Key Points
-
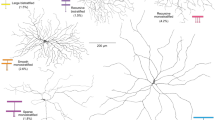
Direction-selective retinal ganglions cells (DSGCs) consist of several distinct types that branch at different levels in the inner retina. Most types are comprised of multiple subtypes, each of which responds to image motion in a different preferred direction. Recent studies have identified specific molecular markers that are expressed either endogenously or transgenically by particular subtypes of DSGC.
-
In most cases, the key player in the generation of direction selectivity in the retina is the starburst amacrine cell (SAC), which is a morphologically symmetrical interneuron that contains both GABA and acetylcholine (ACh). The release of GABA from individual distal dendrites of SACs is itself direction selective, owing to the sequential activation of excitatory inputs along the dendrite together with intrinsic nonlinearities in the SAC; inhibitory interactions between overlapping SACs also seem to have a role.
-
The directional output from individual dendrites is preserved because dendrites on different sides of the SAC make selective inhibitory synapses on different subtypes of DSGCs, thus establishing their preferred direction. The centrifugal separation of input and output synapses along the SAC dendrites provides the fundamental spatial asymmetry that underlies the generation of direction selectivity in the retina.
-
The asymmetric GABAergic inhibition from SACs interacts with symmetric cholinergic excitation from SACs and glutamatergic excitation from bipolar cells within local regions of the DSGC's dendritic tree. The summed inputs within each of these functional subunits are locally thresholded, producing dendritic spikes that propagate to the soma independently of the activity in other subunits.
-
The development of the different subtypes of DSGCs and their selective connectivity with the SACs seems to be mainly governed by intrinsic mechanisms, with visual stimulation and spontaneous neuronal activity playing negligible roles.
Abstract
Visual information is processed in the retina to a remarkable degree before it is transmitted to higher visual centres. Several types of retinal ganglion cells (the output neurons of the retina) respond preferentially to image motion in a particular direction, and each type of direction-selective ganglion cell (DSGC) is comprised of multiple subtypes with different preferred directions. The direction selectivity of the cells is generated by diverse mechanisms operating within microcircuits that rely on independent neuronal processing in individual dendrites of both the DSGCs and the presynaptic neurons that innervate them.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
10 February 2012
In the reference list of this article, the text underneath reference 86 should have been placed under reference 87 and should have referred to reference 79, rather than reference 78. This has now been corrected in the online pdf.
References
Barlow, H. B. & Hill, R. M. Selective sensitivity to direction of movement in ganglion cells of the rabbit retina. Science 139, 412–414 (1963).
Barlow, H. B., Hill, R. M. & Levick, W. R. Retinal ganglion cells responding selectively to direction and speed of image motion in the rabbit. J. Physiol. 173, 377–407 (1964).
Hubel, D. H. & Wiesel, T. N. Receptive fields of single neurones in the cat's striate cortex. J. Physiol. 148, 574–591 (1959).
Cajal, S. R. La rétine des vertébrés. La Cellule 9, 119–257 (1893) (in Spanish).
Wässle, H. Parallel processing in the mammalian retina. Nature Rev. Neurosci. 5, 747–757 (2004).
Masland, R. H. & Martin, P. R. The unsolved mystery of vision. Curr. Biol. 17, R577–R582 (2007).
Kuffler, S. W. Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 16, 37–68 (1953).
Demb, J. B. Cellular mechanisms for direction selectivity in the retina. Neuron 55, 179–186 (2007).
Barlow, H. B. & Levick, W. R. The mechanism of directionally selective units in rabbit's retina. J. Physiol. 178, 477–504 (1965). This extracellular recording study showed that null-direction inhibition is the key mechanism underlying the generation of direction selectivity in DSGCs.
Reichardt, W. in Sensory Communication (ed. Rosenblith, W.) 303–317 (John Wiley, New York, 1961).
Wyatt, H. J. & Day, N. W. Specific effects of neurotransmitter antagonists on ganglion cells in rabbit retina. Science 191, 204–205 (1976).
Caldwell, J. H., Daw, N. W. & Wyatt, H. J. Effects of picrotoxin and strychnine on rabbit retinal ganglion cells: lateral interactions for cells with more complex receptive fields. J. Physiol. 276, 277–298 (1978).
Ariel, M. & Daw, N. W. Pharmacological analysis of directionally sensitive rabbit retinal ganglion cells. J. Physiol. 324, 161–185 (1982).
Kittila, C. A. & Massey, S. C. Pharmacology of directionally selective ganglion cells in the rabbit retina. J. Neurophysiol. 77, 675–689 (1997).
Massey, S. C., Linn. D. M., Kittila, C. A. & Mirza, W. Contributions of GABAA receptors and GABAC receptors to acetylcholine release and directional selectivity in the rabbit retina. Vis. Neurosci. 14, 939–948 (1997).
He, S. & Masland, R. H. Retinal direction selectivity after targeted laser ablation of starburst amacrine cells. Nature 389, 378–382 (1997).
Oyster, C. W. The analysis of image motion by the rabbit retina. J. Physiol. 199, 613–635 (1968).
