Key Points
-
Neurodevelopmental programming is defined as the implementation of the genetic and epigenetic blueprints that guide and coordinate normal brain development.
-
Epigenetic processes are also responsible for the reprogramming that occurs in response to environmental challenges, such as maternal stress or infection, making the organism more or less adaptive depending on the future environment and the challenges presented.
-
Developmental windows of susceptibility exist such that exposures occurring during these dynamic periods are more likely to produce marked and broad changes to the epigenome.
-
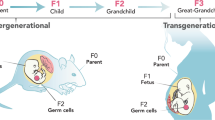
Alterations to the germ cell epigenome as a result of environmental influences throughout life can result in transgenerational transmission of traits that are able to increase or to decrease disease risk in future offspring.
-
Seemingly different environmental perturbations, such as maternal stress or infection, can produce similar neurodevelopmental changes and phenotypes, which suggests that common downstream cellular mechanisms are responsible for transmitting information from the in utero environment to the developing fetus.
-
Sex specificity of the parent and the offspring is also an important factor in determining the effect of the exposures and the epigenetic mechanisms involved.
Abstract
Neurodevelopmental programming — the implementation of the genetic and epigenetic blueprints that guide and coordinate normal brain development — requires tight regulation of transcriptional processes. During prenatal and postnatal time periods, epigenetic processes fine-tune neurodevelopment towards an end product that determines how an organism interacts with and responds to exposures and experiences throughout life. Epigenetic processes also have the ability to reprogramme the epigenome in response to environmental challenges, such as maternal stress, making the organism more or less adaptive depending on the future challenges presented. Epigenetic marks generated within germ cells as a result of environmental influences throughout life can also shape future generations long before conception occurs.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Bale, T. L. et al. Early life programming and neurodevelopmental disorders. Biol. Psychiatry 68, 314–319 (2010).
Curley, J. P., Jensen, C. L., Mashoodh, R. & Champagne, F. A. Social influences on neurobiology and behavior: epigenetic effects during development. Psychoneuroendocrinology 36, 352–371 (2011).
McCarthy, M. M. & Nugent, B. M. Epigenetic contributions to hormonally mediated sexual differentiation of the brain. J. Neuroendocrinol. 25, 1133–1140 (2013).
Hodes, G. E. Sex, stress, and epigenetics: regulation of behavior in animal models of mood disorders. Biol. Sex Differ. 4, 1 (2013).
Bohacek, J., Gapp, K., Saab, B. J. & Mansuy, I. M. Transgenerational epigenetic effects on brain functions. Biol. Psychiatry 73, 313–320 (2013).
Hales, C. N. & Barker, D. J. The thrifty phenotype hypothesis. Br. Med. Bull. 60, 5–20 (2001). In this paper, the thrifty phenotype hypothesis describes how a mismatch between fetal programming and the postnatal environment can result in disease risk.
Binder, E. B. & Nemeroff, C. B. The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol. Psychiatry 15, 574–588 (2010).
Hackman, D. A., Farah, M. J. & Meaney, M. J. Socioeconomic status and the brain: mechanistic insights from human and animal research. Nature Rev. Neurosci. 11, 651–659 (2010).
Sweatt, J. D. The emerging field of neuroepigenetics. Neuron 80, 624–632 (2013).
Glynn, L. M., Wadhwa, P. D., Dunkel-Schetter, C., Chicz-Demet, A. & Sandman, C. A. When stress happens matters: effects of earthquake timing on stress responsivity in pregnancy. Am. J. Obstet. Gynecol. 184, 637–642 (2001).
Deverman, B. E. & Patterson, P. H. Cytokines and CNS development. Neuron 64, 61–78 (2009).
Howerton, C. L. & Bale, T. L. Prenatal programing: at the intersection of maternal stress and immune activation. Horm. Behav. 62, 237–242 (2012).
Brown, A. S. Epidemiologic studies of exposure to prenatal infection and risk of schizophrenia and autism. Dev. Neurobiol. 72, 1272–1276 (2012).
Brown, A. S. et al. Prenatal infection and cavum septum pellucidum in adult schizophrenia. Schizophr. Res. 108, 285–287 (2009).
Brown, A. S. et al. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am. J. Psychiatry 166, 683–690 (2009).
Selevan, S. G., Kimmel, C. A. & Mendola, P. Identifying critical windows of exposure for children's health. Environ. Health Perspect. 108 (Suppl. 3), 451–455 (2000).
