Abstract
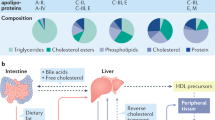
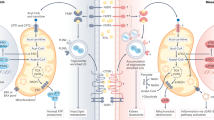
Chronic kidney disease (CKD) is associated with development of atherosclerosis and premature death from cardiovascular disease. The predisposition of patients with CKD to atherosclerosis is driven by inflammation, oxidative stress and dyslipidemia, all of which are common features of this condition. Markers of dyslipidemia in patients with advanced CKD are impaired clearance and heightened oxidation of apolipoprotein-B-containing lipoproteins and their atherogenic remnants, and a reduction of the plasma concentration, antioxidant, and anti-inflammatory properties of high-density lipoprotein (HDL). Studies in animal models of CKD indicate that the disease promotes lipid accumulation in the artery wall and kidney, leading to atherosclerosis, glomerulosclerosis and tubulointerstitial injury. These effects seem to be mediated by an increased cellular influx of lipids, elevated cellular production and reduced cellular catabolism of fatty acids, and impaired antioxidant, anti-inflammatory and reverse lipid transport properties of HDL. Available pharmacological therapies have been largely ineffective in ameliorating oxidative stress, inflammation, HDL deficiency and/or dysfunction, and the associated atherosclerosis and cardiovascular disease in patients with end-stage renal disease. This Review aims to provide an overview of the mechanisms and consequences of CKD-induced HDL deficiency and dysfunction.
Key Points
-
Chronic kidney disease (CKD) is associated with oxidative stress, inflammation and profound dysregulation of lipid metabolism
-
The combination of dyslipidemia, proteinuria, oxidative stress and inflammation in patients with CKD results in an accumulation of lipids in the artery wall and in the diseased kidney tissue
-
The harmful effect of increased lipid influx in the kidney and arterial tissues is compounded by impaired cellular lipid efflux caused by high-density lipoprotein (HDL) deficiency and dysfunction
-
The increase in tissue lipid burden associated with CKD leads to foam cell formation, cytotoxicity and progression of renal disease and atherosclerosis
-
Efficacy of therapeutic interventions to retard progression of renal disease and atherosclerosis in patients with CKD depends on the ability to attenuate oxidative stress and inflammation, reverse HDL deficiency and restore HDL antioxidant and anti-inflammatory properties
-
Currently available pharmacological options that target dyslipidemia are ineffective, unsafe and/or poorly tolerated by patients with end-stage renal disease
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Collins, A. J. et al. Excerpts from the United States Renal Data system 2005 Annual Data Report. Atlas of end-stage renal disease in the United States. Am. J. Kidney Dis. 45 (Suppl. 1), S1–S280 (2005).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. & Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Kovesdy, C. P., Trivedi, B. K. & Anderson, J. E. Association of kidney function with mortality in patients with chronic kidney disease not yet on dialysis: a historical prospective cohort study. Adv. Chronic Kidney Dis. 13, 183–188 (2006).
Muntner, P., He, J., Astor, B. C., Folsom, A. R. & Coresh, J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J. Am. Soc. Nephrol. 16, 529–538 (2005).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 62, 1524–1538 (2002).
McCullough, P. A. Why is chronic kidney disease the ''spoiler'' for cardiovascular outcomes? J. Am. Coll. Cardiol. 41, 725–728 (2003).
Stenvinkel, P. & Alvestrand, A. Inflammation in end-stage renal disease: sources, consequences, and therapy. Semin. Dial. 15, 329–337 (2002).
Vaziri, N. D. Oxidative stress in uremia: nature, mechanisms and consequences. Semin. Nephrol. 24, 469–473 (2004).
Vaziri, N. D. et al. Intra-renal angiotensin II/AT1 receptor, oxidative stress, inflammation, and progressive injury in renal mass reduction. J. Pharmacol. Exp. Ther. 323, 85–93 (2007).
Vaziri, N. D. Dyslipidemia of chronic renal failure: the nature, mechanisms and potential consequences. Am. J. Physiol. Renal Physiol. 290, F262–F272 (2006).
