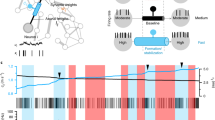
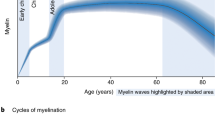
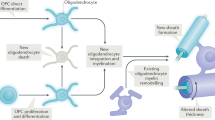
Abstract
The synapse is the focus of experimental research and theory on the cellular mechanisms of nervous system plasticity and learning, but recent research is expanding the consideration of plasticity into new mechanisms beyond the synapse, notably including the possibility that conduction velocity could be modifiable through changes in myelin to optimize the timing of information transmission through neural circuits. This concept emerges from a confluence of brain imaging that reveals changes in white matter in the human brain during learning, together with cellular studies showing that the process of myelination can be influenced by action potential firing in axons. This Opinion article summarizes the new research on activity-dependent myelination, explores the possible implications of these studies and outlines the potential for new research.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Fields, R. D. in Neuroglia 3rd edn (eds Kettenmann, H. & Ransom, B. R.) 573–585 (Oxford Univ. Press, 2013).
Fields, R. D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 31, 361–370 (2008).
Yakovlev, P. I. & Lecours, A.-R. in Regional Development of the Brain in Early Life (ed. Minkowski, A.) 3–70 (Blackwell Scientific, 1987).
Mabbott, D. J., Noseworthy, M., Bouffet, E., Laughlin, S. & Rockel, C. White matter growth as a mechanism of cognitive development in children. Neuroimage 33, 936–946 (2006).
Nagy, Z. et al. Maturation of white matter is associated with the development of cognitive functions during childhood. J. Cogn. Neurosci. 16, 1227–1233 (2004).
Kraft, R. H., Mitchell, O. R., Languis, M. L. & Wheatley, G. H. Hemispheric asymmetries during six- to eight-year-olds performance of Piagetian conservation and reading tasks. Neuropsychologia 18, 637–643 (1980).
Pujol, J. et al. Myelination of language-related areas in the developing brain. Neurology 66, 339–343 (2006).
Liston, C. et al. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cereb. Cortex 16, 553–560 (2006).
Giedd, J. N. Structural magnetic resonance imaging of the adolescent brain. Ann. NY Acad. Sci. 1021, 77–85 (2004).
Schrager, P. & Novakovic, S. D. Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Brain Res. Dev. Brain Res. 88, 68–78 (1995).
Demerens, C. et al. Induction of myelination in the central nervous system by electrical activity. Proc. Natl Acad. Sci. USA 93, 9887–9892 (1996).
Stevens, B., Tanner, S. & Fields, R. D. Control of myelination by specific patterns of neural impulses. J. Neurosci. 18, 9303–9311 (1998).
Stevens, B., Porta, S., Haak, L. L., Gallo, V. & Fields, R. D. Adenosine: a neuron-glial transmitter promoting myelination in the CNS in response to action potentials. Neuron 36, 855–868 (2002).
Stevens, B., Ishibashi, T., Chen, J. F. & Fields, R. D. Adenosine: an activity-dependent axonal signal regulating MAP kinase and proliferation in developing Schwann cells. Neuron Glia Biol. 1, 23–34 (2004).
Ishibashi, T. et al. Astrocytes promote myelination in response to electrical impulses. Neuron 49, 823–823 (2006).
Wake, H., Lee, P. R. & Fields, R. D. Control of local protein synthesis and initial events in myelination by action potentials. Science 333, 1647–1651 (2011).
Yang, I. H. et al. Axon myelination and electrical stimulation in a microfluidic compartmentalized cell culture platform. Neuromolecular Med. 14, 112–118 (2012).
Malone, M. et al. Neuronal activity promotes myelination via a cAMP pathway. Glia 61, 843–854 (2013).
Luundgard, L. et al. Neuregulin and BDNF induce a switch to NMDA receptor-dependent myelination by oligodendrocytes. PLoS Biol. 11, e1001743 (2013).
