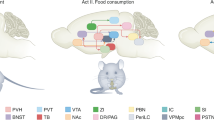
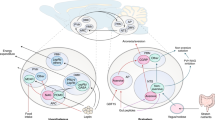
Key Points
-
The energy homeostasis system effectively ensures stability of body fat stores under usual conditions by processing humoral inputs related to both total body energy stores and current nutrient availability and transducing them into neuronal signals that powerfully influence the perception of satiety on the one hand and of food reward on the other.
-
During conditions of negative energy balance (for example, starvation or a calorically restricted diet), homeostatic responses are engaged to promote increased food intake by reducing the satiating while enhancing the rewarding properties of food.
-
Two well-studied leptin-sensing neuronal subpopulations involved in feeding are those that co-express neuropeptide Y, agouti-related protein (AGRP; an antagonist of melanocortin signalling) and GABA, and those that express pro-opiomelanocortin (POMC).
-
Optogenetic and pharmacogenetic (designer receptors exclusively activated by a designer drug) strategies demonstrate that AGRP neuronal activation is sufficient to rapidly and potently stimulate food and increase the motivation to work for food.
-
Conversely, stimulation and inhibition of POMC neurons using either optogenetics or DREADDs in mice reduces and increases food intake, respectively through the melanocortin receptor 4.
-
Neurocircuits exist that are normally inhibited, but when activated in response to emergent or stressful stimuli, can override the homeostatic control of energy balance.
-
In response to the lack of either immediately available fuel (such as hypoglycaemia) or the amount of stored fuel (leptin deficiency), the brain triggers an overlapping set of responses that drive increased food intake.
-
During conditions of stress, trauma and illness, a neurocircuit involving calcitonin gene-related peptide-containing neurons in the parabrachial nucleus is activated that functions as an 'off' switch' for feeding.
-
Improved knowledge of how these emergency-activated circuits interact with the energy homeostasis system is fundamental for understanding the control of food intake and may bear on the pathogenesis of disorders at both ends of the body weight spectrum.
Abstract
Under normal conditions, food intake and energy expenditure are balanced by a homeostatic system that maintains stability of body fat content over time. However, this homeostatic system can be overridden by the activation of 'emergency response circuits' that mediate feeding responses to emergent or stressful stimuli. Inhibition of these circuits is therefore permissive for normal energy homeostasis to occur, and their chronic activation can cause profound, even life-threatening, changes in body fat mass. This Review highlights how the interplay between homeostatic and emergency feeding circuits influences the biologically defended level of body weight under physiological and pathophysiological conditions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Edholm, O. G. Energy balance in man studies carried out by the Division of Human Physiology, National Institute for Medical Research. J. Hum. Nutr. 31, 413–431 (1977).
Edholm, O. G., Fletcher, J. G., Widdowson, E. M. & McCance, R. A. The energy expenditure and food intake of individual men. Br. J. Nutr. 9, 286–300 (1955).
Bray, G. A., Flatt, J. P., Volaufova, J., Delany, J. P. & Champagne, C. M. Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am. J. Clin. Nutr. 88, 1504–1510 (2008).
Schwartz, M. W. et al. Central nervous system control of food intake. Nature 404, 661–671 (2000).
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S. & Schwartz, M. W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006). This comprehensive review provides a new conceptual understanding of how energy homeostasis is achieved.
Saper, C. B., Chou, T. C. & Elmquist, J. K. The need to feed: homeostatic and hedonic control of eating. Neuron 36, 199–211 (2002).
Nguyen, D. M. & El-Serag, H. B. The epidemiology of obesity. Gastroenterol. Clin. North Am. 39, 1–7 (2010).
Braun, T. P. & Marks, D. L. Pathophysiology and treatment of inflammatory anorexia in chronic disease. J. Cachexia Sarcopenia Muscle 1, 135–145 (2010).
Ritter, R. C. & Slusser, P. 5-Thio-D-glucose causes increased feeding and hyperglycemia in the rat. Am. J. Physiol. 238, E141–E144 (1980). This study provides evidence that the hindbrain is important in responding to neuroglucopenia.