Vaziri, N. D. & Liang, K. Downregulation of tissue lipoprotein lipase expression in experimental chronic renal failure. Kidney Int. 50, 1928–1935 (1996).
Vaziri, N. D., Wang, X. Q. & Liang, K. Secondary hyperparathyroidism downregulates lipoprotein lipase expression in chronic renal failure. Am. J. Physiol. Renal Physiol. 273, F925–F930 (1997).
Vaziri, N. D. & Liang, K. Downregulation of VLDL receptor expression in chronic experimental renal failure. Kidney Int. 51, 913–919 (1997).
Sato, T., Liang, K. & Vaziri, N. D. Protein restriction and AST-120 improve lipoprotein lipase and VLDL receptor in focal glomerulosclerosis. Kidney Int. 64, 1780–1786 (2003).
Kim, C. & Vaziri, N. D. Downregulation of hepatic LDL receptor-related protein (LRP) in chronic renal failure. Kidney Int. 67, 1028–1032 (2005).
Moradi, H., Pahl, M. V., Elahimehr, R. & Vaziri, N. D. Impaired antioxidant activity of HDL in chronic kidney disease. Transl. Res. 153, 77–85 (2009).
Vaziri, N. D., Liang, K. & Parks, J. S. Downregulation of hepatic lecithin:cholesterol acyltransferase (LCAT) gene expression in chronic renal failure. Kidney Int. 59, 2192–2196 (2001).
Liang, K. & Vaziri, N. D. Upregulation of acyl-CoA: cholesterol acyltransferase (ACAT) in chronic renal failure. Am. J. Physiol. Endocrinol. Metab. 283, E676–E681 (2002).
Vaziri, N. D. & Liang, K. ACAT inhibition reverses LCAT deficiency and improves plasma HDL in chronic renal failure. Am. J. Physiol. Renal Physiol. 287, F1038–F1043 (2004).
Botham, K. M., Moore, E. H., De Pascale, C. & Bejta, F. The induction of macrophage foam cell formation by chylomicron remnants. Biochem. Soc. Trans. 35, 454–458 (2007).
Gleissner, C. A., Leitinger, N. & Ley, K. Effects of native and modified low-density lipoproteins on monocyte recruitment in atherosclerosis. Hypertension 50, 276–283 (2007).
Hansson, G. K., Robertson, A. K. & Söderberg-Nauclér, C. Inflammation and atherosclerosis. Annu. Rev. Pathol. 1, 297–329 (2006).
Shashkin, P., Dragulev, B. & Ley, K. Macrophage differentiation to foam cells. Curr. Pharm. Des. 11, 3061–3072 (2005).
Bobryshev, Y. V. Monocyte recruitment and foam cell formation in atherosclerosis. Micron 37, 208–222 (2006).
Wilensky, R. L. & Hamamdzic, D. The molecular basis of vulnerable plaque: potential therapeutic role for immunomodulation. Curr. Opin. Cardiol. 22, 545–551 (2007).
Shao, B., Oda, M. N., Oram, J. F. & Heinecke, J. W. Myeloperoxidase: an inflammatory enzyme for generating dysfunctional high density lipoprotein. Curr. Opin. Cardiol. 21, 322–328 (2006).
Ansell, B. J. et al. Inflammatory/antiinflammatory properties of high-density lipoprotein distinguish patients from control subjects better than high-denisty lipoprotein cholesterol levels and are favorably affected by simvastatin treatment. Circulation 108, 2751–2756 (2003).
Navab, M. et al. The oxidation hypothesis of atherogenesis: the role of oxidized phospholipids and HDL. J. Lipid Res. 45, 993–1007 (2004).
Huang, Y. H., Rönnelid, J. & Frostegård, J. Oxidized LDL induces enhanced antibody formation and MCH class II-dependent INF-gamma production in lymphocytes from healthy individuals. Arterioscler. Thromb. Vasc. Biol. 15, 1577–1583 (1995).
Jovinge, S., Ares, M. P., Kallin, B. & Nilsson, J. Human monocytes/macrophages release TNF alpha in response to oxidized LDL. Arterioscler. Thromb. Vasc. Biol. 16, 1573–1579 (1996).