Dan, Y. & Poo, M. M. Spike timing-dependent plasticity: from synapse to perception. Physiol. Rev. 86, 1033–1048 (2006).
Fields, R. D. Myelination: an overlooked mechanism of synaptic plasticity? Neuroscientist 11, 528–531 (2005).
Buzsáki, G. Rhythms of the Brain (Oxford Univ. Press, 2006).
Ainsworth, M. et al. Rates and rhythms: a synergistic view of frequency and temporal coding in neuronal networks. Neuron 75, 572–583 (2012).
Nunez, P. L. Srinivasan, R. & Fields, R. D. EEG functional connectivity, axon delays and white matter disease. Clin. Neurophysiol. 126, 110–120 (2015).
Pajevic, S., Basser, P. J. & Fields, R. D. Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147 (2014).
Zatorre, R. J., Fields, R. D. & Johansen-Berg, H. Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536 (2012).
Bengtsson, S. L. et al. Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150 (2005).
Carreiras, M. et al. An anatomical signature for literacy. Nature 461, 983–986 (2009).
Scholz, J., Klein, M. C., Behrens, T. E. & Johansen-Berg, H. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 (2009).
Fields, R. D. Imaging learning: The search for a memory trace. Neuroscientist 17, 185–196 (2011).
Gibson, E. M. et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 (2014).
Hines, H. H., Ravanelli, A. M., Schwindt, R., Scott, E. K. & Appel, B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 (2015).
Mensch, S. et al. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 (2015).
Swadlow, H. A. Physiological properties of individual cerebral axons studied in vivo for as long as one year. J. Neurophysiol. 54, 1346–1362 (1985).
Liu, J. et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 (2012).
Makinodan, M., Rosen, K. M., Ito, S. & Corfas, G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 (2012).
Okuda, H. et al. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J. Neurosci. Res. 87, 3546–3553 (2009).
Zhao, U. Y. et al. Enriched environment increases the total number of CNPase positive cells in the corpus callosum of middle-aged rats. Acta Neurobiol. Exp. 71, 322–330 (2011).
Simon, C., Gotz, M. & Dimou, L. Progenitors in adult cerebral cortex: cell cycle properties and regulation by physiological stimuli and injury. Glia 59, 869–881 (2011).
McKenzie, I. et al. Motor skill learning requires central myelination. Science 346, 318–322 (2014).
Blumenfeld-Katzir, T., Pasternak, O., Dagan, M. & Assaf, Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS ONE 6, e20678 (2011).
Kleim, J. A. et al. Motor learning-dependent synaptogenesis is localized to functionally reorganized motor cortex. Neurobiol. Learn. Mem. 77, 63–77 (2002).
Sampaio-Baptista, C. et al. Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503 (2013).
Hofstetter, S., Tavor, I., Moryosef, S. T. & Assaf, Y. Short-term learning induces white matter plasticity in the fornix. J. Neurosci. 33, 12844–12850 (2013).
Yang, S. et al. Effects of an enriched environment on myelin sheaths in the white matter of rats during normal aging: A stereological study. Neuroscience 234, 13–21 (2013).
Engvig, A. et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Hum. Brain Mapp. 33, 2390–2406 (2012).
Engvig, A. et al. Effects of memory training on cortical thickness in the elderly. Neuroimage 52, 667–1676 (2010).
Kennedy, K. M. & Raz, N. Aging white matter and cognition: Differential effects of regional variations in diffusion properties on memory, executive funcitons, and speed. Neuropsychologia 47, 916–927 (2009).
Marner, L., Nyengaard, J. R., Tang, Y. & Pakkenberg, B. Marked loss of myelinated nerve fibers in the human brain with age. J. Comp. Neurol. 462, 144–152 (2003).
Salat, D. H. et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol. Aging 26, 1215–1227 (2005).