Kennedy, G. The role of depot fat in the hypothalamic control of food intake in the rat. Proc. R. Soc. Lond. B 140, 578–592 (1953).
Gao, Q. & Horvath, T. L. Neurobiology of feeding and energy expenditure. Annu. Rev. Neurosci. 30, 367–398 (2007).
Rosenbaum, M. & Leibel, R. L. Adaptive thermogenesis in humans. Int. J. Obes. (Lond.) 34 (Suppl. 1), 47–55 (2010).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This study describes the mapping and sequencing of the gene that encodes the hormone leptin.
Considine, R. V. et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 334, 292–295 (1996).
Schwartz, M. W., Peskind, E., Raskind, M., Boyko, E. J. & Porte, D. Jr. Cerebrospinal fluid leptin levels: relationship to plasma levels and to adiposity in humans. Nature Med. 2, 589–593 (1996).
Baskin, D. G., Breininger, J. F. & Schwartz, M. W. Leptin receptor mRNA identifies a subpopulation of neuropeptide Y neurons activated by fasting in rat hypothalamus. Diabetes 48, 828–833 (1999).
Elmquist, J. K., Bjorbaek, C., Ahima, R. S., Flier, J. S. & Saper, C. B. Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 395, 535–547 (1998).
Campfield, L. A., Smith, F. J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 (1995).
Halaas, J. L. et al. Physiological response to long-term peripheral and central leptin infusion in lean and obese mice. Proc. Natl Acad. Sci. USA 94, 8878–8883 (1997).
Lee, G.-H. et al. Abnormal splicing of the leptin receptor in diabetic mice. Nature 379, 632–635 (1996).
Bagdade, J. D., Bierman, E. L. & Porte, D. Jr. The significance of basal insulin levels in the evaluation of the insulin response to glucose in diabetic and nondiabetic subjects. J. Clin. Invest. 46, 1549–1557 (1967).
Woods, S. C., Lotter, E. C., McKay, L. D. & Porte, D. Jr. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282, 503–505 (1979). An early study demonstrating that insulin is an adiposity signal that acts in the brain of non-human primates to regulate food intake and body weight.
Bruning, J. C. et al. Role of brain insulin receptor in control of body weight and reproduction. Science 289, 2122–2125 (2000).
Batterham, R. L. et al. Gut hormone PYY3–36 physiologically inhibits food intake. Nature 418, 650–654 (2002).
Turton, M. D. et al. A role for glucagon-like peptide-1 in the central regulation of feeding. Nature 379, 69–72 (1996).
Gibbs, J., Young, R. C. & Smith, G. P. Cholecystokinin decreases food intake in rats. J. Comp. Physiol. Psychol. 84, 488–495 (1973).
Tschop, M., Smiley, D. L. & Heiman, M. L. Ghrelin induces adiposity in rodents. Nature 407, 908–913 (2000).
Lam, T. K. Neuronal regulation of homeostasis by nutrient sensing. Nature Med. 16, 392–395 (2010).
Strubbe, J. H. & Woods, S. C. The timing of meals. Psychol. Rev. 111, 128–141 (2004).
West, D. B., Fey, D. & Woods, S. C. Cholecystokinin persistently suppresses meal size but not food intake in free-feeding rats. Am. J. Physiol. 246, R776–R787 (1984).
McMinn, J. E., Sindelar, D. K., Havel, P. J. & Schwartz, M. W. Leptin deficiency induced by fasting impairs the satiety response to cholecystokinin. Endocrinology 141, 4442–4448 (2000).
Barrachina, M. D., Martinez, V., Wang, L., Wei, J. Y. & Tache, Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc. Natl Acad. Sci. USA 94, 10455–10460 (1997).
Figlewicz, D. P., Stein, L. J., West, D., Porte, D. Jr & Woods, S. C. Intracisternal insulin alters sensitivity to CCK-induced meal suppression in baboons. Am. J. Physiol. 250, R856–R860 (1986).
