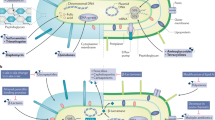
Abstract
Antibiotics are among the most frequently prescribed drugs in medicine. Their use, however, is often limited by associated renal toxic effects. The most common manifestation of these toxic effects is decreased glomerular filtration rate. However, they can also occur while renal function remains near to normal. This Review focuses on antibiotic-associated fluid, electrolyte and acid–base disorders that do not greatly reduce glomerular filtration. Renal tubules can be affected by antibiotics at various locations. In the proximal tubule, toxic effects of tetracyclines and aminoglycosides can result in complete proximal tubular dysfunction, also known as Fanconi syndrome. Aminoglycosides (and capreomycin) can also affect the loop of Henle and lead to a Bartter-like syndrome. In the collecting ducts, antibiotics can cause a diverse range of disorders, including hyponatremia, hypokalemia, hyperkalemia, renal tubular acidosis, and nephrogenic diabetes insipidus. Causative antibiotics include trimethoprim, amphotericin B, penicillins, ciprofloxacin, demeclocycline and various antitubercular agents. Here, we describe the mechanisms that disrupt renal tubular function. Integrated with the physiology of each successive nephron segment, we discuss the receptors, transporters, channels or pores that are affected by antibiotics. This insight should pave the way for pathophysiology-directed treatment of these disorders.
Key Points
-
Renal tubular function can be affected by antibiotic treatment without a concurrent reduction in glomerular filtration rate
-
Hypokalemia is a frequent complication of antimicrobial therapy
-
Treatment with aminoglycosides can affect renal tubular function in several ways and can lead to hypokalemia, as well as acidosis and alkalosis
-
If unexpected disturbances in electrolyte and/or acid–base balance occur in a patient, their prescribed medications should be carefully checked
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rougier, F. et al. Aminoglycoside nephrotoxicity. Curr. Drug Targets Infect. Disord. 4, 153–162 (2004).
Gonzalez, E. et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int. 73, 940–946 (2008).
Martinez-Salgado, C., Lopez-Hernandez, F. J. & Lopez-Novoa, J. M. Glomerular nephrotoxicity of aminoglycosides. Toxicol. Appl. Pharmacol. 223, 86–98 (2007).
Rodriguez-Barbero, A., Lopez-Novoa, J. M. & Arevalo, M. Involvement of platelet-activating factor in gentamicin nephrotoxicity in rats. Exp. Nephrol. 5, 47–54 (1997).
Brown, R. S. Potassium homeostasis and clinical implications. Am. J. Med. 77, 3–10 (1984).
Izzedine, H., Launay-Vacher, V. & Deray, G. Antiviral drug-induced nephrotoxicity. Am. J. Kidney Dis. 45, 804–817 (2005).
Knepper, M. A. & Brooks, H. L. Regulation of the sodium transporters NHE3, NKCC2 and NCC in the kidney. Curr. Opin. Nephrol. Hypertens. 10, 655–659 (2001).
Boron, W. F. Acid–base transport by the renal proximal tubule. J. Am. Soc. Nephrol. 17, 2368–2382 (2006).
Igarashi, T. et al. Unraveling the molecular pathogenesis of isolated proximal renal tubular acidosis. J. Am. Soc. Nephrol. 13, 2171–2177 (2002).
Giebisch, G., Krapf, R. & Wagner, C. Renal and extrarenal regulation of potassium. Kidney Int. 72, 397–410 (2007).
Davies, J. & Davis, B. D. Misreading of ribonucleic acid code words induced by aminoglycoside antibiotics. The effect of drug concentration. J. Biol. Chem. 243, 3312–3316 (1968).
Schmitz, C. et al. Megalin deficiency offers protection from renal aminoglycoside accumulation. J. Biol. Chem. 277, 618–622 (2002).
Beauchamp, D., Gourde, P. & Bergeron, M. G. Subcellular distribution of gentamicin in proximal tubular cells, determined by immunogold labeling. Antimicrob. Agents Chemother. 35, 2173–2179 (1991).
Bennett, W. M. et al. Microsomal protein synthesis inhibition: an early manifestation of gentamicin nephrotoxicity. Am. J. Physiol. 255, F265–F269 (1988).