Bartzokis, G. Age-related myelin breakdown: A developmental model of cognitive decline and Alzheimer's disease. Neurobiol. Aging 25, 5–18 (2004).
Ransom, B. R. & Orkand, R. K. Glial-neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. Trends Neurosci. 19, 352–358 (1996).
Káradóttir, R., Cavelier, P., Bergersen, L. H. & Attwell, D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 438, 1162–1166 (2005).
Stevens, B. & Fields, R. D. Response of Schwann cells to action potentials in development. Science. 287, 2267–2271 (2000).
Gallo, V. et al. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K1 channel block. J. Neurosci. 16, 2659–2670 (1996).
Mangin, J. M., Li, P., Scafidi, J. & Gallo, V. Experience-dependent regulation of NG2 progenitors in the developing barrel cortex. Nat. Neurosci. 15, 1192–1194 (2012).
Zonouzi, M. et al. GABAergic regulation of cerebellar NG2 cell development is altered in perinatal white matter injury. Nat. Neurosci. 18, 674–682 (2015).
Fields, R. D. Nonsynaptic and nonvesicular ATP release from neurons and relevance to neuron–glia signaling. Semin. Cell Dev. Biol. 22, 214–219 (2011).
Burnstock, G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 (2007).
Zhang, Z. W., Kang, J. I. & Vaucher, E. Axonal varicosity density as an index of local neuronal interactions. PLoS ONE 6, e22543 (2011).
Kriegler, S. & Chiu, S. Y. Calcium signaling of glial cells along mammalian axons. J. Neurosci. 13, 4229–4245 (1993).
Orkand, R. K., Nicholls, J. G. & Kuffler, S. W. Effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 29, 788–806 (1966).
Fields, R. D. & Ni, Y. Nonsynaptic communication through ATP release from volume-activated anion channels in axons. Sci. Signal. 3, ra73 (2010).
Fields, R. D. Imaging single photons and intrinsic optical signals for studies of vesicular and non-vesicular ATP release from axons. Front. Neuroanat. 5, 32 (2011).
Micu, I. et al. NMDA receptors mediate calcium accumulation in myelin during chemical ischaemia. Nature 439, 988–992 (2006).
Butt, A. M., Fern, R. F. & Matute, C. Neurotransmitter signaling in white matter. Glia. 62, 1762–1779 (2014).
Fern, R. F., Matute, C. & Stys, P. K. White matter injury: Ischemic and nonischemic. Glia 62, 1780–1789 (2014).
Garthwaite, G., Hampden-Smith, K., Wilson, G. W., Goodwin, D. A. & Garthwaite, J. Nitric oxide targets oligodendrocytes and promotes their morphological differentiation. Glia 63, 383–399 (2015).
Itoh, K., Stevens, B., Schachner, M. & Fields, R. D. Regulated expression of the neural cell adhesion molecule L1 by specific patterns of neural impulses. Science. 270, 1369–1372 (1995).
Itoh, K., Ozaki, M., Stevens, B. & Fields, R. D. Activity-dependent regulation of N-cadherin in DRG neurons: differential regulation of N-cadherin, NCAM, and L1 by distinct patterns of action potentials. J. Neurobiol. 33, 735–748 (1997).
Yuen, T. J. et al. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 158, 383–396 (2014).
Bergles, D. E., Roberts, J. D., Somogyi, P. & Jahr, C. E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 405, 187–191 (2000).
Horner, P. J. et al. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 20, 2218–2228 (2000).
Dawson, M. R. L., Polito, A., Levine, J. M. & Reynolds, R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 24, 476–488 (2003).
Young, K. M. et al. Oligodendrocyte dynamics in healthy adult CNS: evidence for myelin remodeling. Neuron 77, 873–853 (2013).
Lin, S. C. et al. Climbing fiber innervation of NG2-expressing glia in the mammalian cerebellum. Neuron. 46, 773–785 (2005).