Emond, M., Schwartz, G. J., Ladenheim, E. E. & Moran, T. H. Central leptin modulates behavioral and neural responsivity to CCK. Am. J. Physiol. 276, R1545–R1549 (1999).
Williams, D. L., Baskin, D. G. & Schwartz, M. W. Evidence that intestinal glucagon-like peptide-1 plays a physiological role in satiety. Endocrinology 150, 1680–1687 (2009).
Hulsey, M. G., Lu, H., Wang, T., Martin, R. J. & Baile, C. A. Intracerebroventricular (i.c.v.) administration of mouse leptin in rats: behavioral specificity and effects on meal patterns. Physiol. Behav. 65, 445–455 (1998).
Kahler, A. et al. Chronic administration of OB protein decreases food intake by selectively reducing meal size in male rats. Am. J. Physiol. 275, R180–R185 (1998).
McLaughlin, C. L. & Baile, C. A. Decreased sensitivity of Zucker obese rats to the putative satiety agent cholecystokinin. Physiol. Behav. 25, 543–548 (1980).
Morton, G. J. et al. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell. Metab. 2, 411–420 (2005).
Grill, H. J. et al. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology 143, 239–246 (2002).
Morton, G. J. et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J. Clin. Invest. 115, 703–710 (2005).
Barrera, J. G., Sandoval, D. A., D'Alessio, D. A. & Seeley, R. J. GLP-1 and energy balance: an integrated model of short-term and long-term control. Nature Rev. Endocrinol. 7, 507–516 (2011).
Berthoud, H. R. Homeostatic and non-homeostatic pathways involved in the control of food intake and energy balance. Obesity, 14, (Suppl. 5), 197S–200S (2006).
Figlewicz, D. P. Adiposity signals and food reward: expanding the CNS roles of insulin and leptin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 284, R882–R892 (2003). This is one of the first reviews to describe the role of the adiposity hormones in the regulation of the reward system.
Dossat, A. M., Lilly, N., Kay, K. & Williams, D. L. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. J. Neurosci. 31, 14453–14457 (2011).
Mehta, S. et al. Regional brain response to visual food cues is a marker of satiety that predicts food choice. Am. J. Clin. Nutr. 96, 989–999 (2012).
Nijs, I. M., Muris, P., Euser, A. S. & Franken, I. H. Differences in attention to food and food intake between overweight/obese and normal-weight females under conditions of hunger and satiety. Appetite 54, 243–254 (2010).
Figlewicz, D. P. & Sipols, A. J. Energy regulatory signals and food reward. Pharmacol. Biochem. Behav. 97, 15–24 (2010).
Fulton, S., Woodside, B. & Shizgal, P. Modulation of brain reward circuitry by leptin. Science 287, 125–128 (2000).
Farooqi, I. S. et al. Leptin regulates striatal regions and human eating behavior. Science 317, 1355 (2007).
Berthoud, H. R., Lenard, N. R. & Shin, A. C. Food reward, hyperphagia, and obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1266–R1277 (2011).
Stice, E., Figlewicz, D. P., Gosnell, B. A., Levine, A. S. & Pratt, W. E. The contribution of brain reward circuits to the obesity epidemic. Neurosci. Biobehav. Rev. 37, 2047–2058 (2012).
Kelley, A. E., Baldo, B. A., Pratt, W. E. & Will, M. J. Corticostriatal-hypothalamic circuitry and food motivation: integration of energy, action and reward. Physiol. Behav. 86, 773–795 (2005).
Leinninger, G. M. et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell. Metab. 10, 89–98 (2009).
Hahn, T. M., Breininger, J. F., Baskin, D. G. & Schwartz, M. W. Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nature Neurosci. 1, 271–272 (1998).
Hagan, M. M. et al. Long-term orexigenic effects of AgRP-(83—132) involve mechanisms other than melanocortin receptor blockade. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R47–R52 (2000).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001). A groundbreaking study identifying POMC neurons in the ARC as being leptin-responsive and under GABA inhibition.