Rapoport, J. L., Addington, A. M., Frangou, S. & Psych, M. R. The neurodevelopmental model of schizophrenia: update 2005. Mol. Psychiatry 10, 434–449 (2005).
Meyer, U., Feldon, J. & Dammann, O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr. Res. 69, 26R–33R (2011). This study examines the shared mechanistic link between neurodevelopmental disorders that are related to prenatal inflammation.
Morrison, K. E., Rodgers, A. B., Morgan, C. P. & Bale, T. L. Epigenetic mechanisms in pubertal brain maturation. Neuroscience 264, 7–24 (2013).
Davis, E. P., Sandman, C. A., Buss, C., Wing, D. A. & Head, K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol. Psychiatry 74, 647–655 (2013).
Malter Cohen, M. et al. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc. Natl Acad. Sci. USA 110, 18274–18278 (2013).
Bale, T. L. Sex differences in prenatal epigenetic programming of stress pathways. Stress 14, 348–356 (2011).
Susser, E., St Clair, D. & He, L. Latent effects of prenatal malnutrition on adult health: the example of schizophrenia. Ann. NY Acad. Sci. 1136, 185–192 (2008).
Stoner, R. et al. Patches of disorganization in the neocortex of children with autism. N. Engl. J. Med. 370, 1209–1219 (2014).
Avishai-Eliner, S., Brunson, K. L., Sandman, C. A. & Baram, T. Z. Stressed-out, or in (utero)? Trends Neurosci. 25, 518–524 (2002).
Wadhwa, P. D., Sandman, C. A. & Garite, T. J. The neurobiology of stress in human pregnancy: implications for prematurity and development of the fetal central nervous system. Prog. Brain Res. 133, 131–142 (2001).
Wadhwa, P. D., Sandman, C. A., Porto, M., Dunkel-Schetter, C. & Garite, T. J. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am. J. Obstet. Gynecol. 169, 858–865 (1993).
Khashan, A. S. et al. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch. Gen. Psychiatry 65, 146–152 (2008).
Atladottir, H. O. et al. Association of hospitalization for infection in childhood with diagnosis of autism spectrum disorders: a Danish cohort study. Arch. Pediatr. Adolesc. Med. 164, 470–477 (2010).
Atladottir, H. O. et al. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. J. Autism Dev. Disord. 40, 1423–1430 (2010).
Goines, P. E. et al. Increased midgestational IFNγ, IL-4 and IL-5 in women bearing a child with autism: a case-control study. Mol. Autism 2, 13 (2011).
Brown, A. S. & Susser, E. S. Prenatal nutritional deficiency and risk of adult schizophrenia. Schizophr. Bull. 34, 1054–1063 (2008).
Xu, M. Q. et al. Prenatal malnutrition and adult schizophrenia: further evidence from the 1959–1961 Chinese famine. Schizophr. Bull. 35, 568–576 (2009).
Ravelli, G. P., Stein, Z. A. & Susser, M. W. Obesity in young men after famine exposure in utero and early infancy. N. Engl. J. Med. 295, 349–353 (1976).
Heijmans, B. T. et al. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl Acad. Sci. USA 105, 17046–17049 (2008).
Van Lieshout, R. J., Taylor, V. H. & Boyle, M. H. Pre-pregnancy and pregnancy obesity and neurodevelopmental outcomes in offspring: a systematic review. Obes. Rev. 12, e548–e559 (2011).
Buss, C. et al. Impaired executive function mediates the association between maternal pre-pregnancy body mass index and child ADHD symptoms. PLoS ONE 7, e37758 (2012).
Howerton, C. L., Morgan, C. P., Fischer, D. B. & Bale, T. L. O-GlcNAc transferase (OGT) as a placental biomarker of maternal stress and reprogramming of CNS gene transcription in development. Proc. Natl Acad. Sci. USA 110, 5169–5174 (2013).
Mao, J. et al. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc. Natl Acad. Sci. USA 107, 5557–5562 (2010).
Weaver, J. R., Susiarjo, M. & Bartolomei, M. S. Imprinting and epigenetic changes in the early embryo. Mamm. Genome 20, 532–543 (2009).
Mueller, B. R. & Bale, T. L. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol. Behav. 88, 605–614 (2006).
Mueller, B. R. & Bale, T. L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 28, 9055–9065 (2008). This study identifies sex-specific changes in the placenta in response to early gestational stress that are associated with changes in offspring neurodevelopment.
Brunton, P. J. & Russell, J. A. Prenatal social stress in the rat programmes neuroendocrine and behavioural responses to stress in the adult offspring: sex-specific effects. J. Neuroendocrinol. 22, 258–271 (2010).