Weinberg, J. M., Harding, P. G. & Humes, H. D. Mechanisms of gentamicin-induced dysfunction of renal cortical mitochondria. II. Effects on mitochondrial monovalent cation transport. Arch. Biochem. Biophys. 205, 232–239 (1980).
Ramsammy, L. S., Josepovitz, C. & Kaloyanides, G. J. Gentamicin inhibits agonist stimulation of the phosphatidylinositol cascade in primary cultures of rabbit proximal tubular cells and in rat renal cortex. J. Pharmacol. Exp. Ther. 247, 989–996 (1988).
Powell, J. H. & Reidenberg, M. M. Further studies of the response of kidney lysosomes to aminoglycosides and other cations. Biochem. Pharmacol. 32, 3213–3220 (1983).
Sassen, M. C. et al. Dysregulation of renal sodium transporters in gentamicin-treated rats. Kidney Int. 70, 1026–1037 (2006).
Nix, D. E. et al. Assessment of the enzymuria resulting from gentamicin alone and combinations of gentamicin with various β-lactam antibiotics. Ann. Pharmacother. 31, 696–703 (1997).
Banday, A. A. et al. Time dependent effects of gentamicin on the enzymes of carbohydrate metabolism, brush border membrane and oxidative stress in rat kidney tissues. Life Sci. 82, 450–459 (2008).
Chou, C. L. et al. Acquired Bartter-like syndrome associated with gentamicin administration. Am. J. Med. Sci. 329, 144–149 (2005).
Riccardi, D. et al. Localization of the extracellular Ca2+/polyvalent cation-sensing protein in rat kidney. Am. J. Physiol. 274, F611–F622 (1998).
Ward, D. T., McLarnon, S. J. & Riccardi, D. Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular calcium-sensing receptor. J. Am. Soc. Nephrol. 13, 1481–1489 (2002).
Ward, D. T. et al. Aminoglycosides induce acute cell signaling and chronic cell death in renal cells that express the calcium-sensing receptor. J. Am. Soc. Nephrol. 16, 1236–1244 (2005).
Gibbons, C. E. et al. Calcium-sensing receptor antagonism or lithium treatment ameliorates aminoglycoside-induced cell death in renal epithelial cells. Biochim. Biophys. Acta 1782, 188–195 (2008).
Alexandridis, G., Liberopoulos, E. & Elisaf, M. Aminoglycoside-induced reversible tubular dysfunction. Pharmacology 67, 118–120 (2003).
Melnick, J. Z., Baum, M. & Thompson, J. R. Aminoglycoside-induced Fanconi's syndrome. Am. J. Kidney Dis. 23, 118–122 (1994).
Ghiculescu, R. A. & Kubler, P. A. Aminoglycoside-associated Fanconi syndrome. Am. J. Kidney Dis. 48, e89–e93 (2006).
Etherington, C. et al. Measurement of urinary N-acetyl-β-D-glucosaminidase in adult patients with cystic fibrosis: before, during and after treatment with intravenous antibiotics. J. Cyst. Fibros. 6, 67–73 (2007).
Watanabe, A. et al. Targeted prevention of renal accumulation and toxicity of gentamicin by aminoglycoside binding receptor antagonists. J. Control Release 95, 423–433 (2004).
Nagai, J. & Takano, M. Molecular aspects of renal handling of aminoglycosides and strategies for preventing the nephrotoxicity. Drug Metab. Pharmacokinet. 19, 159–170 (2004).
Hemstreet, B. A. Antimicrobial-associated renal tubular acidosis. Ann. Pharmacother. 38, 1031–1038 (2004).
Babu, E. et al. Human organic anion transporters mediate the transport of tetracycline. Jpn J. Pharmacol. 88, 69–76 (2002).
Montoliu, J. et al. Lactic acidosis and Fanconi's syndrome due to degraded tetracycline. Br. Med. J. (Clin. Res. Ed.) 283, 1576–1577 (1981).
Budkevich, T. V., El'skaya, A. V. & Nierhaus, K. H. Features of 80S mammalian ribosome and its subunits. Nucleic Acids Res. 36, 4736–4744 (2008).
Connell, S. R. et al. Ribosomal protection proteins and their mechanism of tetracycline resistance. Antimicrob. Agents Chemother. 47, 3675–3681 (2003).