Spanswick, D., Smith, M. A., Groppi, V. E., Logan, S. D. & Ashford, M. L. Leptin inhibits hypothalamic neurons by activation of ATP-sensitive potassium channels. Nature 390, 521–525 (1997).
Spanswick, D., Smith, M. A., Mirshamsi, S., Routh, V. H. & Ashford, M. L. Insulin activates ATP-sensitive K+ channels in hypothalamic neurons of lean, but not obese rats. Nature Neurosci. 3, 757–758 (2000).
Cowley, M. A. et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 37, 649–661 (2003).
Schwartz, M. W. et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45, 531–535 (1996).
Schwartz, M. W. et al. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46, 2119–2123 (1997).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997). This paper was one of the first to identify the melanocortin system and the MC4R in the control of feeding behaviour.
Williams, K. W. et al. Segregation of acute leptin and insulin effects in distinct populations of arcuate proopiomelanocortin neurons. J. Neurosci. 30, 2472–2479 (2010).
Hill, J. W. et al. Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell. Metab. 11, 286–297 (2010).
Sohn, J. W. et al. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron 71, 488–497 (2011).
Sohn, J. W., Elmquist, J. K. & Williams, K. W. Neuronal circuits that regulate feeding behavior and metabolism. Trends Neurosci. 36, 504–512 (2013).
Gautron, L. & Elmquist, J. K. Sixteen years and counting: an update on leptin in energy balance. J. Clin. Invest. 121, 2087–2093 (2011).
van de Wall, E. et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149, 1773–1785 (2008).
Ring, L. E. & Zeltser, L. M. Disruption of hypothalamic leptin signaling in mice leads to early-onset obesity, but physiological adaptations in mature animals stabilize adiposity levels. J. Clin. Invest. 120, 2931–2941 (2010).
Dhillon, H. et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006).
Fulton, S. et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51, 811–822 (2006).
Hommel, J. D. et al. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 51, 801–810 (2006).
Hayes, M. R. et al. Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell. Metab. 11, 77–83 (2010).
Vong, L. et al. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron 71, 142–154 (2011).
Balthasar, N. et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42, 983–991 (2004).
Leshan, R. L., Greenwald-Yarnell, M., Patterson, C. M., Gonzalez, I. E. & Myers, M. G. Jr. Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nature Med. 18, 820–823 (2012).
Tong, Q., Ye, C. P., Jones, J. E., Elmquist, J. K. & Lowell, B. B. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature Neurosci. 11, 998–1000 (2008).
Wu, Q., Boyle, M. P. & Palmiter, R. D. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell 137, 1225–1234 (2009).
Wu, Q., Clark, M. S. & Palmiter, R. D. Deciphering a neuronal circuit that mediates appetite. Nature 483, 594–597 (2012). An excellent paper identifying the PBN as an integration centre of visceral toxicity via hindbrain glutamatergic signals and GABAergic homeostatic feeding circuits.
Carter, M. E., Soden, M. E., Zweifel, L. S. & Palmiter, R. D. Genetic identification of a neural circuit that suppresses appetite. Nature 503, 111–114 (2013). In this paper, the authors identify a neural circuit that suppresses appetite.
Kramer, R. H., Mourot, A. & Adesnik, H. Optogenetic pharmacology for control of native neuronal signaling proteins. Nature Neurosci. 16, 816–823 (2013).
Packer, A. M., Roska, B. & Hausser, M. Targeting neurons and photons for optogenetics. Nature Neurosci. 16, 805–815 (2013).
Aponte, Y., Atasoy, D. & Sternson, S. M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature Neurosci. 14, 351–355 (2011). The first use of optogenetics in the field of energy balance, examining the effect of AGRP neuronal activation on feeding.
Atasoy, D., Betley, J. N., Su, H. H. & Sternson, S. M. Deconstruction of a neural circuit for hunger. Nature 488, 172–177 (2012).
Krashes, M. J. et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 121, 1424–1428 (2011). Another pioneering study using DREADD technology to manipulate neurons involved in energy homeostasis.