Brunton, P. J. & Russell, J. A. Allopregnanolone and suppressed hypothalamo–pituitary–adrenal axis stress responses in late pregnancy in the rat. Stress 14, 6–12 (2011).
Weinstock, M. Gender differences in the effects of prenatal stress on brain development and behaviour. Neurochem. Res. 32, 1730–1740 (2007).
Kapoor, A., Kostaki, A., Janus, C. & Matthews, S. G. The effects of prenatal stress on learning in adult offspring is dependent on the timing of the stressor. Behav. Brain Res. 197, 144–149 (2009).
Kapoor, A., Leen, J. & Matthews, S. G. Molecular regulation of the hypothalamic–pituitary–adrenal axis in adult male guinea pigs after prenatal stress at different stages of gestation. J. Physiol. 586, 4317–4326 (2008).
Schneider, M. L., Moore, C. F., Kraemer, G. W., Roberts, A. D. & DeJesus, O. T. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology 27, 285–298 (2002).
Kapoor, A. & Matthews, S. G. Short periods of prenatal stress affect growth, behaviour and hypothalamo–pituitary–adrenal axis activity in male guinea pig offspring. J. Physiol. 566, 967–977 (2005).
Mueller, B. R. & Bale, T. L. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol. Behav. 91, 55–65 (2007).
Lemaire, V., Koehl, M., Le Moal, M. & Abrous, D. N. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc. Natl Acad. Sci. USA 97, 11032–11037 (2000).
Darnaudery, M. & Maccari, S. Epigenetic programming of the stress response in male and female rats by prenatal restraint stress. Brain Res. Rev. 57, 571–585 (2008).
Weinstock, M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog. Neurobiol. 65, 427–451 (2001).
Coe, C. L. et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry 54, 1025–1034 (2003).
Welberg, L. A., Seckl, J. R. & Holmes, M. C. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience 104, 71–79 (2001).
Welberg, L. A. & Seckl, J. R. Prenatal stress, glucocorticoids and the programming of the brain. J. Neuroendocrinol. 13, 113–128 (2001).
Ward, I. L. Prenatal stress feminizes and demasculinizes the behavior of males. Science 175, 82–84 (1972). This is one of the first studies to determine the importance of sex-specific neurodevelopmental programming that can be disrupted by maternal stress.
Ward, I. L. & Stehm, K. E. Prenatal stress feminizes juvenile play patterns in male rats. Physiol. Behav. 50, 601–605 (1991).
Baron-Cohen, S. et al. Elevated fetal steroidogenic activity in autism. Mol. Psychiatry 20, 369–376 (2015).
Moore, L. et al. Serum testosterone levels are related to cognitive function in men with schizophrenia. Psychoneuroendocrinology 38, 1717–1728 (2013).
Lombardo, M. V. et al. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J. Neurosci. 32, 674–680 (2012).
Gur, R. E. et al. A sexually dimorphic ratio of orbitofrontal to amygdala volume is altered in schizophrenia. Biol. Psychiatry 55, 512–517 (2004). This paper reports the dysmasculinization of limbic brain regions in men with schizophrenia.
Fenoglio, K. A. et al. Enduring, handling-evoked enhancement of hippocampal memory function and glucocorticoid receptor expression involves activation of the corticotropin-releasing factor type 1 receptor. Endocrinology 146, 4090–4096 (2005).
Fenoglio, K. A., Brunson, K. L. & Baram, T. Z. Hippocampal neuroplasticity induced by early-life stress: functional and molecular aspects. Front. Neuroendocrinol. 27, 180–192 (2006).
Ivy, A. S., Brunson, K. L., Sandman, C. & Baram, T. Z. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 154, 1132–1142 (2008).
Ivy, A. S. et al. Hippocampal dysfunction and cognitive impairments provoked by chronic early-life stress involve excessive activation of CRH receptors. J. Neurosci. 30, 13005–13015 (2010).
Korosi, A. & Baram, T. Z. Plasticity of the stress response early in life: mechanisms and significance. Dev. Psychobiol. 52, 661–670 (2010).
Korosi, A. et al. Early-life experience reduces excitation to stress-responsive hypothalamic neurons and reprograms the expression of corticotropin-releasing hormone. J. Neurosci. 30, 703–713 (2010).
McClelland, S., Korosi, A., Cope, J., Ivy, A. & Baram, T. Z. Emerging roles of epigenetic mechanisms in the enduring effects of early-life stress and experience on learning and memory. Neurobiol. Learn. Mem. 96, 79–88 (2011).