Wirmer, J. & Westhof, E. Molecular contacts between antibiotics and the 30S ribosomal particle. Methods Enzymol. 415, 180–202 (2006).
Izzedine, H. et al. Drug-induced Fanconi's syndrome. Am. J. Kidney Dis. 41, 292–309 (2003).
Inselmann, G., Balaschke, M. & Heidemann, H. T. Enzymuria following amphotericin B application in the rat. Mycoses 46, 169–173 (2003).
Hebert, S. C: Bartter syndrome. Curr. Opin. Nephrol. Hypertens. 12, 527–532 (2003).
Shiah, C. J. et al. Acute muscular paralysis in an adult with subclinical Bartter's syndrome associated with gentamicin administration. Am. J. Kidney Dis. 24, 932–935 (1994).
Landau, D. & Kher, K. K. Gentamicin-induced Bartter-like syndrome. Pediatr. Nephrol. 11, 737–740 (1997).
Pollak, M. R. et al. Autosomal dominant hypocalcaemia caused by a Ca2+-sensing receptor gene mutation. Nat. Genet. 8, 303–307 (1994).
Shin, S. et al. Hypokalemia among patients receiving treatment for multidrug-resistant tuberculosis. Chest 125, 974–980 (2004).
Steiner, R. W. & Omachi, A. S. A Bartter's-like syndrome from capreomycin, and a similar gentamicin tubulopathy. Am. J. Kidney Dis. 7, 245–249 (1986).
Ecelbarger, C. A. & Tiwari, S. Sodium transporters in the distal nephron and disease implications. Curr. Hypertens. Rep. 8, 158–165 (2006).
Alexander, R. T., Hoenderop, J. G. & Bindels, R. J. Molecular determinants of magnesium homeostasis: insights from human disease. J. Am. Soc. Nephrol. 19, 1451–1458 (2008).
Nijenhuis, T. et al. Enhanced passive Ca2+ reabsorption and reduced Mg2+ channel abundance explains thiazide-induced hypocalciuria and hypomagnesemia. J. Clin. Invest. 115, 1651–1658 (2005).
Finton, C. K. et al. Gentamicin-induced hypomagnesemia. Am. Surg. 49, 576–578 (1983).
Elliott, C., Newman, N. & Madan, A. Gentamicin effects on urinary electrolyte excretion in healthy subjects. Clin. Pharmacol. Ther. 67, 16–21 (2000).
Kang, H. S. et al. Aminoglycosides inhibit hormone-stimulated Mg2+ uptake in mouse distal convoluted tubule cells. Can. J. Physiol. Pharmacol. 78, 595–602 (2000).
Fenton, R. A. & Knepper, M. A. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol. Rev. 87, 1083–1112 (2007).
Kamel, K. S. et al. A new classification for renal defects in net acid excretion. Am. J. Kidney Dis. 29, 136–146 (1997).
Mori, H. et al. Hyponatremia and/or hyperkalemia in patients treated with the standard dose of trimethoprim–sulfamethoxazole. Intern. Med. 42, 665–669 (2003).
Perazella, M. A. Trimethoprim is a potassium-sparing diuretic like amiloride and causes hyperkalemia in high-risk patients. Am. J. Ther. 4, 343–348 (1997).
Ahn, Y. H. & Goldman, J. M. Trimethoprim–sulfamethoxazole and hyponatremia. Ann. Intern. Med. 103, 161–162 (1985).
Muto, S. et al. Effect of trimethoprim–sulfamethoxazole on Na+ and K+ transport properties in the rabbit cortical collecting duct perfused in vitro. Nephron Physiol. 102, p51–p60 (2006).
Don, B. R. The effect of trimethoprim on potassium and uric acid metabolism in normal human subjects. Clin. Nephrol. 55, 45–52 (2001).
Eiam-Ong, S., Kurtzman, N. A. & Sabatini, S. Studies on the mechanism of trimethoprim-induced hyperkalemia. Kidney Int. 49, 1372–1378 (1996).
Perazella, M. A. Trimethoprim-induced hyperkalaemia: clinical data, mechanism, prevention and management. Drug Saf. 22, 227–236 (2000).
Reiser, I. W. et al. Reversal of trimethoprim-induced antikaliuresis. Kidney Int. 50, 2063–2069 (1996).