Wyatt, H. J. & Daw, N. W. Directionally sensitive ganglion cells in the rabbit retina: specificity for stimulus direction, size, and speed. J. Neurophysiol. 38, 613–626 (1975).
Ölveczky, B. P., Baccus, S. A. & Meister, M. Segregation of object and background motion in the retina. Nature 423, 401–408 (2003).
Simpson, J. I. The accessory optic system. Annu. Rev. Neurosci. 7, 13–41 (1984).
Vaney, D. I., He, S., Taylor, W. R. & Levick, W. R. in Motion Vision: Computational, Neural, and Ecological Constraints (eds Zanker, J. M. & Zeil, J.) 13–56 (Springer, Berlin, 2001).
Kim, I. J., Zhang, Y., Yamagata, M., Meister, M. & Sanes, J. R. Molecular identification of a retinal cell type that responds to upward motion. Nature 452, 478–482 (2008). This study characterized a novel type of DSGC, named the J-RGC, that stratifies in the Off sublamina of the IPL.
Oyster, C. W. & Barlow, H. B. Direction-selective units in rabbit retina: distribution of preferred directions. Science 155, 841–842 (1967).
Elstrott, J. et al. Direction selectivity in the retina is established independent of visual experience and cholinergic retinal waves. Neuron 58, 499–506 (2008).
Briggman, K. L., Helmstaedter, M. & Denk, W. Wiring specificity in the direction-selectivity circuit of the retina. Nature 471, 183–188 (2011). By combining two-photon population calcium imaging of RGCs with serial block-face scanning electron microscopy and neuronal reconstruction, this study showed that DSGCs receive numerous putative synapses from null-side SACs but very few from preferred-side SACs.
Sun, L., Han, X. & He, S. Direction-selective circuitry in rat retina develops independently of GABAergic, cholinergic and action potential activity. PLoS ONE 6, e19477 (2011).
Amthor, F. R., Oyster, C. W. & Takahashi, E. S. Morphology of on-off direction-selective ganglion cells in the rabbit retina. Brain Res. 298, 187–190 (1984).
Oyster, C. W., Amthor, F. R. & Takahashi, E. S. Dendritic architecture of ON-OFF direction-selective ganglion cells in the rabbit retina. Vision Res. 33, 579–608 (1993).
Yang, G. & Masland, R. H. Receptive fields and dendritic structure of directionally selective retinal ganglion cells. J. Neurosci. 14, 5267–5280 (1994).
Weng, S., Sun, W. & He, S. Identification of ON-OFF direction-selective ganglion cells in the mouse retina. J. Physiol. 562, 915–923 (2005).
Famiglietti, E. V. Dendritic co-stratification of ON and ON-OFF directionally selective ganglion cells with starburst amacrine cells in rabbit retina. J. Comp. Neurol. 324, 322–335 (1992).
Vaney, D. I. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci. Lett. 125, 187–190 (1991).
Vaney, D. I. Territorial organization of direction-selective ganglion cells in rabbit retina. J. Neurosci. 14, 6301–6316 (1994).
DeBoer, D. J. & Vaney, D. I. Gap-junction communication between subtypes of direction-selective ganglion cells in the developing retina. J. Comp. Neurol. 482, 85–93 (2005).
Kanjhan, R. & Vaney, D. I. Semi-loose seal Neurobiotin electroporation for combined structural and functional analysis of neurons. Pflügers Arch. 457, 561–568 (2008).
Amthor, F. R. & Oyster, C. W. Spatial organization of retinal information about the direction of image motion. Proc. Natl Acad. Sci. USA 92, 4002–4005 (1995).
Yonehara, K. et al. Expression of SPIG1 reveals development of a retinal ganglion cell subtype projecting to the medial terminal nucleus in the mouse. PLoS ONE 3, e1533 (2008).
Yonehara, K. et al. Identification of retinal ganglion cells and their projections involved in central transmission of information about upward and downward image motion. PLoS ONE 4, e4320 (2009). References 37 and 38 selectively labelled the superior-preferring On DSGCs by transgenic methods and showed that this subtype and the inferior-preferring subtype provide most of the retinal input to the MTN.
Huberman, A. D. et al. Genetic identification of an On-Off direction-selective retinal ganglion cell subtype reveals a layer-specific subcortical map of posterior motion. Neuron 62, 327–334 (2009). References 39 to 42 showed that individual subtypes of On–Off DSGCs can be selectively labelled by transgenic methods. This has enabled DSGCs with a known preferred direction to be efficiently targeted for physiological recording, even before they are visually responsive.
Rivlin-Etzion, M. et al. Transgenic mice reveal unexpected diversity of on-off direction-selective retinal ganglion cell subtypes and brain structures involved in motion processing. J. Neurosci. 31, 8760–8769 (2011). This study provided evidence that the posterior-preferring DSGCs comprise two subtypes.
Kay, J. N. et al. Retinal ganglion cells with distinct directional preferences differ in molecular identity, structure, and central projections. J. Neurosci. 31, 7753–7762 (2011). This study showed that each subtype of DSGC endogenously expresses a different complement of genes.