Rice, C. J., Sandman, C. A., Lenjavi, M. R. & Baram, T. Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 149, 4892–4900 (2008). This novel and reproducible model shows how disruptions in maternal care can manifest in reprogramming of offspring stress pathways in the brain.
Ladd, C. O. et al. Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog. Brain Res. 122, 81–103 (2000).
Liu, D. et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277, 1659–1662 (1997).
Meaney, M. J. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu. Rev. Neurosci. 24, 1161–1192 (2001).
Weaver, I. C. et al. Epigenetic programming by maternal behavior. Nature Neurosci. 7, 847–854 (2004). This important paper was the first to establish epigenetic mechanisms in the developing brain that were altered by the quality of maternal care.
Barha, C. K., Pawluski, J. L. & Galea, L. A. Maternal care affects male and female offspring working memory and stress reactivity. Physiol. Behav. 92, 939–950 (2007).
Sanchez, M. M., Ladd, C. O. & Plotsky, P. M. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 13, 419–449 (2001).
Caldji, C. et al. Maternal care during infancy regulates the development of neural systems mediating the expression of fearfulness in the rat. Proc. Natl Acad. Sci. USA 95, 5335–5340 (1998).
Weaver, I. C., Diorio, J., Seckl, J. R., Szyf, M. & Meaney, M. J. Early environmental regulation of hippocampal glucocorticoid receptor gene expression: characterization of intracellular mediators and potential genomic target sites. Ann. NY Acad. Sci. 1024, 182–212 (2004).
Korosi, A. & Baram, T. Z. The pathways from mother's love to baby's future. Front. Behav. Neurosci. 3, 27 (2009).
Graham, Y. P., Heim, C., Goodman, S. H., Miller, A. H. & Nemeroff, C. B. The effects of neonatal stress on brain development: implications for psychopathology. Dev. Psychopathol. 11, 545–565 (1999).
Lyons, D. M., Martel, F. L., Levine, S., Risch, N. J. & Schatzberg, A. F. Postnatal experiences and genetic effects on squirrel monkey social affinities and emotional distress. Horm. Behav. 36, 266–275 (1999).
Barr, C. S. et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc. Natl Acad. Sci. USA 101, 12358–12363 (2004).
McGowan, P. O. et al. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nature Neurosci. 12, 342–348 (2009).
Vukojevic, V. et al. Epigenetic modification of the glucocorticoid receptor gene is linked to traumatic memory and post-traumatic stress disorder risk in genocide survivors. J. Neurosci. 34, 10274–10284 (2014).
Koshy, T. S., Sara, V. R., King, T. L. & Lazarus, L. The influence of protein restriction imposed at various stages of pregnancy on fetal and placental development. Growth 39, 497–506 (1975).
Vucetic, Z. et al. Early life protein restriction alters dopamine circuitry. Neuroscience 168, 359–370 (2010).
Pankevich, D. E., Teegarden, S. L., Hedin, A. D., Jensen, C. L. & Bale, T. L. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J. Neurosci. 30, 16399–16407 (2010).
Teegarden, S. L. & Bale, T. L. Decreases in dietary preference produce increased emotionality and risk for dietary relapse. Biol. Psychiatry 61, 1021–1029 (2007).
Teegarden, S. L. & Bale, T. L. Effects of stress on dietary preference and intake are dependent on access and stress sensitivity. Physiol. Behav. 93, 713–723 (2008).
Teegarden, S. L., Scott, A. N. & Bale, T. L. Early life exposure to a high fat diet promotes long-term changes in dietary preferences and central reward signaling. Neuroscience 162, 924–932 (2009).
Will, M. J., Franzblau, E. B. & Kelley, A. E. Nucleus accumbens μ-opioids regulate intake of a high-fat diet via activation of a distributed brain network. J. Neurosci. 23, 2882–2888 (2003).
Pecina, S., Cagniard, B., Berridge, K. C., Aldridge, J. W. & Zhuang, X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J. Neurosci. 23, 9395–9402 (2003).
Cottrell, E. C., Holmes, M. C., Livingstone, D. E., Kenyon, C. J. & Seckl, J. R. Reconciling the nutritional and glucocorticoid hypotheses of fetal programming. FASEB J. 26, 1866–1874 (2012).
Lesage, J., Blondeau, B., Grino, M., Breant, B. & Dupouy, J. P. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo–pituitary–adrenal axis in the newborn rat. Endocrinology 142, 1692–1702 (2001).