Schreiber, M. et al. Antikaliuretic action of trimethoprim is minimized by raising urine pH. Kidney Int. 49, 82–87 (1996).
Lin, S. H. et al. Reversible voltage-dependent distal renal tubular acidosis in a patient receiving standard doses of trimethoprim sulfamethoxazole. Nephrol. Dial. Transplant. 12, 1031–1033 (1997).
Sawaya, B. P., Briggs, J. P. & Schnermann, J. Amphotericin B nephrotoxicity: the adverse consequences of altered membrane properties. J. Am. Soc. Nephrol. 6, 154–164 (1995).
Wingard, J. R. et al. Clinical significance of nephrotoxicity in patients treated with amphotericin B for suspected or proven aspergillosis. Clin. Infect. Dis. 29, 1402–1407 (1999).
Burges, J. L. & Birchall, R. Nephrotoxicity of amphotericin B, with emphasis on changes in tubular function. Am. J. Med. 53, 77–84 (1972).
Lucas da Silva, P. S., Iglesias, S. B. & Waisberg, J. Hypokalemic rhabdomyolysis in a child due to amphotericin B therapy. Eur. J. Pediatr. 166, 169–171 (2007).
Gerkens, J. F. & Branch, R. A. The influence of sodium status and furosemide on canine acute amphotericin B nephrotoxicity. J. Pharmacol. Exp. Ther. 214, 306–311 (1980).
Bernardo, J. F. et al. Potassium depletion potentiates amphotericin-B-induced toxicity to renal tubules. Nephron 70, 235–241 (1995).
Wazny, L. D. & Brophy, D. F. Amiloride for the prevention of amphotericin B-induced hypokalemia and hypomagnesemia. Ann. Pharmacother. 34, 94–97 (2000).
Ural, A. U. et al. Spironolactone: is it a novel drug for the prevention of amphotericin B-related hypokalemia in cancer patients? Eur. J. Clin. Pharmacol. 57, 771–773 (2002).
Mohan, S. et al. Proteinuria lowers the risk of amphotericin B-associated hypokalaemia. J. Antimicrob. Chemother. 60, 690–693 (2007).
Steinmetz, P. R. & Lawson, L. R. Defect in urinary acidification induced in vitro by amphotericin B. J. Clin. Invest. 49, 596–601 (1970).
Barbour, G. L. et al. Vasopressin-resistant nephrogenic diabetes insipidus. A result of amphotericin B therapy. Arch. Intern. Med. 139, 86–88 (1979).
Frokiaer, J. et al. Pathophysiology of aquaporin-2 in water balance disorders. Am. J. Med. Sci. 316, 291–299 (1998).
Butler, W. T. et al. Nephrotoxicity of amphotericin B; early and late effects in 81 patients. Ann. Intern. Med. 61, 175–187 (1964).
Canada, T. W., Weavind, L. M. & Augustin, K. M. Possible liposomal amphotericin B-induced nephrogenic diabetes insipidus. Ann. Pharmacother. 37, 70–73 (2003).
Yano, Y., Monteiro, J. L. & Seguro, A. C. Effect of amphotericin B on water and urea transport in the inner medullary collecting duct. J. Am. Soc. Nephrol. 5, 68–74 (1994).
Kim, S. W. et al. Amphotericin B decreases adenylyl cyclase activity and aquaporin-2 expression in rat kidney. J. Lab. Clin. Med. 138, 243–249 (2001).
Hoorn, E. J. & Zietse, R. Combined renal tubular acidosis and diabetes insipidus in hematological disease. Nat. Clin. Pract. Nephrol. 3, 171–175 (2007).
El-Sheikh, A. A., Masereeuw, R. & Russel, F. G. Mechanisms of renal anionic drug transport. Eur. J. Pharmacol. 585, 245–255 (2008).
Hirji, M. R. & Mucklow, J. C. Transepithelial water movement in response to carbamazepine, chlorpropamide and demeclocycline in toad urinary bladder. Br. J. Pharmacol. 104, 550–553 (1991).
Kinzie, B. J. Management of the syndrome of inappropriate secretion of antidiuretic hormone. Clin. Pharm. 6, 625–633 (1987).
Brunner, F. P. & Frick, P. G. Hypokalaemia, metabolic alkalosis, and hypernatraemia due to “massive” sodium penicillin therapy. Br. Med. J. 4, 550–552 (1968).