White, J. D. & Kershaw, M. Increased hypothalamic neuropeptide Y expression following food deprivation. Mol. Cell Neurosci. 1, 41–48 (1990).
Sipols, A. J., Baskin, D. G. & Schwartz, M. W. Effect of intracerebroventricular insulin infusion on diabetic hyperphagia and hypothalamic neuropeptide gene expression. Diabetes 44, 147–151 (1995).
Wilding, J. P. et al. Increased neuropeptide-Y messenger ribonucleic acid (mRNA) and decreased neurotensin mRNA in the hypothalamus of the obese (ob/ob) mouse. Endocrinology 132, 1939–1944 (1993).
Krashes, M. J., Shah, B. P., Koda, S. & Lowell, B. B. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell. Metab. 18, 588–595 (2013).
Leibowitz, S. F., Hammer, N. J. & Chang, K. Hypothalamic paraventricular nucleus lesions produce overeating and obesity in the rat. Physiol. Behav. 27, 1031–1040 (1981).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Vaisse, C., Clement, K., Guy Grand, B. & Froguel, P. A frameshift mutation in human MC4R is associated with a dominant form of obesity. Nature Genet. 20, 113–114 (1998).
Yeo, G. S. et al. A frameshift mutation in MC4R associated with dominantly inherited human obesity. Nature Genet. 20, 111–112 (1998).
Farooqi, I. S. et al. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N. Engl. J. Med. 348, 1085–1095 (2003).
Mountjoy, K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, 1298–1308 (1994).
Cowley, M. A. et al. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24, 155–163 (1999).
Giraudo, S. Q., Billington, C. J. & Levine, A. S. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 809, 302–306 (1998).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Rossi, J. et al. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell. Metab. 13, 195–204 (2011).
Arletti, R., Benelli, A. & Bertolini, A. Influence of oxytocin on feeding behavior in the rat. Peptides 10, 89–93 (1989).
Arletti, R., Benelli, A. & Bertolini, A. Oxytocin inhibits food and fluid intake in rats. Physiol. Behav. 48, 825–830 (1990).
Olson, B. R. et al. Oxytocin and an oxytocin agonist administered centrally decrease food intake in rats. Peptides 12, 113–118 (1991).
Morton, G. J. et al. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am. J. Physiol. Endocrinol. Metab. 302, E134–E144 (2012).
Blevins, J. E. & Ho, J. M. Role of oxytocin signaling in the regulation of body weight. Rev. Endocr. Metab. Disord. 14, 311–329 (2013).
Blevins, J. E., Eakin, T. J., Murphy, J. A., Schwartz, M. W. & Baskin, D. G. Oxytocin innervation of caudal brainstem nuclei activated by cholecystokinin. Brain Res. 993, 30–41 (2003).
Blevins, J. E., Schwartz, M. W. & Baskin, D. G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brainstem nuclei controlling meal size. Am. J. Physiol. Regul. Integr. Comp. Physiol. 287, R87–R96 (2004).
Kublaoui, B. M., Gemelli, T., Tolson, K. P., Wang, Y. & Zinn, A. R. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol. Endocrinol. 22, 1723–1734 (2008).
Bonnefond, A. et al. Loss-of-function mutations in SIM1 contribute to obesity and Prader–Willi-like features. J. Clin. Invest. 123, 3037–3041 (2013).
Holder, J. L. Jr, Butte, N. F. & Zinn, A. R. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum. Mol. Genet. 9, 101–108 (2000).
Ramachandrappa, S. et al. Rare variants in single-minded 1 (SIM1) are associated with severe obesity. J. Clin. Invest. 123, 3042–3050 (2013).
Swaab, D. F., Purba, J. S. & Hofman, M. A. Alterations in the hypothalamic paraventricular nucleus and its oxytocin neurons (putative satiety cells) in Prader–Willi syndrome: a study of five cases. J. Clin. Endocrinol. Metab. 80, 573–579 (1995).
Camerino, C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity 17, 980–984 (2009).