Navab, M. et al. The Yin and Yang of oxidation in the development of the fatty streak. A review based on the 1994 George Lyman Duff Memorial Lecture. Arterioscler. Thromb. Vasc. Biol. 16, 831–842 (1996).
Tsimikas, S. et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353, 46–57 (2005).
Berliner, J. A. & Watson, A. D. A role for oxidized phospholipids in atherosclerosis. N. Engl. J. Med. 353, 9–11 (2005).
Nicholls, S. J., Zheng, L. & Hazen, S. L. Formation of dysfunctional high-density lipoprotein by myeloperoxidase. Trends Cardiovasc. Med. 15, 212–219 (2005).
Kalantar-Zadeh, K., Brennan, M. L. & Hazen, S. L. Serum myeloperoxidase and mortality in maintenance hemodialysis patients. Am. J. Kidney Dis. 48, 59–68 (2006).
Navab, M. et al. HDL and the inflammatory response induced by LDL-derived oxidized phospholipids. Arterioscler. Thromb. Vasc. Biol. 21, 481–488 (2001).
Navab, M. et al. The double jeopardy of HDL. Ann. Med. 37, 173–178 (2005).
Navab, M. et al. Oxidized lipids as mediators of coronary heart disease. Curr. Opin. Lipidol. 13, 363–372 (2002).
Calabresi, L., Gomaraschi, M. & Franceschini, G. Endothelial protection by high-density lipoproteins: from bench to bedside. Arterioscler. Thromb. Vasc. Biol. 23, 1724–1731 (2003).
Davidson, M. H. & Toth, P. P. High-density lipoprotein metabolism: potential therapeutic targets. Am. J. Cardiol. 100, n32–n40 (2007).
Feig, J. E., Shamir, R. & Fisher, E. A. Atheroprotective effects of HDL: beyond reverse cholesterol transport. Curr. Drug Targets 9, 196–203 (2008).
Navab, M. et al. Monocyte transmigration induced by modification of low density lipoprotein in cocultures of human aortic wall cells is due to induction of monocyte chemotactic protein 1 synthesis and is abolished by high density lipoprotein. J. Clin. Invest. 88, 2039–2046 (1991).
Watson, A. D. et al. Protective effect of high density lipoprotein associated paraoxonase: inhibition of the biological activity of minimally oxidized low density lipoprotein. J. Clin. Invest. 96, 2882–2891 (1995).
Walter, M. Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 29, 1244–1250 (2009).
Attman, P. O. & Alaupovic, P. Lipid and apolipoprotein profiles of uremic dyslipoproteinemia—relation to renal function and dialysis. Nephron 57, 401–410 (1991).
Vaziri, N. D., Deng, G. & Liang, K. Hepatic HDL receptor, SR-B1 and Apo A-I expression in chronic renal failure. Nephrol. Dial. Transplant. 14, 1462–1466 (1999).
Kamanna, V. S. et al. Uremic serum subfraction inhibits apolipoprotein A-I production by a human hepatoma cell line. J. Am. Soc. Nephrol. 5, 193–200 (1994).
Shah, G. M. et al. Effect of serum subfractions from peritoneal dialysis patients on Hep-G2 cell apolipoprotein A-I and B metabolism. Kidney Int. 50, 2079–2087 (1996).
Zhao, Y. & Marcel, Y. L. Serum albumin is a significant intermediate in cholesterol transfer between cells and lipoproteins. Biochemistry 35, 7174–7180 (1996).
Shoji, T., Nishizawa, Y., Nishitani, H., Yamakawa, M. & Morii, H. Impaired metabolism of high density lipoprotein in uremic patients. Kidney Int. 41, 1653–1661 (1992).
Liang, K., Kim, C. & Vaziri, N. D. HMG-CoA reductase inhibition reverses LCAT and LDL receptor deficiencies and improves HDL in rats with chronic renal failure. Am. J. Physiol. Renal Physiol. 288, F539–F544 (2005).
Vaziri, N. D., Liang, K. & Parks, J. S. Downregulation of hepatic lecithin: cholesterol acyltransferase gene expression in chronic renal failure. Kidney Int. 59, 2192–2196 (2001).