Lingas, R., Dean, F. & Matthews, S. G. Maternal nutrient restriction (48 h) modifies brain corticosteroid receptor expression and endocrine function in the fetal guinea pig. Brain Res. 846, 236–242 (1999).
Cottrell, E. C. & Seckl, J. R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 3, 19 (2009).
Seckl, J. R. & Holmes, M. C. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal 'programming' of adult pathophysiology. Nature Clin. Pract. Endocrinol. Metab. 3, 479–488 (2007).
Welberg, L. A., Thrivikraman, K. V. & Plotsky, P. M. Chronic maternal stress inhibits the capacity to up-regulate placental 11β-hydroxysteroid dehydrogenase type 2 activity. J. Endocrinol. 186, R7–R12 (2005).
Grayson, B. E. et al. Changes in melanocortin expression and inflammatory pathways in fetal offspring of nonhuman primates fed a high-fat diet. Endocrinology 151, 1622–1632 (2010).
Sullivan, E. L. et al. Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. J. Neurosci. 30, 3826–3830 (2010).
Bilbo, S. D. & Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 24, 2104–2115 (2010).
Frias, A. E. et al. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology 152, 2456–2464 (2011).
Shen, Q. et al. The role of pro-inflammatory factors in mediating the effects on the fetus of prenatal undernutrition: implications for schizophrenia. Schizophr. Res. 99, 48–55 (2008).
Ilievski, V., Lu, S. J. & Hirsch, E. Activation of toll-like receptors 2 or 3 and preterm delivery in the mouse. Reprod. Sci. 14, 315–320 (2007).
Hsiao, E. Y. & Patterson, P. H. Placental regulation of maternal–fetal interactions and brain development. Dev. Neurobiol. 72, 1317–1326 (2012).
Malkova, N. V., Yu, C. Z., Hsiao, E. Y., Moore, M. J. & Patterson, P. H. Maternal immune activation yields offspring displaying mouse versions of the three core symptoms of autism. Brain Behav. Immun. 26, 607–616 (2012).
Bilbo, S. D. & Schwarz, J. M. Early-life programming of later-life brain and behavior: a critical role for the immune system. Front. Behav. Neurosci. 3, 14 (2009).
Bronson, S. L. & Bale, T. L. Prenatal stress-induced increases in placental inflammation and offspring hyperactivity are male-specific and ameliorated by maternal antiinflammatory treatment. Endocrinology 155, 2635–2646 (2014).
Hsiao, E. Y. & Patterson, P. H. Activation of the maternal immune system induces endocrine changes in the placenta via IL-6. Brain Behav. Immun. 25, 604–615 (2011).
Lucassen, P. J. et al. Prenatal stress reduces postnatal neurogenesis in rats selectively bred for high, but not low, anxiety: possible key role of placental 11β-hydroxysteroid dehydrogenase type 2. Eur. J. Neurosci. 29, 97–103 (2009).
Mairesse, J. et al. Maternal stress alters endocrine function of the feto-placental unit in rats. Am. J. Physiol. Endocrinol. Metab. 292, E1526–E1533 (2007).
Garbett, K. A., Hsiao, E. Y., Kalman, S., Patterson, P. H. & Mirnics, K. Effects of maternal immune activation on gene expression patterns in the fetal brain. Transl. Psychiatry 2, e98 (2012).
Hodge, D. R. et al. Interleukin-6 regulation of the human DNA methyltransferase (HDNMT) gene in human erythroleukemia cells. J. Biol. Chem. 276, 39508–39511 (2001).
Howerton, C. L. & Bale, T. L. Targeted placental deletion of OGT recapitulates the prenatal stress phenotype including hypothalamic mitochondrial dysfunction. Proc. Natl Acad. Sci. USA 111, 9639–9644 (2014).
Dunn, G. A., Morgan, C. P. & Bale, T. L. Sex-specificity in transgenerational epigenetic programming. Horm. Behav. 59, 290–295 (2011).
Pembrey, M. E. et al. Sex-specific, male-line transgenerational responses in humans. Eur. J. Hum. Genet. 14, 159–166 (2006).
Kaati, G., Bygren, L. O. & Edvinsson, S. Cardiovascular and diabetes mortality determined by nutrition during parents' and grandparents' slow growth period. Eur. J. Hum. Genet. 10, 682–688 (2002).
Pembrey, M., Saffery, R. & Bygren, L. O. Human transgenerational responses to early-life experience: potential impact on development, health and biomedical research. J. Med. Genet. 51, 563–572 (2014). This is a comprehensive review that covers the field of transgenerational epigenetics in relation to human health and disease.