Takayanagi, Y. et al. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 19, 951–955 (2008).
Beall, C., Ashford, M. L. & McCrimmon, R. J. The physiology and pathophysiology of the neural control of the counterregulatory response. Am. J. Physiol. Regul. Integr. Comp. Physiol. 302, R215–R223 (2012).
Gerich, J. E. et al. Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N. Engl. J. Med. 292, 985–989 (1975).
Garber, A. J. et al. The role of adrenergic mechanisms in the substrate and hormonal response to insulin-induced hypoglycemia in man. J. Clin. Invest. 58, 7–15 (1976).
Rizza, R. A., Cryer, P. E. & Gerich, J. E. Role of glucagon, catecholamines, and growth hormone in human glucose counterregulation. Effects of somatostatin and combined α- and β-adrenergic blockade on plasma glucose recovery and glucose flux rates after insulin-induced hypoglycemia. J. Clin. Invest. 64, 62–71 (1979).
Ritter, S., Li, A. J., Wang, Q. & Dinh, T. T. Minireview: the value of looking backward: the essential role of the hindbrain in counterregulatory responses to glucose deficit. Endocrinology 152, 4019–4032 (2011).
McCrimmon, R. J. & Sherwin, R. S. Hypoglycemia in type 1 diabetes. Diabetes 59, 2333–2339 (2010).
Routh, V. H. Glucose-sensing neurons: are they physiologically relevant? Physiol. Behav. 76, 403–413 (2002).
Watts, A. G. & Donovan, C. M. Sweet talk in the brain: glucosensing, neural networks, and hypoglycemic counterregulation. Front. Neuroendocrinol. 31, 32–43 (2010).
Dryden, S., Pickavance, L., Henderson, L. & Williams, G. Hyperphagia induced by hypoglycemia in rats is independent of leptin and hypothalamic neuropeptide Y. (NPY). Peptides 19, 1549–1555 (1998).
Russell-Jones, D. & Khan, R. Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes. Metab. 9, 799–812 (2007).
Sindelar, D. K. et al. Neuropeptide Y is required for hyperphagic feeding in response to neuroglucopenia. Endocrinology 145, 3363–3368 (2004).
Sergeyev, V., Broberger, C., Gorbatyuk, O. & Hokfelt, T. Effect of 2-mercaptoacetate and 2-deoxy-D-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport 11, 117–121 (2000).
Luquet, S., Phillips, C. T. & Palmiter, R. D. NPY/AgRP neurons are not essential for feeding responses to glucoprivation. Peptides 28, 214–225 (2007).
Corrin, S. E., McCarthy, H. D., McKibbin, P. E. & Williams, G. Unchanged hypothalamic neuropeptide Y concentrations in hyperphagic, hypoglycemic rats: evidence for specific metabolic regulation of hypothalamic NPY. Peptides 12, 425–430 (1991).
Ritter, S., Bugarith, K. & Dinh, T. T. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J. Comp. Neurol. 432, 197–216 (2001).
Havel, P. J. et al. Marked and rapid decreases of circulating leptin in streptozotocin diabetic rats: reversal by insulin. Am. J. Physiol. 274, R1482–R1491 (1998).
American Diabetes Association. Standards of medical care in diabetes—2011. Diabetes Care 34 (Suppl. 1), 11–61 (2011).
Kumaresan, P. & Turner, C. W. Effect of alloxan on feed consumption and on replacement therapy with graded levels of insulin in rats. Proc. Soc. Exp. Biol. Med. 122, 526–527 (1966).
Ahima, R. S. et al. Role of leptin in the neuroendocrince response to fasting. Nature 382, 250–252 (1996).
Bray, G. A., Fisler, J. & York, D. A. Neuroendocrine control of the development of obesity: understanding gained from studies of experimental animal models. Front. Neuroendocrinol. 11, 128–181 (1990).
Fujikawa, T., Chuang, J. C., Sakata, I., Ramadori, G. & Coppari, R. Leptin therapy improves insulin-deficient type 1 diabetes by CNS-dependent mechanisms in mice. Proc. Natl Acad. Sci. USA 107, 17391–17396 (2010).
