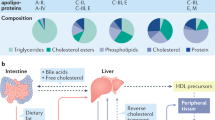
Abstract
Patients with chronic kidney disease (CKD) are at increased risk of atherosclerotic cardiovascular disease and loss of renal parenchyma accelerates atherosclerosis in animal models. Macrophages are central to atherogenesis because they regulate cholesterol traffic and inflammation in the arterial wall. CKD influences macrophage behavior at multiple levels, rendering them proatherogenic. Even at normal creatinine levels, macrophages from uninephrectomized Apoe−/− mice are enriched in cholesterol owing to downregulation of cholesterol transporter ATP-binding cassette subfamily A member 1 levels and activation of nuclear factor κB, which leads to impaired cholesterol efflux. Interestingly, treatment with an angiotensin-II-receptor blocker (ARB) improves these effects. Moreover, atherosclerotic aortas from Apoe−/− mice transplanted into renal-ablated normocholesterolemic recipients show plaque progression and increased macrophage content instead of the substantial regression seen in recipient mice with intact kidneys. ARBs reduce atherosclerosis development in mice with partial renal ablation. These results, combined with the clinical benefits of angiotensin-converting-enzyme (ACE) inhibitors and ARBs in patients with CKD, suggest an important role for the angiotensin system in the enhanced susceptibility to atherosclerosis seen across the spectrum of CKD. The role of macrophages could explain why these therapies may be effective in end-stage renal disease, one of the few conditions in which statins show no clinical benefit.
Key Points
-
Even subtle chronic kidney disease (CKD) increases the risk of cardiovascular events in humans and worsens atherosclerosis in experimental models
-
CKD-associated inflammatory and oxidant stress promotes the infiltration and trapping of circulating monocytes in the arterial intima
-
CKD promotes the formation of foam cells by downregulating cholesterol efflux through activation of nuclear factor κB and repression of cholesterol transporter ATP-binding cassette subfamily A member 1
-
CKD modifies lipoproteins and makes them more susceptible to scavenging by macrophages and less capable of acting as acceptors of cellular cholesterol
-
Systemic and macrophage angiotensin II actions contribute to the enhanced susceptibility of patients with CKD to atherosclerosis
-
Treatment with antagonists and inhibitors of angiotensin II might be of particular value in the prevention of cardiovascular disease in patients with CKD
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Foley, R. N., Parfrey, P. S. & Sarnak, M. J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 32 (Suppl.), S112–S119 (1998).
Mann, J. F., Gerstein, H. C., Pogue, J., Bosch, J. & Yusuf, S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann. Intern. Med. 134, 629–636 (2001).
Muntner, P., He, J., Hamm, L., Loria, C. & Whelton, P. K. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J. Am. Soc. Nephrol. 13, 745–753 (2002).
Go, A. S., Chertow, G. M., Fan, D., McCulloch, C. E. & Hsu, C. Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 (2004).
Hillege, H. L. et al. Urinary albumin excretion predicts cardiovascular and noncardiovascular mortality in general population. Circulation 106, 1777–1782 (2002).
Glass, C. K. & Witztum, J. L. Atherosclerosis: the road ahead. Cell 104, 503–516 (2001).
Huo, Y. & Ley, K. Adhesion molecules and atherogenesis. Acta Physiol. Scand. 173, 35–43 (2001).
Peters, W. & Charo, I. F. Involvement of chemokine receptor 2 and its ligand, monocyte chemoattractant protein-1, in the development of atherosclerosis: lessons from knockout mice. Curr. Opin. Lipidol. 12, 175–180 (2001).
Smith, J. D. et al. Decreased atherosclerosis in mice deficient in both macrophage colony-stimulating factor (op) and apolipoprotein E. Proc. Natl Acad. Sci. USA 92, 8264–8268 (1995).
Michelsen, K. S. et al. Lack of Toll-like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein E. Proc. Natl Acad. Sci. USA 101, 10679–10684 (2004).
Mach, F. et al. Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40–CD40 ligand signaling in atherosclerosis. Proc. Natl Acad. Sci. USA 94, 1931–1936 (1997).
Gupta, S. et al. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J. Clin. Invest. 99, 2752–2761 (1997).
Pinderski, L. J. et al. Overexpression of interleukin-10 by activated T lymphocytes inhibits atherosclerosis in LDL receptor-deficient mice by altering lymphocyte and macrophage phenotypes. Circ. Res. 90, 1064–1071 (2002).
Endemann, G. et al. CD36 is a receptor for oxidized low density lipoprotein. J. Biol. Chem. 268, 11811–11816 (1993).