Kukley, M., Capetillo-Zarate, E. & Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 10, 311–320 (2007).
Kukley, M. et al. Glial cells are born with synapses. FASEB J. 22, 2957–2969 (2008).
Maldonado, P. P., Velez-Fort, M. & Angulo, M. C. Is neuronal communication with NG2 cells synaptic or extrasynaptic? J. Anat. 219, 8–17 (2011).
Wake, H. et al. Non-synaptic junctions on myelinating glia promote preferential myelination of electrically-active axons. Nat. Commun. 6, 7844 (2015).
Sakry, D. et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of NG2. PLoS Biol 12, 1001–1092 (2014).
Bakhti, M., Winter, C. & Simons, M. Inhibition of myelin membrane sheath formation by oligodendrocyte-derived exosome-like vesicles. J. Biol. Chem. 286, 787–796 (2011).
Frühbeis, C. et al. Neurotransmitter-triggered transfer of exosomes mediates oligodendrocyte-neuron communication. PLoS Biol. 11, e1001604 (2013).
Pusic, A. D. & Kraig, R. P. Youth and environmental enrichment generate serum exosomes containing miR-219 that promote CNS myelination. Glia 62, 284–299 (2014).
Luse, S. A. in The Biology of Myelin (ed. Korey, S. R.) 59 (Paul B. Hoeber, 1959).
Krämer, E. M., Klein, C., Koch, T., Boytinck, M. & Trotter, J. Compartmentation of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem. 274, 29042–29049 (1999).
Laursen, L. S., Chan, C. W. & ffrench-Constant, C. An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J. Neurosci. 29, 9174–9185 (2009).
White, R. et al. Activation of oligodendroglial Fyn kinase enhances translation of mRNAs transported in hnRNP A2-dependent RNA granules. J. Cell Biol. 181, 579–586 (2008).
Barbin, G. et al. Axonal cell-adhesion molecule L1 in CNS myelination. Neuron Glia Biol. 1, 65–72 (2004).
Seilheimer, B., Persohn, E. & Schachner, M. Neural cell adhesion molecule expression is regulated by Schwann cell-neuron interactions in culture. J. Cell Biol. 108, 1909–1915 (1989).
Wood, P. M., Schachner, M. & Bunge, R. P. Inhibition of Schwann cell myelination in vitro by antibody to the L1 adhesion molecule. J. Neurosci. 10, 3635–3645 (1990).
Chiu, S. Y. & Wilson, G. F. The role of potassium channels in Schwann cell proliferation in Wallerian degeneration of explant rabbit sciatic nerves. J. Physiol. 408, 199–222 (1989).
Pappas, C. A., Ullrich, N. & Sontheimer, H. Reduction of glial proliferation by K+ channel blockers is mediated by changes in pH. Neuroreport 6, 193–196 (1994).
Knutson, P. et al. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J. Neurosci. 17, 2669–2682 (1997).
Gudz, T. I., Komuro, H. & Macklin, W. B. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J. Neurosci. 26, 2458–2466 (2006).
Xiao, L. et al. NMDA receptor couples Rac1-GEF Tiam1 to direct oligodendrocyte precursor cell migration. Glia. 61, 2078–2099 (2013).
Fields, R. D. & Burnstock, G. Purinergic signaling in neuron-glia interactions. Nat. Rev. Neurosci. 7, 423–436 (2006).
Barres, B. A. & Raff, M. C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 361, 258–260 (1993).
Van't Veer, A. et al. Brain-derived neurotrophic factor effects on oligodendrocyte progenitors of the basal forebrain are mediated through trkB and the MAP kinase pathway. J. Neurosci. Res. 87, 69–78 (2009).
Fulmer, C. G. et al. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J. Neurosci. 34, 8186–8196 (2014).
Xiao, J., Wong, A. W., Willingham, M. M. vanden Buuse, M., Kilpatrick, T. J. & Murray, S. S. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals 18, 186–202 (2010).