Lipner, H. I. et al. The behavior of carbenicillin as a nonreabsorbable anion. J. Lab. Clin. Med. 86, 183–194 (1975).
Hoorn, E. J. & Zietse, R. Severe hypokalaemia caused by flucloxacillin. J. Antimicrob. Chemother. 61, 1396–1398 (2008).
Bustamante, M. et al. Calcium-sensing receptor attenuates AVP-induced aquaporin-2 expression via a calmodulin-dependent mechanism. J. Am. Soc. Nephrol. 19, 109–116 (2008).
Gordon, J. A. et al. The renal concentrating defect after gentamicin administration in the rat. J. Lab. Clin. Med. 101, 903–910 (1983).
Blazes, D. L. & Decker, C. F. Symptomatic hyperlactataemia precipitated by the addition of tetracycline to combination antiretroviral therapy. Lancet Infect. Dis. 6, 249–252 (2006).
Wiener, M. et al. Lactic acidosis after treatment with linezolid. Infection 35, 278–281 (2007).
De Vriese, A. S. et al. Linezolid-induced inhibition of mitochondrial protein synthesis. Clin. Infect. Dis. 42, 1111–1117 (2006).
Coronado, B. E., Opal, S. M. & Yoburn, D. C. Antibiotic-induced D-lactic acidosis. Ann. Intern. Med. 122, 839–842 (1995).
Halperin, M. L. & Kamel, K. S. D-lactic acidosis: turning sugar into acids in the gastrointestinal tract. Kidney Int. 49, 1–8 (1996).
Fenves, A. Z. et al. Increased anion gap metabolic acidosis as a result of 5-oxoproline (pyroglutamic acid): a role for acetaminophen. Clin. J. Am. Soc. Nephrol. 1, 441–447 (2006).
Peter, J. V. et al. An unusual cause of severe metabolic acidosis. Med. J. Aust. 185, 223–225 (2006).
Rolleman, E. J. et al. Guilty as charged: unmeasured urinary anions in a case of pyroglutamic acidosis. Neth. J. Med. 66, 351–353 (2008).
Adler, D. et al. SIADH consecutive to ciprofloxacin intake. Eur. J. Intern. Med. 15, 463–464 (2004).
Kushner, J. M., Peckman, H. J. & Snyder, C. R. Seizures associated with fluoroquinolones. Ann. Pharmacother. 35, 1194–1198 (2001).
Chitre, M. M. & Berenson, C. S. Idiosyncratic rifabutin-induced leukopenia and SIADH: case report and review. Pharmacotherapy 21, 493–497 (2001).
Holmes, A. M., Hesling, C. M. & Wilson, T. M. Capreomycin-induced serum electrolyte abnormalities. Thorax 25, 608–611 (1970).
Nakashita, T. & Motojima, S. Case of SIADH caused by ethionamide in a patient with pulmonary tuberculosis [Japanese]. Kekkaku 81, 731–735 (2006).
Thiele, A. & Rehman, H. U. Hyperkalemia caused by penicillin. Am. J. Med. 121, e1–e2 (2008).
Barcia, J. P. Hyperkalemia associated with rapid infusion of conventional and lipid complex formulations of amphotericin B. Pharmacotherapy 18, 874–876 (1998).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Zietse, R., Zoutendijk, R. & Hoorn, E. Fluid, electrolyte and acid–base disorders associated with antibiotic therapy. Nat Rev Nephrol 5, 193–202 (2009). https://doi.org/10.1038/nrneph.2009.17
Issue Date:
DOI: https://doi.org/10.1038/nrneph.2009.17
This article is cited by
-
The pathophysiology of distal renal tubular acidosis
Nature Reviews Nephrology (2023)
-
Acquired autoimmune Bartter syndrome in a patient with primary hypothyroidism
Rheumatology International (2021)
-
Prevalence, diagnosis, and management of secondary pseudohypoaldosteronism
Pediatric Nephrology (2020)
-
Magnetic Functionalized Nanoparticles for Biomedical, Drug Delivery and Imaging Applications
Nanoscale Research Letters (2019)
-
Na+, K+, Cl−, acid–base or H2O homeostasis in children with urinary tract infections: a narrative review
Pediatric Nephrology (2016)