Morgan, C. P. & Bale, T. L. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J. Neurosci. 31, 11748–11755 (2011).
Franklin, T. B. et al. Epigenetic transmission of the impact of early stress across generations. Biol. Psychiatry 68, 408–415 (2010).
King, V. et al. Maternal obesity has little effect on the immediate offspring but impacts on the next generation. Endocrinology 154, 2514–2524 (2013).
Dunn, G. A. & Bale, T. L. Maternal high-fat diet promotes body length increases and insulin insensitivity in second-generation mice. Endocrinology 150, 4999–5009 (2009).
Dunn, G. A. & Bale, T. L. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 152, 2228–2236 (2011).
Jimenez-Chillaron, J. C. et al. Intergenerational transmission of glucose intolerance and obesity by in utero undernutrition in mice. Diabetes 58, 460–468 (2009).
Thamotharan, M. et al. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am. J. Physiol. Endocrinol. Metab. 292, E1270–E1279 (2007).
Burdge, G. C. et al. Dietary protein restriction of pregnant rats in the F0 generation induces altered methylation of hepatic gene promoters in the adult male offspring in the F1 and F2 generations. Br. J. Nutr. 97, 435–439 (2007).
Boney, C. M., Verma, A., Tucker, R. & Vohr, B. R. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 115, e290–e296 (2005).
McCurdy, C. E. et al. Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J. Clin. Invest. 119, 323–335 (2009).
Sullivan, E. L., Smith, M. S. & Grove, K. L. Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 93, 1–8 (2011). This paper examines the sex-specific neurodevelopmental programming and resulting increased anxiety in the non-human primate that follows exposure to a maternal high-fat diet during pregnancy.
Tamashiro, K. L., Terrillion, C. E., Hyun, J., Koenig, J. I. & Moran, T. H. Prenatal stress or high-fat diet increases susceptibility to diet-induced obesity in rat offspring. Diabetes 58, 1116–1125 (2009).
Shiell, A. W., Campbell, D. M., Hall, M. H. & Barker, D. J. Diet in late pregnancy and glucose-insulin metabolism of the offspring 40 years later. BJOG 107, 890–895 (2000).
Dietz, D. M. & Nestler, E. J. From father to offspring: paternal transmission of depressive-like behaviors. Neuropsychopharmacology 37, 311–312 (2012).
Dias, B. G. & Ressler, K. J. Parental olfactory experience influences behavior and neural structure in subsequent generations. Naure Neurosci. 17, 89–96 (2014). This is a comprehensive examination, in adult male mice, of epigenetic changes in germ cells that occur in response to exposure to an odour cue in fear conditioning that is related to reprogramming of offspring brain and behaviour.
Rodgers, A. B., Morgan, C. P., Bronson, S. L., Revello, S. & Bale, T. L. Paternal stress exposure alters sperm microRNA content and reprograms offspring HPA stress axis regulation. J. Neurosci. 33, 9003–9012 (2013). This paper identifies important changes in specific sperm miRNAs produced by chronic stress in adult mice that correlate with programming of offspring stress reactivity.
Ng, S. F. et al. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 467, 963–966 (2010).
Vassoler, F. M., White, S. L., Schmidt, H. D., Sadri-Vakili, G. & Pierce, R. C. Epigenetic inheritance of a cocaine-resistance phenotype. Nature Neurosci. 16, 42–47 (2013).
Carone, B. R. et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 143, 1084–1096 (2010).
Anway, M. D., Cupp, A. S., Uzumcu, M. & Skinner, M. K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005).
Anderson, L. M. et al. Preconceptional fasting of fathers alters serum glucose in offspring of mice. Nutrition 22, 327–331 (2006).
Jenkins, T. G. & Carrell, D. T. The sperm epigenome and potential implications for the developing embryo. Reproduction 143, 727–734 (2012).
van Os, J. & Selten, J. P. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br. J. Psychiatry 172, 324–326 (1998).
Gerardin, P. et al. Depression during pregnancy: is the developmental impact earlier in boys? A prospective case-control study. J. Clin. Psychiatry 72, 378–387 (2010).
Newschaffer, C. J. et al. The epidemiology of autism spectrum disorders. Annu. Rev. Publ. Health 28, 235–258 (2007).
Beversdorf, D. Q. et al. Timing of prenatal stressors and autism. J. Autism Dev. Disord. 35, 471–478 (2005).
Sandman, C. A., Glynn, L. M. & Davis, E. P. Is there a viability–vulnerability tradeoff? Sex differences in fetal programming. J. Psychosomat. Res. 75, 327–335 (2013).