Kodama, T. et al. Type I macrophage scavenger receptor contains α-helical and collagen-like coiled coils. Nature 343, 531–535 (1990).
Babaev, V. R. et al. Reduced atherosclerotic lesions in mice deficient for total or macrophage-specific expression of scavenger receptor-A. Arterioscler. Thromb. Vasc. Biol. 20, 2593–2599 (2000).
Overton, C. D., Yancey, P. G., Major, A. S., Linton, M. F. & Fazio, S. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ. Res. 100, 670–677 (2007).
Yancey, P. G. et al. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 23, 712–719 (2003).
Cuchel, M. & Rader, D. J. Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation 113, 2548–2555 (2006).
Yancey, P. G., Yu, H., Linton, M. F. & Fazio, S. A pathway-dependent on ApoE, ApoAI, and ABCA1 determines formation of buoyant high-density lipoprotein by macrophage foam cells. Arterioscler. Thromb. Vasc. Biol. 27, 1123–1131 (2007).
Linton, M. F., Atkinson, J. B. & Fazio, S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science 267, 1034–1037 (1995).
Fazio, S. et al. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc. Natl Acad. Sci. USA 94, 4647–4652 (1997).
Trogan, E. et al. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc. Natl Acad. Sci. USA 103, 3781–3786 (2006).
Tabas, I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler. Thromb. Vasc. Biol. 25, 2255–2264 (2005).
Erbay, E. et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 15, 1383–1391 (2009).
Babaev, V. R. et al. Macrophage EP4 deficiency increases apoptosis and suppresses early atherosclerosis. Cell. Metab. 8, 492–501 (2008).
Tabas, I. Macrophage death and defective inflammation resolution in atherosclerosis. Nat. Rev. Immunol. 10, 36–46 (2010).
Yancey, P. G. et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler. Thromb. Vasc. Biol. 30, 787–795 (2010).
Amann, K. Media calcification and intima calcification are distinct entities in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 3, 1599–1605 (2008).
Miyagi, M. et al. Impact of renal function on coronary plaque composition. Nephrol. Dial. Transplant. 25, 175–181 (2010).
Nicholls, S. J. et al. Comparison of coronary atherosclerotic volume in patients with glomerular filtration rates ≤60 versus >60 ml/min/1.73 m2: a meta-analysis of intravascular ultrasound studies. Am. J. Cardiol. 99, 813–816 (2007).
Rigatto, C. et al. Atheroma progression in chronic kidney disease. Clin. J. Am. Soc. Nephrol. 4, 291–298 (2009).
Bro, S. et al. Chronic renal failure accelerates atherogenesis in apolipoprotein E-deficient mice. J. Am. Soc. Nephrol. 14, 2466–2474 (2003).
Bro, S., Moeller, F., Andersen, C. B., Olgaard, K. & Nielsen, L. B. Increased expression of adhesion molecules in uremic atherosclerosis in apolipoprotein-E-deficient mice. J. Am. Soc. Nephrol. 15, 1495–1503 (2004).
Suganuma, E. et al. Antiatherogenic effects of angiotensin receptor antagonism in mild renal dysfunction. J. Am. Soc. Nephrol. 17, 433–441 (2006).
Bro, S., Binder, C. J., Witztum, J. L., Olgaard, K. & Nielsen, L. B. Inhibition of the renin–angiotensin system abolishes the proatherogenic effect of uremia in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 27, 1080–1086 (2007).
Buzello, M. et al. The apolipoprotein E knockout mouse: a model documenting accelerated atherogenesis in uremia. J. Am. Soc. Nephrol. 14, 311–316 (2003).
Massy, Z. A. et al. Uremia accelerates both atherosclerosis and arterial calcification in apolipoprotein E knockout mice. J. Am. Soc. Nephrol. 16, 109–116 (2005).
Carrero, J. J., Yilmaz, M. I., Lindholm, B. & Stenvinkel, P. Cytokine dysregulation in chronic kidney disease: how can we treat it? Blood Purif. 26, 291–299 (2008).
Honda, H. et al. Serum albumin, C-reactive protein, interleukin 6, and fetuin a as predictors of malnutrition, cardiovascular disease, and mortality in patients with ESRD. Am. J. Kidney Dis. 47, 139–148 (2006).
Pachaly, M. A. et al. Interleukin-6 is a better predictor of mortality as compared to C-reactive protein, homocysteine, pentosidine and advanced oxidation protein products in hemodialysis patients. Blood Purif. 26, 204–210 (2008).
Stinghen, A. E. et al. Increased plasma and endothelial cell expression of chemokines and adhesion molecules in chronic kidney disease. Nephron Clin. Pract. 111, c117–c126 (2009).