Kimura, H., Miyazaki, R., Suzuki, S., Gejyo, F. & Yoshida, H. Cholesteryl ester transfer protein as a protective factor against vascular disease in hemodialysis patients. Am. J. Kidney Dis. 38, 70–76 (2001).
Kimura, H. et al. Hepatic lipase mutation may reduce vascular disease prevalence in hemodialysis patients with high CETP levels. Kidney Int. 64, 1829–1837 (2003).
Pahl, M. V., Ni, Z., Sepassi, L. & Vaziri, N. D. Plasma phospholipid transfer protein, cholesteryl ester transfer protein and lecithin: cholesterol acyltransferase in end-stage renal disease. Nephrol. Dial. Transplant. 24, 2541–2546 (2009).
Dullaart, R. P. et al. Role of elevated lecithin: cholesterol acyltransferase and cholesteryl ester transfer protein in lipoproteins from proteinuric patients. Kidney Int. 44, 91–97 (1993).
Vaziri, N. D. Molecular mechanisms of lipid disorders in nephrotic syndrome. Kidney Int. 63, 1964–1976 (2003).
Klin, M., Smogorzewski, M., Ni, Z., Zhang, G. & Massry, S. G. Abnormalities in hepatic lipase in chronic renal failure: role of excess parathyroid hormone. J. Clin. Invest. 97, 2167–2173 (1996).
Sato, T., Liang, K. & Vaziri, N. D. Protein restriction and AST-120 improve lipoprotein lipase and VLDL receptor deficiencies in focal glomerulosclerosis. Kidney Int. 64, 1780–1786 (2003).
Acton, S. et al. Identification of scavenger receptor SR-BI as a high density lipoprotein receptor. Science 271, 518–520 (1996).
Liang, K. & Vaziri, N. D. Downregulation of hepatic high-density lipoprotein receptor, SRB-1, in nephrotic syndrome. Kidney Int. 56, 621–626 (1999).
Fabre, A. C. et al. Cell surface adenylate kinase activity regulates the F (1)-ATPase/P2Y (13)-mediated HDL endocytosis pathway on human hepatocytes. Cell. Mol. Life Sci. 63, 2829–2837 (2006).
Martinez, L. O. et al. Ectopic beta-chain of ATP synthase is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 421, 75–79 (2003).
Van Lenten, B. J., Reddy, S. T., Navab, M. & Fogelman, A. M. Understanding changes in high density lipoproteins during the acute phase response. Arterioscler. Thromb. Vasc. Biol. 26, 1687–1688 (2006).
Morena, M. et al. Protective effects of high-density lipoprotein against oxidative stress are impaired in hemodialysis patients. Nephrol. Dial. Transplant. 15, 389–395 (2000).
Kalantar-Zadeh, K., Kopple, J. D., Kamranpour, N., Fogelman, A. M. & Navab, M. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 72, 1149–1156 (2007).
Vaziri, N. D., Moradi, H., Pahl, M. V., Fogelman, A. M. & Navab, M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 76, 437–444 (2009).
Ansell, B. J., Fonarow, G. C. & Fogelman, A. M. The paradox of dysfunctional high-density lipoprotein. Curr. Opin. Lipidol. 18, 427–434 (2007).
Deigner, H. P. & Hermetter, A. Oxidized phospholipids: emerging lipid mediators in pathophysiology. Curr. Opin. Lipidol. 19, 289–294 (2008).
Mehta, J. L., Chen, J., Hermonat, P. L., Romeo, F. & Novelli, G. Lectin-like, oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovasc. Res. 69, 36–45 (2006).
Cominacini, L. et al. Oxidized low density lipoprotein (ox-LDL) binding to ox-LDL receptor-1 in endothelial cells induces the activation of NF-kappaB through an increased production of intracellular reactive oxygen species. J. Biol. Chem. 275, 12633–12638 (2000).
Cominacini, L. et al. The binding of oxidized low density lipoprotein (ox-LDL) to ox-LDL receptor-1 reduces the intracellular concentration of nitric oxide in endothelial cells through an increased production of superoxide. J. Biol. Chem. 276, 13750–13755 (2001).