German, J. P. et al. Leptin activates a novel CNS mechanism for insulin-independent normalization of severe diabetic hyperglycemia. Endocrinology 152, 394–404 (2011).
Hidaka, S. et al. Chronic central leptin infusion restores hyperglycemia independent of food intake and insulin level in streptozotocin-induced diabetic rats. FASEB J. 16, 509–518 (2002).
Lin, C. Y., Higginbotham, D. A., Judd, R. L. & White, B. D. Central leptin increases insulin sensitivity in streptozotocin-induced diabetic rats. Am. J. Physiol. Endocrinol. Metab. 282, E1084–E1091 (2002).
Peters, A. et al. The selfish brain: competition for energy resources. Neurosci. Biobehav. Rev. 28, 143–180 (2004).
Schwartz, M. W. & Niswender, K. D. Adiposity signaling and biological defense against weight gain: absence of protection or central hormone resistance? J. Clin. Endocrinol. Metab. 89, 5889–5897 (2004).
Ryan, K. K., Woods, S. C. & Seeley, R. J. Central nervous system mechanisms linking the consumption of palatable high-fat diets to the defense of greater adiposity. Cell. Metab. 15, 137–149 (2012).
Knowler, W. C. et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet 374, 1677–1686 (2009).
Safer, D. J. Diet, behavior modification, and exercise: a review of obesity treatments from a long-term perspective. South Med. J. 84, 1470–1474 (1991).
Hagan, M. M. et al. Role of the CNS melanocortin system in the response to overfeeding. J. Neurosci. 19, 2362–2367 (1999).
Sims, E. A. et al. Experimental obesity in man. Trans. Assoc. Am. Physicians 81, 153–170 (1968).
Deriaz, O., Fournier, G., Tremblay, A., Despres, J. P. & Bouchard, C. Lean-body-mass composition and resting energy expenditure before and after long-term overfeeding. Am. J. Clin. Nutr. 56, 840–847 (1992).
Diaz, E. O., Prentice, A. M., Goldberg, G. R., Murgatroyd, P. R. & Coward, W. A. Metabolic response to experimental overfeeding in lean and overweight healthy volunteers. Am. J. Clin. Nutr. 56, 641–655 (1992).
Leibel, R. L., Rosenbaum, M. & Hirsch, J. Changes in energy expenditure resulting from altered body weight. N. Engl. J. Med. 332, 621–628 (1995).
Sims, E. A. et al. Endocrine and metabolic effects of experimental obesity in man. Recent Prog. Horm. Res. 29, 457–496 (1973).
Farooqi, I. S. et al. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N. Engl. J. Med. 341, 879–884 (1999). A seminal study showing leptin replacement can correct metabolic and endocrine abnormalities in humans with congenital leptin deficiency.
El-Haschimi, K., Pierroz, D. D., Hileman, S. M., Bjorbaek, C. & Flier, J. S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Invest. 105, 1827–1832 (2000).
Heymsfield, S. B. et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA 282, 1568–1575 (1999).
Myers, M. G. Jr., Leibel, R. L., Seeley, R. J. & Schwartz, M. W. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol. Metab. 21, 643–651 (2010).
Xu, H. et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J. Clin. Invest. 112, 1821–1830 (2003).
Weisberg, S. P. et al. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 (2003).
Hotamisligil, G. S. Inflammation and metabolic disorders. Nature 444, 860–867 (2006).
Posey, K. et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 296, E1003–E1012 (2008).
De Souza, C. T. et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199 (2005).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012). A notable study postulating that neuronal injury due to high-fat feeding underlies the development of obesity.
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
Thaler, J. P., Guyenet, S. J., Dorfman, M. D., Wisse, B. E. & Schwartz, M. W. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes 62, 2629–2634 (2013).
Reilly, S. The parabrachial nucleus and conditioned taste aversion. Brain Res. Bull. 48, 239–254 (1999).