Suliman, M. E., Qureshi, A. R., Heimbürger, O., Lindholm, B. & Stenvinkel, P. Soluble adhesion molecules in end-stage renal disease: a predictor of outcome. Nephrol. Dial. Transplant. 21, 1603–1610 (2006).
Serradell, M. et al. Uremic medium causes expression, redistribution and shedding of adhesion molecules in cultured endothelial cells. Haematologica 87, 1053–1061 (2002).
Campean, V. et al. CD40–CD154 expression in calcified and non-calcified coronary lesions of patients with chronic renal failure. Atherosclerosis 190, 156–166 (2007).
Sela, S. et al. Primed peripheral polymorphonuclear leukocyte: a culprit underlying chronic low-grade inflammation and systemic oxidative stress in chronic kidney disease. J. Am. Soc. Nephrol. 16, 2431–2438 (2005).
Yoon, J. W., Pahl, M. V. & Vaziri, N. D. Spontaneous leukocyte activation and oxygen-free radical generation in end-stage renal disease. Kidney Int. 71, 167–172 (2007).
Pahl, M. V., Vaziri, N. D., Yuan, J. & Adler, S. G. Upregulation of monocyte/macrophage HGFIN (Gpnmb/Osteoactivin) expression in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 5, 56–61 (2010).
Himmelfarb, J., Stenvinkel, P., Ikizler, T. A. & Hakim, R. M. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 62, 1524–1538 (2002).
Kaysen, G. A. & Eiserich, J. P. The role of oxidative stress-altered lipoprotein structure and function and microinflammation on cardiovascular risk in patients with minor renal dysfunction. J. Am. Soc. Nephrol. 15, 538–548 (2004).
Oberg, B. P. et al. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 65, 1009–1016 (2004).
Pupim, L. B., Himmelfarb, J., McMonagle, E., Shyr, Y. & Ikizler, T. A. Influence of initiation of maintenance hemodialysis on biomarkers of inflammation and oxidative stress. Kidney Int. 65, 2371–2379 (2004).
D'Apolito, M. et al. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Invest. 120, 203–213 (2010).
Touyz, R. M., Yao, G., Quinn, M. T., Pagano, P. J. & Schiffrin, E. L. p47phox associates with the cytoskeleton through cortactin in human vascular smooth muscle cells: role in NAD(P)H oxidase regulation by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 25, 512–518 (2005).
Wang, Z. et al. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 13, 1176–1184 (2007).
Düzgünçinar, O. et al. Plasma myeloperoxidase is related to the severity of coronary artery disease. Acta Cardiol. 63, 147–152 (2008).
Meuwese, M. C. et al. Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC–Norfolk Prospective Population Study. J. Am. Coll. Cardiol. 50, 159–165 (2007).
Grahl, D. A. et al. Associations between the CYBA 242C/T and the MPO −463G/A polymorphisms, oxidative stress and cardiovascular disease in chronic kidney disease patients. Blood Purif. 25, 210–218 (2007).
Pecoits-Filho, R. et al. A functional variant of the myeloperoxidase gene is associated with cardiovascular disease in end-stage renal disease patients. Kidney Int. Suppl. 84, S172–S176 (2003).
Bro, S. Cardiovascular effects of uremia in apolipoprotein E-deficient mice. Dan. Med. Bull. 56, 177–192 (2009).
Ivanovski, O. et al. The antioxidant N-acetylcysteine prevents accelerated atherosclerosis in uremic apolipoprotein E knockout mice. Kidney Int. 67, 2288–2294 (2005).
Boaz, M. et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): randomised placebo-controlled trial. Lancet 356, 1213–1218 (2000).
Tepel, M., van der Giet, M., Statz, M., Jankowski, J. & Zidek, W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: a randomized, controlled trial. Circulation 107, 992–995 (2003).
Himmelfarb, J. et al. Gamma-tocopherol and docosahexaenoic acid decrease inflammation in dialysis patients. J. Ren. Nutr. 17, 296–304 (2007).
Nanayakkara, P. W. et al. Randomized placebo-controlled trial assessing a treatment strategy consisting of pravastatin, vitamin E, and homocysteine lowering on plasma asymmetric dimethylarginine concentration in mild to moderate CKD. Am. J. Kidney Dis. 53, 41–50 (2009).
Mafra, D. et al. Alpha-tocopherol supplementation decreases electronegative low-density lipoprotein concentration [LDL(-)] in haemodialysis patients. Nephrol. Dial. Transplant. 24, 1587–1592 (2009).
Lee, I. M. et al. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA 294, 56–65 (2005).
Sesso, H. D. et al. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA 300, 2123–2133 (2008).