Guarnieri, G. F. et al. Lecithin-cholesterol acyltransferase (LCAT) activity in chronic uremia. Kidney Int. Suppl. 8, S26–S30 (1978).
Boadu, E., Bilbey, N. J. & Francis, G. A. Cellular cholesterol substrate pools for adenosine-triphosphate cassette transporter A1-dependent high-density lipoprotein formation. Curr. Opin. Lipidol. 19, 270–276 (2008).
Bowry, V. W., Stanley, K. K. & Stocker, R. High density lipoprotein is the major carrier of lipid hydroperoxides in human blood plasma from fasting donors. Proc. Natl Acad. Sci. USA 89, 10316–10320 (1992).
Navab, M. et al. Mechanisms of disease: proatherogenic HDL-an evolving field. Nat. Clin. Pract. Endocrinol. Metab. 2, 504–511 (2006).
Abrass, C. K. Cellular lipid metabolism and the role of lipids in progressive renal disease. Am. J. Nephrol. 24, 46–53 (2004).
Schaffer, J. E. Lipotoxicity: when tissues overeat. Curr. Opin. Lipidol. 14, 281–287 (2003).
Johnson, A. C., Yabu, J. M., Hanson, S., Shah, V. O. & Zager, R. A. Experimental glomerulopathy alters renal cortical cholesterol, SR-B1, ABCA1, and HMG CoA reductase expression. Am. J. Pathol. 162, 283–291 (2003).
Susztak, K., Ciccone, E., McCue, P., Sharma, K. & Böttinger, E. P. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PloS Med. 2, e45 (2005).
Moorhead, J. F., Chan, M. K., El-Nahas, M. & Varghese, Z. Lipid nephrotoxicity in chronic progressive glomerular and tubulo-interstitial disease. Lancet 2, 1309–1311 (1982).
Zager, R. A., Johnson, A. C., Hanson, S. Y. & Shah, V. O. Acute tubular injury causes dysregulation of cellular cholesterol transport proteins. Am. J. Pathol. 163, 313–320 (2003).
Glass, C. K. & Witztum, J. L. Atherosclerosis: the road ahead. Cell 104, 503–516 (2001).
Li, D. & Mehta, J. L. Antisense to LOX-1 inhibits oxidized LDL-mediated upregulation of monocyte chemoattractant protein-1 and monocyte adhesion to human coronary artery endothelial cells. Circulation 101, 2889–2895 (2000).
Tontonoz, P. & Mangelsdorf, D. J. Liver X receptor signaling pathways in cardiovascular disease. Mol. Endocrinol. 17, 985–993 (2003).
Brown, M. S. & Goldstein, J. L. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell 89, 331–340 (1997).
Ishii, S., Iizuka, K., Miller, B. C. & Uyeda, K. Carbohydrate response element binding protein directly promotes lipogenic enzyme gene transcription. Proc. Natl Acad. Sci. USA 101, 15597–15602 (2004).
Brown, M. S. & Goldstein, J. L. A proteolytic pathway that controls the cholesterol content of membranes, cells, and blood. Proc. Natl Acad. Sci. USA 96, 11041–11048 (1999).
Guan, Y. & Breyer, M. D. Peroxisome proliferator-activated receptors (PPARs): novel therapeutic targets in renal disease. Kidney Int. 60, 14–30 (2001).
Guan, Y. Peroxisome proliferator-activated receptor family and its relationship to renal complications of the metabolic syndrome. J. Am. Soc. Nephrol. 15, 2801–2815 (2004).
Kim, H. J., Moradi, H., Yuan, J., Norris, K. & Vaziri, N. D. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am. J. Physiol. Renal Physiol. 296, F1297–F1306 (2009).
Moradi, H., Yuan, J., Ni, Z., Norris, K. & Vaziri, N. D. Reverse cholesterol transport pathway in experimental chronic kidney disease. Am. J. Nephrol. 30, 147–154 (2009).
Remuzzi, G., Benigni, A. & Remuzzi, A. Mechanisms of progression and regression of renal lesions of chronic nephropathies and diabetes. J. Clin. Invest. 116, 288–296 (2006).