Smith, S. E., Li, J., Garbett, K., Mirnics, K. & Patterson, P. H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 27, 10695–10702 (2007).
Morgan, C. P. & Bale, T. L. Sex differences in microRNA regulation of gene expression: no smoke, just miRs. Biol. Sex Differ. 3, 22 (2012).
Cowell, P. E., Kostianovsky, D. J., Gur, R. C., Turetsky, B. I. & Gur, R. E. Sex differences in neuroanatomical and clinical correlations in schizophrenia. Am. J. Psychiatry 153, 799–805 (1996).
McCarthy, M. M. et al. The epigenetics of sex differences in the brain. J. Neurosci. 29, 12815–12823 (2009).
Nugent, B. M. & McCarthy, M. M. Epigenetic underpinnings of developmental sex differences in the brain. Neuroendocrinology 93, 150–158 (2011).
Westberry, J. M., Trout, A. L. & Wilson, M. E. Epigenetic regulation of estrogen receptor-α gene expression in the mouse cortex during early postnatal development. Endocrinology 151, 731–740 (2010).
Kurian, J. R., Olesen, K. M. & Auger, A. P. Sex differences in epigenetic regulation of the estrogen receptor-α promoter within the developing preoptic area. Endocrinology 151, 2297–2305 (2010).
Murray, E. K., Hien, A., de Vries, G. J. & Forger, N. G. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150, 4241–4247 (2009).
McCarthy, M. M. Molecular aspects of sexual differentiation of the rodent brain. Psychoneuroendocrinology 19, 415–427 (1994).
Bale, T. L. Stress sensitivity and the development of affective disorders. Horm. Behav. 50, 529–533 (2006).
Bale, T. L. Neuroendocrine and immune influences on the CNS: it's a matter of sex. Neuron 64, 13–16 (2009).
Cryan, J. F. & Dinan, T. G. Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nature Rev. Neurosci. 13, 701–712 (2012).
Dinan, T. G. & Cryan, J. F. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology 37, 1369–1378 (2012).
Clarke, G. et al. The microbiome–gut–brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry 18, 666–673 (2013).
Borrelli, E., Nestler, E. J., Allis, C. D. & Sassone-Corsi, P. Decoding the epigenetic language of neuronal plasticity. Neuron 60, 961–974 (2008).
Colvis, C. M. et al. Epigenetic mechanisms and gene networks in the nervous system. J. Neurosci. 25, 10379–10389 (2005).
Tsankova, N., Renthal, W., Kumar, A. & Nestler, E. J. Epigenetic regulation in psychiatric disorders. Nature Rev. Neurosci. 8, 355–367 (2007).
Day, J. J. et al. DNA methylation regulates associative reward learning. Nature Neurosci. 16, 1445–1452 (2013).
Roth, T. L. & Sweatt, J. D. Regulation of chromatin structure in memory formation. Curr. Opin. Neurobiol. 19, 336–342 (2009).
Sweatt, J. D. Experience-dependent epigenetic modifications in the central nervous system. Biol. Psychiatry 65, 191–197 (2009).
Zovkic, I. B., Guzman-Karlsson, M. C. & Sweatt, J. D. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 20, 61–74 (2013).
Zovkic, I. B. & Sweatt, J. D. Epigenetic mechanisms in learned fear: implications for PTSD. Neuropsychopharmacology 38, 77–93 (2013).
Mahgoub, M. & Monteggia, L. M. A role for histone deacetylases in the cellular and behavioral mechanisms underlying learning and memory. Learn. Mem. 21, 564–568 (2014).
Adachi, M. & Monteggia, L. M. Decoding transcriptional repressor complexes in the adult central nervous system. Neuropharmacology 80, 45–52 (2014).
Lester, B. M. et al. Behavioral epigenetics. Ann. NY Acad. Sci. 1226, 14–33 (2011).
Morrison, K. E., Rodgers, A. B., Morgan, C. P. & Bale, T. L. Epigenetic mechanisms in pubertal brain maturation. Neuroscience 264, 17–24 (2014).
Waddington, C. H. Canalization of development and genetic assimilation of acquired characters. Nature 183, 1654–1655 (1959). This paper laid the foundation for the field of epigenetic programming by defining the 'epigenetic landscape'.
Berger, S. L., Kouzarides, T., Shiekhattar, R. & Shilatifard, A. An operational definition of epigenetics. Genes Dev. 23, 781–783 (2009).