Llodrá, J. et al. Emigration of monocyte-derived cells from atherosclerotic lesions characterizes regressive, but not progressive, plaques. Proc. Natl Acad. Sci. USA 101, 11779–11784 (2004).
Williams, K. J., Feig, J. E. & Fisher, E. A. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat. Clin. Pract. Cardiovasc. Med. 5, 91–102 (2008).
Trogan, E. et al. Serial studies of mouse atherosclerosis by in vivo magnetic resonance imaging detect lesion regression after correction of dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 24, 1714–1719 (2004).
Ponda, M. P., Barash, I., Feig, J. E., Fisher, E. A. & Skolnik, E. Y. Moderate kidney disease inhibits atherosclerosis regression. Atherosclerosis 210, 57–62 (2010).
Osada, S. et al. Alterations in proteoglycan components and histopathology of the peritoneum in uraemic and peritoneal dialysis (PD) patients. Nephrol. Dial. Transplant. 24, 3504–3512 (2009).
Yamamoto, Y. et al. Possible involvement of increased glycoxidation and lipid peroxidation of elastin in atherogenesis in haemodialysis patients. Nephrol. Dial. Transplant. 17, 630–636 (2002).
Nagao, T., Qin, C., Grosheva, I., Maxfield, F. R. & Pierini, L. M. Elevated cholesterol levels in the plasma membranes of macrophages inhibit migration by disrupting RhoA regulation. Arterioscler. Thromb. Vasc. Biol. 27, 1596–1602 (2007).
Zerbinatti, C. V. & Gore, R. W. Uptake of modified low-density lipoproteins alters actin distribution and locomotor forces in macrophages. Am. J. Physiol. Cell. Physiol. 284, C555–C561 (2003).
Chmielewski, M. et al. Expression of scavenger receptor CD36 in chronic renal failure patients. Artif. Organs 29, 608–614 (2005).
Wu, C. C. et al. Aberrant activation of the TNF-α system and production of Fas and scavenger receptors on monocytes in patients with end-stage renal disease. Artif. Organs 29, 701–707 (2005).
Park, Y. M., Febbraio, M. & Silverstein, R. L. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J. Clin. Invest. 119, 136–145 (2009).
Harb, D. et al. The role of the scavenger receptor CD36 in regulating mononuclear phagocyte trafficking to atherosclerotic lesions and vascular inflammation. Cardiovasc. Res. 83, 42–51 (2009).
Kuchibhotla, S. et al. Absence of CD36 protects against atherosclerosis in ApoE knock-out mice with no additional protection provided by absence of scavenger receptor A I/II. Cardiovasc. Res. 78, 185–196 (2008).
Marleau, S. et al. EP 80317, a ligand of the CD36 scavenger receptor, protects apolipoprotein E-deficient mice from developing atherosclerotic lesions. FASEB J. 19, 1869–1871 (2005).
Ando, M., Lundkvist, I., Bergström, J. & Lindholm, B. Enhanced scavenger receptor expression in monocyte-macrophages in dialysis patients. Kidney Int. 49, 773–780 (1996).
Kim, H. J., Moradi, H., Yuan, J., Norris, K. & Vaziri, N. D. Renal mass reduction results in accumulation of lipids and dysregulation of lipid regulatory proteins in the remnant kidney. Am. J. Physiol. Renal Physiol. 296, F1297–F1306 (2009).
Moradi, H., Yuan, J., Ni, Z., Norris, K. & Vaziri, N. D. Reverse cholesterol transport pathway in experimental chronic renal failure. Am. J. Nephrol. 30, 147–154 (2009).
Zager, R. A., Johnson, A. C., Hanson, S. Y. & Shah, V. O. Acute tubular injury causes dysregulation of cellular cholesterol transport proteins. Am. J. Pathol. 163, 313–320 (2003).
Ando, M., Gafvels, M., Bergström, J., Lindholm, B. & Lundkvist, I. Uremic serum enhances scavenger receptor expression and activity in the human monocytic cell line U937. Kidney Int. 51, 785–792 (1997).
Konishi, Y. et al. Enhanced gene expression of scavenger receptor in peripheral blood monocytes from patients on cuprophane haemodialysis. Nephrol. Dial. Transplant. 12, 1167–1172 (1997).
Wang, X. & Rader, D. J. Molecular regulation of macrophage reverse cholesterol transport. Curr. Opin. Cardiol. 22, 368–372 (2007).
Yvan-Charvet, L., Wang, N. & Tall, A. R. Role of HDL, ABCA1, and ABCG1 transporters in cholesterol efflux and immune responses. Arterioscler. Thromb. Vasc. Biol. 30, 139–143 (2010).