Mestrup, S. K. & Nielsen, L. B. The role of the kidney in lipid metabolism. Curr. Opin. Lipidol. 16, 301–306 (2005).
Baines, R. J. & Brunskill, N. J. The molecular interactions between filtered proteins and proximal tubular cells in proteinuria. Nephron Exp. Nephrol. 110, e67–e71 (2008).
Bocan, T. M. et al. The ACAT inhibitor avasimibe reduces macrophages and matrix metalloproteinase expression in atherosclerotic lesions of hypercholesterolemic rabbits. Arterioscler. Thromb. Vasc. Biol. 20, 70–79 (2000).
Wang, X., Sato, R., Brown, M. S., Hua, X. & Goldstein, J. L. SREBP-1, a membrane-bound transcription factor released by sterol-regulated proteolysis. Cell 77, 53–62 (1994).
Harper, C. R. & Jacobson, T. A. Managing dyslipidemia in chronic kidney disease. J. Am. Coll. Cardiol. 51, 2375–2384 (2008).
Montague, T. & Murphy, B. Lipid management in chronic kidney disease, hemodialysis, and transplantation. Endocrinol. Metab. Clin. North Am. 38, 223–234 (2009).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353, 238–248 (2005).
Fellström, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360, 1395–1407 (2009).
Krane, V. et al. Effect of atorvastatin on inflammation and outcome in patients with type 2 diabetes mellitus on hemodialysis. Kidney Int. 74, 1461–1467 (2008).
Ruan, X. Z., Varghese, Z. & Moorhead, J. F. An update on the lipid nephrotoxicity hypothesis. Nat. Rev. Nephrol. 5, 713–721 (2009).
Duval, C., Müller, M. & Kersten, S. PPAR alpha and dyslipidemia. Biochim. Biophys. Acta 1771, 961–971 (2007).
Tonelli, M., Collins, D., Robins, S., Bloomfield, H. & Curhan, G. C. Effect of gemfibrozil on change in renal function in men with moderate chronic renal insufficiency and coronary disease. Am. J. Kidney Dis. 44, 832–839 (2004).
Tonelli, M., Collins, D., Robins, S., Bloomfield, H. & Curhan, G. C. ; Veterans' Affairs High-Density Lipoprotein Intervention Trial (VA-HIT) Investigators. Gemfibrozil for secondary prevention of cardiovascular events in mild to moderate chronic renal insufficiency. Kidney Int. 66, 1123–1130 (2004).
Carlson, L. A. Nicotinic acid: the broad-spectrum lipid drug. A 50th anniversary review. J. Intern. Med. 258, 94–114 (2005).
Cho, K. H., Kim, H. J., Rodriguez-Iturbe, B. & Vaziri, N. D. Niacin ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am. J. Physiol. Renal Physiol. 297, F106–F113 (2009).
Cho, K. H., Kim, H. J., Kamanna, V. S. & Vaziri, N. D. Niacin improves renal lipid metabolism and slows progression in chronic kidney disease. Biochim. Biophys. Acta 1800, 6–15 (2010).
Belalcazar, L. M. et al. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation 107, 2726–2732 (2003).
Tangirala, R. K. et al. Regression of atherosclerosis induced by liver-directed gene transfer of apolipoprotein A-I in mice. Circulation 100, 1816–1822 (1999).
Calabresi, L., Sirtori, C. R., Paoletti, R. & Franceschini, G. Recombinant apolipoprotein A-IMilano for the treatment of cardiovascular diseases. Curr. Atheroscler. Rep. 8, 163–167 (2006).
Nissen, S. E. et al. Effect of recombinant apoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA 290, 2292–2300 (2003).
Navab, M. et al. Structure and function of HDL mimetics. Arterioscler. Thromb. Vasc. Biol. 30, 164–168 (2010).
Navab, M. et al. D-4F and statins synergize to render HDL antiinflammatory in mice and monkeys and cause lesion regression in old apolipoprotein E-null mice. Arterioscler. Thromb. Vasc. Biol. 25, 1426–1432 (2005).