Czopka, T., ffrench-Constant, C. & Lyons, D. A. Individual oligodendrocytes have only a few hours in which to generate new myelin sheaths in vivo. Dev. Cell. 25, 599–609 (2013).
Kole, M. H. P. & Stuart, G. J. Signal processing in the axon initial segment. Neuron 73, 235–247 (2012).
Kuba, H., Oichi, Y. & Ohmori, H. Presynaptic activity regulates Na+ channel distribution at the axon initial segment. Nature 465, 1075–1078 (2010).
Grubb, M. S. & Burrone, J. Activity-dependent relocation of the axon initial segment fine-tunes neuronal excitability. Nature 465, 1070–1074 (2010).
Tagoe, T., Barker, M., Jones, A., Allcock, N. & Hamann, M. Auditory nerve perinodal dysmyelination in noise-induced hearing loss. J. Neurosci. 34, 2684–2688 (2014).
Fields, R. D. Myelin — more than insulation. Science 344, 264–266 (2014).
Tomassy, G. S. et al. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science 334, 319–324 (2014).
Schwab, M. E. & Strittmatter, S. M. Nogo limits neural plasticity and recovery from injury. Curr. Opin. Neurobiol. 27, 53–60 (2014).
Bukalo, O. & Fields, R. D. Synaptic plasticity by antidromic firing during hippocampal network oscillations. Proc. Natl Acad. Sci. USA 110, 5175–5180 (2013).
Bullock, T. H., Moore, J. K. & Fields, R. D. Evolution of myelin sheaths: both lamprey and hagfish lack myelin. Neurosci. Lett. 48, 145–148 (1984).
Acknowledgements
This work was supported by funds for intramural research from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD). Electron micrographs in Figure 2 are courtesy of Louis Dye, Microscopy Imaging Core, NICHD. 3D reconstruction is courtesy of Emily Benson and Grahame Kidd, Renovo, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- Axolemma
-
The cell membrane of an axon.
- Fractional anisotropy
-
(FA). A measure of the symmetry of water diffusion in tissue analysed by MRI. Increased FA reflects more restricted diffusion of water, as occurs when axons become myelinated or more highly compacted, thus restricting water diffusion parallel to the axons.
- Non-synaptic transmission
-
Intercellular communication in the nervous system that does not involve synapses. This includes neurotransmitters released from vesicles fusing with the neuronal membrane outside synapses, as well as the release of neurotransmitters through membrane channels and other mechanisms.
- Synaptic coupling
-
A specialized junction between cells in the nervous system for rapid communication by electrical signalling. Neurotransmitters released from synaptic vesicles in the presynaptic neuron in response to electrical depolarization activate neurotransmitter receptors on the membrane of the postsynaptic cell to depolarize or hyperpolarize the postsynaptic membrane potential.
- Uncinate fasciculus
-
A white matter tract that connects the hippocampus, amygdala and temporal lobe with the orbitofrontal cortex of the brain.
Rights and permissions
About this article
Cite this article
Fields, R. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci 16, 756–767 (2015). https://doi.org/10.1038/nrn4023
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn4023
This article is cited by
-
The role of TSC1 and TSC2 proteins in neuronal axons
Molecular Psychiatry (2024)
-
Possible roles of deep cortical neurons and oligodendrocytes in the neural basis of human sociality
Anatomical Science International (2024)
-
A Novel Hybrid of Telmisartan and Borneol Ameliorates Neuroinflammation and White Matter Injury in Ischemic Stroke Through ATF3/CH25H Axis
Translational Stroke Research (2024)
-
Computational conjugate adaptive optics microscopy for longitudinal through-skull imaging of cortical myelin
Nature Communications (2023)
-
Cortical amyloid-beta burden is associated with changes in intracortical myelin in cognitively normal older adults
Translational Psychiatry (2023)