Zuo, Y. et al. Renal dysfunction potentiates foam cell formation by repressing ABCA1. Arterioscler. Thromb. Vasc. Biol. 29, 1277–1282 (2009).
Cardinal, H., Raymond, M. A., Hebert, M. J. & Madore, F. Uraemic plasma decreases the expression of ABCA1, ABCG1 and cell-cycle genes in human coronary arterial endothelial cells. Nephrol. Dial. Transplant. 22, 409–416 (2007).
Baranova, I. et al. Lipopolysaccharide down regulates both scavenger receptor B1 and ATP binding cassette transporter A1 in RAW cells. Infect. Immun. 70, 2995–3003 (2002).
Chen, M., Li, W., Wang, N., Zhu, Y. & Wang, X. ROS and NF-κB but not LXR mediate IL-1β signaling for the downregulation of ATP-binding cassette transporter A1. Am. J. Physiol. Cell. Physiol. 292, C1493–C1501 (2007).
Ferreira, V. et al. Macrophage-specific inhibition of NF-κB activation reduces foam-cell formation. Atherosclerosis 192, 283–290 (2007).
Tang, C., Kanter, J. E., Bornfeldt, K. E., Leboeuf, R. C. & Oram, J. F. Diabetes reduces the cholesterol exporter ABCA1 in mouse macrophages and kidneys. J. Lipid Res. 51, 1719–1728 (2010).
Wang, Y., Kurdi-Haidar, B. & Oram, J. F. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J. Lipid Res. 45, 972–980 (2004).
Bohlender, J. M., Franke, S., Stein, G. & Wolf, G. Advanced glycation end products and the kidney. Am. J. Physiol. Renal Physiol. 289, F645–F659 (2005).
Pedersen, T. X. et al. Effect of treatment with human apolipoprotein A-I on atherosclerosis in uremic apolipoprotein-E deficient mice. Atherosclerosis 202, 372–381 (2009).
Francone, O. L. et al. Increased cholesterol deposition, expression of scavenger receptors, and response to chemotactic factors in Abca1-deficient macrophages. Arterioscler. Thromb. Vasc. Biol. 25, 1198–1205 (2005).
Zhu, X. et al. Increased cellular free cholesterol in macrophage-specific Abca1 knock-out mice enhances pro-inflammatory response of macrophages. J. Biol. Chem. 283, 22930–22941 (2008).
Gollapudi, P., Yoon, J. W., Gollapudi, S., Pahl, M. V. & Vaziri, N. D. Leukocyte toll-like receptor expression in end-stage kidney disease. Am. J. Nephrol. 31, 247–254 (2010).
Kuroki, Y. et al. A study of innate immunity in patients with end-stage renal disease: special reference to toll-like receptor-2 and -4 expression in peripheral blood monocytes of hemodialysis patients. Int. J. Mol. Med. 19, 783–790 (2007).
Tsimikas, S. et al. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 353, 46–57 (2005).
Liu, J. & Rosner, M. H. Lipid abnormalities associated with end-stage renal disease. Semin. Dial. 19, 32–40 (2006).
Podrez, E. A. et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Invest. 105, 1095–1108 (2000).
Hörkkö, S., Huttunen, K., Kervinen, K. & Kesäniemi, Y. A. Decreased clearance of uraemic and mildly carbamylated low-density lipoprotein. Eur. J. Clin. Invest. 24, 105–113 (1994).
Fellström, B. C. et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 360, 1395–1407 (2009).
Wanner, C. et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 353, 238–248 (2005).
Tsimihodimos, V., Dounousi, E. & Siamopoulos, K. C. Dyslipidemia in chronic kidney disease: an approach to pathogenesis and treatment. Am. J. Nephrol. 28, 958–973 (2008).
Mekki, K., Bouchenak, M., Remaoun, M. & Belleville, J. L. Effect of long-term hemodialysis on plasma lecithin: cholesterol acyltransferase activity and the amounts and compositions of HDL2 and HDL3 in hemodialysis-treated patients with chronic renal failure: a 9-year longitudinal study. Med. Sci. Monit. 10, CR439–CR446 (2004).
Moradi, H., Pahl, M. V., Elahimehr, R. & Vaziri, N. D. Impaired antioxidant activity of high-density lipoprotein in chronic kidney disease. Transl. Res. 153, 77–85 (2009).
Gugliucci, A. et al. Acrolein inactivates paraoxonase 1: changes in free acrolein levels after hemodialysis correlate with increases in paraoxonase 1 activity in chronic renal failure patients. Clin. Chim. Acta 384, 105–112 (2007).