Drake, A. J., Walker, B. R. & Seckl, J. R. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 288, R34–R38 (2005).
O'Regan, D., Welberg, L. L., Holmes, M. C. & Seckl, J. R. Glucocorticoid programming of pituitary–adrenal function: mechanisms and physiological consequences. Semin. Neonatol. 6, 319–329 (2001).
Nyirenda, M. J., Welberg, L. A. & Seckl, J. R. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids — fetal effect or maternal influence? J. Endocrinol. 170, 653–660 (2001).
Heller, E. A. et al. Locus-specific epigenetic remodeling controls addiction- and depression-related behaviors. Nature Neurosci. 17, 1720–1727 (2014).
Brunner, A. M., Nanni, P. & Mansuy, I. M. Epigenetic marking of sperm by post-translational modification of histones and protamines. Epigenetics Chromatin 7, 2 (2014).
Bale, T. L. Lifetime stress experience: transgenerational epigenetics and germ cell programming. Dialogues Clin. Neurosci. 16, 297–305 (2014).
Kotaja, N. & Sassone-Corsi, P. The chromatid body: a germ-cell-specific RNA-processing centre. Nature Rev. Mol. Cell Biol. 8, 85–90 (2007).
Acknowledgements
This work was supported by grants from the US National Institutes of Health MH099910, MH104184, MH091258, MH087597 and MH073030.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- Birth cohort studies
-
Longitudinal prospective studies in which pregnancies are followed through birth and into childhood to correlate the risk for given outcomes with specified experiences or exposures during gestation. These cohorts of individuals differ from other epidemiological studies in which the outcome measures are mainly retrospective.
- Biomarkers
-
Specific biochemical, molecular, anatomical or physiological characteristics that are used to predict, measure or indicate the presence or progress of disease or the effects of treatment.
- Executive function
-
A set of cognitive abilities that include inhibition (resisting habits, temptations or distractions), switching (adjusting to change) and working memory (mentally holding and using information).
- Stress responsiveness
-
Behavioural or physiological measures in response to a stress provocation. In all mammals, the physiological hypothalamic–pituitary–adrenal stress axis is the standard measure of stress experience. Behavioural changes in response to stress or threat can also be examined as an indication of stress state.
- Imprinted gene
-
A gene expressed from only one allele in a manner that depends on the parent of origin and that is regulated by DNA methylation.
- Hypothalamic–pituitary–adrenal stress axis
-
(HPA stress axis). The neuroendocrine core of the stress system. Its activation results in the release of corticotropin-releasing factor from the hypothalamus, adrenocorticotropic hormone from the pituitary and cortisol (corticosterone in rats and mice) from the adrenal glands.
- Endophenotypes
-
Quantifiable phenotypes with an assumed intermediate role in the pathway from genes to complex phenotypes. It is thought that the action of an endophenotype is easier to understand biologically and genetically than the action of the complex phenotype of primary interest. They enable the examination of specific aspects of complex human diseases in animal models.
- Barnes maze
-
A spatial learning and memory task in which the animal is placed on a large, open table that has holes around the circumference. Only one of the holes has an escape tunnel, and the animal must learn to use distal cues to identify this hole and escape in the shortest possible time. Learning in this task involves the hippocampus.
- Microglia
-
Glia of mesodermal origin and the resident macrophages of the CNS.
- Social defeat
-
A behavioural model in which rodents (predominantly males) are repeatedly exposed to a more aggressive conspecific. The outcomes of these confrontations are repeated losses for the subject. This exposure is then followed by a period of housing the defeated animal in close proximity to the aggressor to establish the conditioning.
- Pre-pulse inhibition
-
A reduction in the magnitude of the startle reflex that occurs when an organism is presented with a non-startling stimulus (a pre-pulse) before being presented with the startling stimulus. Deficits in pre-pulse inhibition have been observed in patients with schizophrenia.
Rights and permissions
About this article
Cite this article
Bale, T. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci 16, 332–344 (2015). https://doi.org/10.1038/nrn3818
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3818
This article is cited by
-
Plasticity of synapses and reward circuit function in the genesis and treatment of depression
Neuropsychopharmacology (2023)
-
Associations between socioeconomic gradients and racial disparities in preadolescent brain outcomes
Pediatric Research (2023)
-
Interaction of the pre- and postnatal environment in the maternal immune activation model
Discover Mental Health (2023)
-
Should we modulate the neonatal microbiome and what should be the goal?
Microbiome (2022)
-
Cell-type-specific epigenetic effects of early life stress on the brain
Translational Psychiatry (2022)