Navab, M. et al. Oral D-4F causes formation of pre-beta high-density lipoprotein and improves high-density lipoprotein-mediated cholesterol efflux and reverse cholesterol transport from macrophages in apolipoprotein E-null mice. Circulation 109, 3215–3220 (2004).
Li, X. et al. Differential effects of apolipoprotein A-I mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation 110, 1701–1705 (2004).
Shah, P. K. & Chyu, K. Y. Apolipoprotein A-I mimetic peptides: potential role in atherosclerosis management. Trends Cardiovasc. Med. 15, 291–296 (2005).
Ou, J. et al. L-4F, an apolipoprotein A-I mimetic, dramatically improves vasodilation in hypercholesterolemia and sickle cell disease. Circulation 107, 2337–2341 (2003).
Ou, J. et al. Effects of D-4F on vasodilation and vessel wall thickness in hypercholesterolemic LDL receptor-null and LDL receptor/apolipoprotein A-I double-knockout mice on Western diet. Circ. Res. 97, 1190–1197 (2005).
Navab, M., Anantharamaiah, G. M., Reddy, S. T. & Fogelman, A. M. Apolipoprotein A-I mimetic peptides and their role in atherosclerosis prevention. Nat. Clin. Pract. Cardiovasc. Med. 3, 540–547 (2006).
Van Lenten, B. J. et al. High-density lipoprotein loses its anti-inflammatory properties during acute influenza A infection. Circulation 103, 2283–2288 (2001).
Charles-Schoeman, C. et al. Treatment with an apolipoprotein A-1 mimetic peptide in combination with pravastatin inhibits collagen-induced arthritis. Clin. Immunol. 127, 234–244 (2008).
Navab, M., Anantharamaiah, G. M. & Fogelman, A. M. The effect of apolipoprotein mimetic peptides in inflammatory disorders other than atherosclerosis. Trends Cardiovasc. Med. 18, 61–66 (2008).
van der Steeg, W. A. et al. Role of CETP inhibitors in the treatment of dyslipidemia. Curr. Opin. Lipidol. 15, 631–636 (2004).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Kedi, X., Ming, Y., Yongping, W., Yi, Y. & Xiaoxiang, Z. Free cholesterol overloading induced smooth muscle cells death and activated both ER- and mitochondrial-dependent death pathway. Atherosclerosis 207, 123–130 (2009).
Tardif, J. C. et al. Effects of the acyl coenzyme A:cholesterol acyltransferase inhibitor avasimibe on human atherosclerotic lesions. Circulation 110, 3372–3377 (2004).
Vaziri, N. D. & Liang, K. Upregulation of acyl-coenzyme A:cholesterol acyltransferase (ACAT) in nephrotic syndrome. Kidney Int. 61, 1769–1775 (2002).
Vaziri, N. D. & Liang, K. Acyl-coenzymeA:cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, SRB-1 and low density lipoprotein receptor deficiencies in nephrotic syndrome. Circulation 110, 419–425 (2004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M. Navab and A. M. Fogelman declare an association with the following company: Bruin Pharmaceuticals. N. D. Vaziri declares no competing interests.
Rights and permissions
About this article
Cite this article
Vaziri, N., Navab, M. & Fogelman, A. HDL metabolism and activity in chronic kidney disease. Nat Rev Nephrol 6, 287–296 (2010). https://doi.org/10.1038/nrneph.2010.36
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.36
This article is cited by
-
Combinative predictive effect of left ventricular mass index, ratio of HDL and CRP for progression of chronic kidney disease in non-dialysis patient
International Urology and Nephrology (2023)
-
Monocyte to HDL ratio: a novel marker of resistant hypertension in CKD patients
International Urology and Nephrology (2022)
-
Association of proportion of the HDL-cholesterol subclasses HDL-2b and HDL-3 and macrovascular events among patients undergoing hemodialysis
Scientific Reports (2021)
-
There is a U shaped association between non high density lipoprotein cholesterol with overall and cardiovascular mortality in chronic kidney disease stage 3–5
Scientific Reports (2020)
-
Low HDL cholesterol as a predictor of chronic kidney disease progression: a cross-classification approach and matched cohort analysis
Heart and Vessels (2019)