Gugliucci, A. et al. Paraoxonase-1 concentrations in end-stage renal disease patients increase after hemodialysis: correlation with low molecular AGE adduct clearance. Clin. Chim. Acta 377, 213–220 (2007).
Jurek, A., Turyna, B., Kubit, P. & Klein, A. LDL susceptibility to oxidation and HDL antioxidant capacity in patients with renal failure. Clin. Biochem. 39, 19–27 (2006).
Kalantar-Zadeh, K., Kopple, J. D., Kamranpour, N., Fogelman, A. M. & Navab, M. HDL-inflammatory index correlates with poor outcome in hemodialysis patients. Kidney Int. 72, 1149–1156 (2007).
Kronenberg, F., Ikewaki, K., Schaefer, J. R., Konig, P. & Dieplinger, H. Kinetic studies of atherogenic lipoproteins in hemodialysis patients: do they tell us more about their pathology? Semin. Dial. 20, 554–560 (2007).
Vaziri, N. D., Moradi, H., Pahl, M. V., Fogelman, A. M. & Navab, M. In vitro stimulation of HDL anti-inflammatory activity and inhibition of LDL pro-inflammatory activity in the plasma of patients with end-stage renal disease by an apoA-1 mimetic peptide. Kidney Int. 76, 437–444 (2009).
Wong, J., Quinn, C. M., Gelissen, I. C., Jessup, W. & Brown, A. J. The effect of statins on ABCA1 and ABCG1 expression in human macrophages is influenced by cellular cholesterol levels and extent of differentiation. Atherosclerosis 196, 180–189 (2008).
Shoji, T. et al. Advanced atherosclerosis in predialysis patients with chronic renal failure. Kidney Int. 61, 2187–2192 (2002).
Sone, H. et al. Statins downregulate ATP-binding-cassette transporter A1 gene expression in macrophages. Biochem. Biophys. Res. Commun. 316, 790–794 (2004).
Tamehiro, N. et al. Sterol regulatory element-binding protein-2- and liver X receptor-driven dual promoter regulation of hepatic ABC transporter A1 gene expression: mechanism underlying the unique response to cellular cholesterol status. J. Biol. Chem. 282, 21090–21099 (2007).
Argmann, C. A. et al. Regulation of macrophage cholesterol efflux through hydroxymethylglutaryl-CoA reductase inhibition: a role for RhoA in ABCA1-mediated cholesterol efflux. J. Biol. Chem. 280, 22212–22221 (2005).
Becker, L. et al. A macrophage sterol-responsive network linked to atherogenesis. Cell. Metab. 11, 125–135 (2010).
Qiu, G. & Hill, J. S. Atorvastatin inhibits ABCA1 expression and cholesterol efflux in THP-1 macrophages by an LXR-dependent pathway. J. Cardiovasc. Pharmacol. 51, 388–395 (2008).
Lewis, E. J., Hunsicker, L. G., Bain, R. P. & Rohde, R. D. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 329, 1456–1462 (1993).
Maschio, G. et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. The Angiotensin-Converting-Enzyme Inhibition in Progressive Renal Insufficiency Study Group. N. Engl. J. Med. 334, 939–945 (1996).
Baker, W. L. et al. Systematic review: comparative effectiveness of angiotensin-converting enzyme inhibitors or angiotensin II-receptor blockers for ischemic heart disease. Ann. Intern. Med. 151, 861–871 (2009).
Yusuf, S. et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N. Engl. J. Med. 342, 145–153 (2000).
Ayabe, N. et al. Transiently heightened angiotensin II has distinct effects on atherosclerosis and aneurysm formation in hyperlipidemic mice. Atherosclerosis 184, 312–321 (2006).
da Cunha, V. et al. Angiotensin II induces histomorphologic features of unstable plaque in a murine model of accelerated atherosclerosis. J. Vasc. Surg. 44, 364–371 (2006).
da Cunha, V. et al. Enalapril attenuates angiotensin II-induced atherosclerosis and vascular inflammation. Atherosclerosis 178, 9–17 (2005).
Daugherty, A., Manning, M. W. & Cassis, L. A. Angiotensin II promotes atherosclerotic lesions and aneurysms in apolipoprotein E-deficient mice. J. Clin. Invest. 105, 1605–1612 (2000).
Sasaki, T. et al. AT1 blockade attenuates atherosclerotic plaque destabilization accompanied by the suppression of cathepsin S activity in apoE-deficient mice. Atherosclerosis 210, 430–437 (2010).
Xu, H. X. et al. Decreased infiltration of macrophages and inhibited activation of nuclear factor-kappa B in blood vessels: a possible mechanism for the anti-atherogenic effects of losartan. Acta Cardiol. 62, 607–613 (2007).
