glomerular size-selective dysfunction in NIDDM is not ameliorated by ACE inhibition or by calcium channel blockade. Kidney Int. 55, 984–994 (1999).
Remuzzi, G., Benigni, A. & Remuzzi, A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Invest. 116, 288–296 (2006).
Zhang, Z. et al. Importance of baseline distribution of proteinuria in renal outcomes trials: lessons from the reduction of endpoints in NIDDM with the angiotensin II antagonist losartan (RENAAL) study. J. Am. Soc. Nephrol. 16, 1775–1780 (2005).
Bomback, A. S. & Klemmer, P. J. The incidence and implications of aldosterone breakthrough. Nat. Clin. Pract. Nephrol. 3, 486–492 (2007).
Rossi, G. P. Aldosterone breakthrough during RAS blockade: a role for endothelins and their antagonists? Curr. Hypertens. Rep. 8, 262–268 (2006).
Becker, G. J., Hewitson, T. D. & Chrysostomou, A. Aldosterone in clinical nephrology—old hormone, new questions. Nephrol. Dial. Transplant. 24, 2316–2321 (2009).
Struthers, A., Krum, H. & Williams, G. H. A comparison of the aldosterone-blocking agents eplerenone and spironolactone. Clin. Cardiol. 31, 153–158 (2008).
Du, J. et al. Mineralocorticoid receptor blockade and calcium channel blockade have different renoprotective effects on glomerular and interstitial injury in rats. Am. J. Physiol. Renal Physiol. 297, F802–F808 (2009).
Piecha, G. et al. Regression of glomerulosclerosis in subtotally nephrectomized rats: effects of monotherapy with losartan, spironolactone, and their combination. Am. J. Physiol. Renal Physiol. 295, F137–F144 (2008).
Chrysostomou, A. & Becker, G. Spironolactone in addition to ACE inhibition to reduce proteinuria in patients with chronic renal disease. N. Engl. J. Med. 345, 925–926 (2001).
Navaneethan, S. D., Nigwekar, S. U., Sehgal, A. R. & Strippoli, G. F. Aldosterone antagonists for preventing the progression of chronic kidney disease: a systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 4, 542–551 (2009).
Mehdi, U. F., Adams-Huet, B., Raskin, P., Vega, G. L. & Toto, R. D. Addition of angiotensin receptor blockade or mineralocorticoid antagonism to maximal angiotensin-converting enzyme inhibition in diabetic nephropathy. J. Am. Soc. Nephrol. 20, 2641–2650 (2009).
Bianchi, S., Bigazzi, R. & Campese, V. M. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 70, 2116–2123 (2006).
van den Meiracker, A. H. et al. Spironolactone in type 2 diabetic nephropathy: effects on proteinuria, blood pressure and renal function. J. Hypertens. 24, 2285–2292 (2006).
Epstein, M. et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin. J. Am. Soc. Nephrol. 1, 940–951 (2006).
Khosla, N., Kalaitzidis, R. & Bakris, G. L. Predictors of hyperkalemia risk following hypertension control with aldosterone blockade. Am. J. Nephrol. 30, 418–424 (2009).
Estacio, R. O. Renin–angiotensin–aldosterone system blockade in diabetes: role of direct renin inhibitors. Postgrad. Med. 121, 33–44 (2009).
Nguyen, G. et al. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J. Clin. Invest. 109, 1417–1427 (2002).
Nguyen, G. & Danser, A. H. Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp. Physiol. 93, 557–563 (2008).
Ichihara, A. et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the “handle” region for nonproteolytic activation of prorenin. J. Clin. Invest. 114, 1128–1135 (2004).
Eder, J., Hommel, U., Cumin, F., Martoglio, B. & Gerhartz, B. Aspartic proteases in drug discovery. Curr. Pharm. Des. 13, 271–285 (2007).
Persson, F. et al. Renal effects of aliskiren compared with and in combination with irbesartan in patients with type 2 diabetes, hypertension, and albuminuria. Diabetes Care 32, 1873–1879 (2009).
Parving, H. H., Persson, F., Lewis, J. B., Lewis, E. J. & Hollenberg, N. K. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N. Engl. J. Med. 358, 2433–2446 (2008).
Garattini, S. & Bertele, V. Risk:benefit assessment of old medicines. Br. J. Clin. Pharmacol. 58, 581–586 (2004).
Bilous, R. et al. Effect of candesartan on microalbuminuria and albumin excretion rate in diabetes: three randomized trials. Ann. Intern. Med. 151, 11–20 (2009).
Andersen, N. H. et al. Long-term dual blockade with candesartan and lisinopril in hypertensive patients with diabetes: the CALM II study. Diabetes Care 28, 273–277 (2005).
Rossing, K., Christensen, P. K., Jensen, B. R. & Parving, H. H. Dual blockade of the renin–angiotensin system in diabetic nephropathy: a randomized double-blind crossover study. Diabetes Care 25, 95–100 (2002).
Rossing, K., Jacobsen, P., Pietraszek, L. & Parving, H. H. Renoprotective effects of adding angiotensin II receptor blocker to maximal recommended doses of ACE inhibitor in diabetic nephropathy: a randomized double-blind crossover trial. Diabetes Care 26, 2268–2274 (2003).
Tutuncu, N. B., Gurlek, A. & Gedik, O. Efficacy of ACE inhibitors and ATII receptor blockers in patients with microalbuminuria: a prospective study. Acta Diabetol. 38, 157–161 (2001).
Acknowledgements
The authors would like to thank Manuela Passera, secretary of the Mario Negri Institute for Pharmacological Research, Bergamo, Italy, for her editorial assistance in the preparation of this manuscript.
Désirée Lie, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the MedscapeCME-accredited continuing medical education activity associated with this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ruggenenti, P., Cravedi, P. & Remuzzi, G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 6, 319–330 (2010). https://doi.org/10.1038/nrneph.2010.58
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.58
This article is cited by
-
Angiotensin II type 1 receptor-associated protein deletion combined with angiotensin II stimulation accelerates the development of diabetic kidney disease in mice on a C57BL/6 strain
Hypertension Research (2024)
-
SIRT2 alleviated renal fibrosis by deacetylating SMAD2 and SMAD3 in renal tubular epithelial cells
Cell Death & Disease (2023)
-
In vivo mapping of hemodynamic responses mediated by tubuloglomerular feedback in hypertensive kidneys
Scientific Reports (2023)
-
Nanoformulations of flavonoids for diabetes and microvascular diabetic complications
Drug Delivery and Translational Research (2023)
-
Evaluation of N-acetyl-β-D-glucosaminidase as a prognostic marker for diabetic nephropathy in type 2 diabetics: systematic review and meta-analysis
International Urology and Nephrology (2023)