Reilly, S. & Trifunovic, R. Lateral parabrachial nucleus lesions in the rat: aversive and appetitive gustatory conditioning. Brain Res. Bull. 52, 269–278 (2000).
Reilly, S. & Trifunovic, R. Lateral parabrachial nucleus lesions in the rat: neophobia and conditioned taste aversion. Brain Res. Bull. 55, 359–366 (2001).
Grossberg, A. J., Scarlett, J. M. & Marks, D. L. Hypothalamic mechanisms in cachexia. Physiol. Behav. 100, 478–489 (2010).
Stefater, M. A., Wilson-Perez, H. E., Chambers, A. P., Sandoval, D. A. & Seeley, R. J. All bariatric surgeries are not created equal: insights from mechanistic comparisons. Endocr. Rev. 33, 595–622 (2012).
Berglund, E. D. et al. Serotonin 2C receptors in pro-opiomelanocortin neurons regulate energy and glucose homeostasis. J. Clin. Invest. 123, 5061–5070 (2013).
Smith, S. R. et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. N. Engl. J. Med. 363, 245–256 (2010).
Gadde, K. M. et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 377, 1341–1352 (2011).
Marx, J. Cellular warriors at the battle of the bulge. Science 299, 846–849 (2003).
Acknowledgements
This work was supported by a US National Institute of Diabetes and Digestive and Kidney Diseases Grant to M.W.S (DK090320, DK083042, and DK052989) and G.J.M. (DK089053), the US National Institutes of Health (NIH) funded Nutrition Obesity Research Center (DK035816) and Diabetes Research Center (DK17047) at the University of Washington and a NIH Diabetes and Metabolism training Grant (F32 DK097859; T32 DK0007247).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Energy homeostasis
-
The biological process by which the body maintains body fat stores by balancing energy intake with energy expenditure over time.
- Anorexia
-
A disorder that is characterized by a reduction in energy intake and accompanied weight loss.
- Adiposity negative feedback signals
-
Hormones that circulate in direct proportion to body fat and convey the state of total energy stores to the CNS.
- Satiety
-
The state of feeling full to the point of satisfaction after the consumption of food.
- Neuropeptide
-
A small protein-like molecule that is used by neurons to communicate with each other, often in a paracrine manner.
- Optogenetics
-
A technique that uses light to control the activity of specific neurons in living tissue.
- DREADD
-
(Designer receptor exclusively activated by a designer drug). G protein-coupled receptors that are modified for activation by binding to inert small molecules that are used to non-invasively control neuronal signalling.
- Neurotransmitters
-
Chemical messengers that are released by the end of a nerve fibre, causing an impulse to be passed from once cell to another.
- Leptin resistance
-
A state in which the body is no longer responsive to the anorexic effect of exogenous leptin.
- Conditioned taste aversion
-
(CTA). A learned response of an animal to avoid repeated ingestion of certain foods that cause nausea or sickness.
- Cachexia
-
A condition that is characterized by anorexia, weight loss and disproportionate wasting of muscle and adipose tissue.
Rights and permissions
About this article
Cite this article
Morton, G., Meek, T. & Schwartz, M. Neurobiology of food intake in health and disease. Nat Rev Neurosci 15, 367–378 (2014). https://doi.org/10.1038/nrn3745
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3745
This article is cited by
-
Emerging Methods for the Evaluation of Sensory Quality of Food: Technology at Service
Current Food Science and Technology Reports (2024)
-
Association between body mass index (BMI) and [123I]Ioflupane (DaTSCAN) availabilities in patients with parkinsonism using single-photon emission computed tomography–computed tomography (SPECT-CT)
European Journal of Hybrid Imaging (2023)
-
Obese-associated gut microbes and derived phenolic metabolite as mediators of excessive motivation for food reward
Microbiome (2023)
-
Neurocomputational mechanisms of food and physical activity decision-making in male adolescents
Scientific Reports (2023)
-
Programming of metabolism by adipokines during development
Nature Reviews Endocrinology (2023)