Nobuhiko, A. et al. Angiotensin II amplifies macrophage-driven atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 24, 2143–2148 (2004).
Blessing, E. et al. Anti-atherosclerotic properties of telmisartan in advanced atherosclerotic lesions in apolipoprotein E deficient mice. Atherosclerosis 199, 295–303 (2008).
Takahashi, M. et al. Angiotensin II and tumor necrosis factor-α synergistically promote monocyte chemoattractant protein-1 expression: roles of NF-κB, p38, and reactive oxygen species. Am. J. Physiol. Heart Circ. Physiol. 294, H2879–H2888 (2008).
Daugherty, A., Rateri, D. L., Lu, H., Inagami, T. & Cassis, L. A. Hypercholesterolemia stimulates angiotensin peptide synthesis and contributes to atherosclerosis through the AT1A receptor. Circulation 110, 3849–3857 (2004).
Okamura, A. et al. Upregulation of renin–angiotensin system during differentiation of monocytes to macrophages. J. Hypertens. 17, 537–545 (1999).
Sales, V. L. et al. Angiotensin type 2 receptor is expressed in murine atherosclerotic lesions and modulates lesion evolution. Circulation 112, 3328–3336 (2005).
Montecucco, F., Pende, A. & Mach, F. The renin–angiotensin system modulates inflammatory processes in atherosclerosis: evidence from basic research and clinical studies. Mediators Inflamm. doi:10.1155/2009/752406.
AbdAlla, S., Lother, H., Langer, A., el Faramawy, Y. & Quitterer, U. Factor XIIIA transglutaminase crosslinks AT1 receptor dimers of monocytes at the onset of atherosclerosis. Cell 119, 343–354 (2004).
Dörffel, Y. et al. Preactivated monocytes from hypertensive patients as a factor for atherosclerosis? Atherosclerosis 157, 151–160 (2001).
Cassis, L. A., Rateri, D. L., Lu, H. & Daugherty, A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler. Thromb. Vasc. Biol. 27, 380–386 (2007).
Fukuda, D., Sata, M., Ishizaka, N. & Nagai, R. Critical role of bone marrow angiotensin II type 1 receptor in the pathogenesis of atherosclerosis in apolipoprotein E deficient mice. Arterioscler. Thromb. Vasc. Biol. 28, 90–96 (2008).
Lu, H. et al. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J. Clin. Invest. 118, 984–993 (2008).
Tsubakimoto, Y. et al. Bone marrow angiotensin AT1 receptor regulates differentiation of monocyte lineage progenitors from hematopoietic stem cells. Arterioscler. Thromb. Vasc. Biol. 29, 1529–1536 (2009).
Ulrich, C., Heine, G. H., Seibert, E., Fliser, D. & Girndt, M. Circulating monocyte subpopulations with high expression of angiotensin-converting enzyme predict mortality in patients with end-stage renal disease. Nephrol. Dial. Transplant. 25, 2265–2272 (2010).
Nakaya, K. et al. Telmisartan enhances cholesterol efflux from THP-1 macrophages by activating PPARγ. J. Atheroscler. Thromb. 14, 133–141 (2007).
Takata, Y. et al. Transcriptional repression of ATP-binding cassette transporter A1 gene in macrophages: a novel atherosclerotic effect of angiotensin II. Circ. Res. 97, e88–e96 (2005).
Wang, Y. et al. Angiotensin II increases the cholesterol content of foam cells via down-regulating the expression of ATP-binding cassette transporter A1. Biochem. Biophys. Res. Commun. 353, 650–654 (2007).
Takaya, T. et al. Angiotensin II type 1 receptor blocker telmisartan suppresses superoxide production and reduces atherosclerotic lesion formation in apolipoprotein E-deficient mice. Atherosclerosis 186, 402–410 (2006).
Yoshiyama, M. et al. Angiotensin blockade inhibits increased JNKs, AP-1 and NF-κB DNA-binding activities in myocardial infarcted rats. J. Mol. Cell. Cardiol. 33, 799–810 (2001).
Calkin, A. C. et al. The HMG-CoA reductase inhibitor rosuvastatin and the angiotensin receptor antagonist candesartan attenuate atherosclerosis in an apolipoprotein E-deficient mouse model of diabetes via effects on advanced glycation, oxidative stress and inflammation. Diabetologia 51, 1731–1740 (2008).
Koh, K. K., Han, S. H., Oh, P. C., Shin, E. K. & Quon, M. J. Combination therapy for treatment or prevention of atherosclerosis: focus on the lipid–RAAS interaction. Atherosclerosis 209, 307–313 (2010).
Van Antwerpen, P. et al. Captopril inhibits the oxidative modification of apolipoprotein B-100 caused by myeloperoxydase in a comparative in vitro assay of angiotensin converting enzyme inhibitors. Eur. J. Pharmacol. 537, 31–36 (2006).
Kojima, C., Ino, J., Ishii, H., Nitta, K. & Yoshida, M. MMP-9 inhibition by ACE inhibitor reduces oxidized LDL-mediated foam-cell formation. J. Atheroscler. Thromb. 17, 97–105 (2010).
de Nigris, F. et al. Chronic treatment with sulfhydryl angiotensin-converting enzyme inhibitors reduce susceptibility of plasma LDL to in vitro oxidation, formation of oxidation-specific epitopes in the arterial wall, and atherogenesis in apolipoprotein E knockout mice. Int. J. Cardiol. 81, 107–115 (2001).
Tokmakova, M. P. et al. Chronic kidney disease, cardiovascular risk, and response to angiotensin-converting enzyme inhibition after myocardial infarction: the Survival And Ventricular Enlargement (SAVE) study. Circulation 110, 3667–3673 (2004).
Li, P. K., Chow, K. M., Wong, T. Y., Leung, C. B. & Szeto, C. C. Effects of an angiotensin-converting enzyme inhibitor on residual renal function in patients receiving peritoneal dialysis. A randomized, controlled study. Ann. Intern. Med. 139, 105–112 (2003).
Zannad, F. et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 70, 1318–1324 (2006).
Akbari, A. et al. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in peritoneal dialysis: systematic review and meta-analysis of randomized controlled trials. Perit. Dial. Int. 29, 554–561 (2009).
Balamuthusamy, S. et al. Renin angiotensin system blockade and cardiovascular outcomes in patients with chronic kidney disease and proteinuria: a meta-analysis. Am. Heart J. 155, 791–805 (2008).
Berger, A. K., Duval, S. & Krumholz, H. M. Aspirin, beta-blocker, and angiotensin-converting enzyme inhibitor therapy in patients with end-stage renal disease and an acute myocardial infarction. J. Am. Coll. Cardiol. 42, 201–208 (2003).
Efrati, S. et al. ACE inhibitors and survival of hemodialysis patients. Am. J. Kidney Dis. 40, 1023–1029 (2002).
Fang, W., Oreopoulos, D. G. & Bargman, J. M. Use of ACE inhibitors or angiotensin receptor blockers and survival in patients on peritoneal dialysis. Nephrol. Dial. Transplant. 23, 3704–3710 (2008).
Hou, F. F. et al. Efficacy and safety of benazepril for advanced chronic renal insufficiency. N. Engl. J. Med. 354, 131–140 (2006).
McCullough, P. A., Sandberg, K. R., Yee, J. & Hudson, M. P. Mortality benefit of angiotensin-converting enzyme inhibitors after cardiac events in patients with end-stage renal disease. J. Renin Angiotensin Aldosterone Syst. 3, 188–191 (2002).
Suzuki, H. et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am. J. Kidney Dis. 52, 501–506 (2008).
Takahashi, A. et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis—a randomized study. Nephrol. Dial. Transplant. 21, 2507–2512 (2006).
Acknowledgements
The authors' work described in this Review was supported in part by grants from NIH HL087061 (V. Kon) and DK044757 (V. Kon and S. Fazio), HL086988 and HL065405 (M. F. Linton), HL65709 and HL57986 (S. Fazio), and the Lipid, Lipoprotein and Atherosclerosis Core of the Vanderbilt Mouse Metabolic Phenotyping Center (NIH DK59637-01).
Author information
Authors and Affiliations
Contributions
V. Kon, M. F. Linton and S. Fazio contributed equally to all aspects of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kon, V., Linton, M. & Fazio, S. Atherosclerosis in chronic kidney disease: the role of macrophages. Nat Rev Nephrol 7, 45–54 (2011). https://doi.org/10.1038/nrneph.2010.157
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2010.157
This article is cited by
-
Non-invasive chronic kidney disease risk stratification tool derived from retina-based deep learning and clinical factors
npj Digital Medicine (2023)
-
Atorvastatin treatment does not abolish inflammatory mediated cardiovascular risk in subjects with chronic kidney disease
Scientific Reports (2021)
-
A comprehensive review on apolipoproteins as nontraditional cardiovascular risk factors in end-stage renal disease: current evidence and perspectives
International Urology and Nephrology (2019)
-
Chronic kidney disease alters lipid trafficking and inflammatory responses in macrophages: effects of liver X receptor agonism
BMC Nephrology (2018)
-
Epigenetics in diabetic nephropathy, immunity and metabolism
Diabetologia (2018)