Trenholm, S., Johnson, K., Li, X., Smith, R. G. & Awatramani, G. B. Parallel mechanisms encode direction in the retina. Neuron 71, 683–694 (2011). This study on mouse retina showed that DSGCs that have an asymmetric morphology still give direction-selective responses when the GABAergic and nicotinic inputs are blocked.
Williams, R. W., Strom, R. C., Rice, D. S. & Goldowitz, D. Genetic and environmental control of variation in retinal ganglion cell number in mice. J. Neurosci. 16, 7193–7205 (1996).
Jeon, C. J., Strettoi, E. & Masland, R. H. The major cell populations of the mouse retina. J. Neurosci. 18, 8936–8946 (1998).
Erkman, L. et al. A POU domain transcription factor-dependent program regulates axon pathfinding in the vertebrate visual system. Neuron 28, 779–792 (2000).
Levick, W. R., Oyster, C. W. & Takahashi, E. Rabbit lateral geniculate nucleus: sharpener of directional information. Science 165, 712–714 (1969).
Stewart, D. L., Chow, K. L. & Masland, R. H. Receptive-field characteristics of lateral geniculate neurons in the rabbit. J. Neurophysiol. 34, 139–147 (1971).
Vaney, D. I., Peichl, L., Wässle, H. & Illing, R. B. Almost all ganglion cells in the rabbit retina project to the superior colliculus. Brain Res. 212, 447–453 (1981).
Kim, I. J., Zhang, Y., Meister, M. & Sanes, J. R. Laminar restriction of retinal ganglion cell dendrites and axons: subtype-specific developmental patterns revealed with transgenic markers. J. Neurosci. 30, 1452–1462 (2010).
Farajian, R., Raven, M. A., Cusato, K. & Reese, B. E. Cellular positioning and dendritic field size of cholinergic amacrine cells are impervious to early ablation of neighboring cells in the mouse retina. Vis. Neurosci. 21, 13–22 (2004).
Kanjhan, R. & Sivyer, B. Two types of ON direction-selective ganglion cells in rabbit retina. Neurosci. Lett. 483, 105–109 (2010). Together with reference 52, this study showed that there are two types of On DSGCs that differ in their dendritic morphology and receptive-field properties.
Hoshi, H., Tian, L. M., Massey, S. C. & Mills, S. L. Two distinct types of ON directionally selective ganglion cells in the rabbit retina. J. Comp. Neurol. 519, 2509–2521 (2011).
Ackert, J. M. et al. Light-induced changes in spike synchronization between coupled ON direction selective ganglion cells in the mammalian retina. J. Neurosci. 26, 4206–4215 (2006).
Ackert, J. M., Farajian, R., Volgyi, B. & Bloomfield, S. A. GABA blockade unmasks an OFF response in ON direction selective ganglion cells in the mammalian retina. J. Physiol. 587, 4481–4495 (2009).
Buhl, E. H. & Peichl, L. Morphology of rabbit retinal ganglion cells projecting to the medial terminal nucleus of the accessory optic system. J. Comp. Neurol. 253, 163–174 (1986).
Sun, W., Deng, Q., Levick, W. R. & He, S. ON direction-selective ganglion cells in the mouse retina. J. Physiol. 576, 197–202 (2006).
Hayden, S. A., Mills, J. W. & Masland, R. M. Acetylcholine synthesis by displaced amacrine cells. Science 210, 435–437 (1980).
Vaney, D. I., Peichl, L. & Boycott, B. B. Matching populations of amacrine cells in the inner nuclear and ganglion cell layers of the rabbit retina. J. Comp. Neurol. 199, 373–391 (1981).
Famiglietti, E. V. 'Starburst' amacrine cells and cholinergic neurons: mirror-symmetric on and off amacrine cells of rabbit retina. Brain Res. 261, 138–144 (1983).
Vaney, D. I. 'Coronate' amacrine cells in the rabbit retina have the 'starburst' dendritic morphology. Proc. R. Soc. Lond. B 220, 501–508 (1984).
Tauchi, M. & Masland, R. H. The shape and arrangement of the cholinergic neurons in the rabbit retina. Proc. R. Soc. Lond. B 223, 101–119 (1984).
Bloomfield, S. A. & Miller, R. F. A functional organization of ON and OFF pathways in the rabbit retina. J. Neurosci. 6, 1–13 (1986).
Famiglietti, E. V. & Tumosa, N. Immunocytochemical staining of cholinergic amacrine cells in rabbit retina. Brain Res. 413, 398–403 (1987).
Tauchi, M. & Masland, R. H. Local order among the dendrites of an amacrine cell population. J. Neurosci. 5, 2494–2501 (1985).
Brandon, C. Cholinergic neurons in the rabbit retina: dendritic branching and ultrastructural connectivity. Brain Res. 426, 119–130 (1987).
Vaney, D. I., Collin, S. P. & Young, H. M. in Neurobiology of the Inner Retina (eds Weiler, R. & Osborne, N. N.) 157–168 (Springer, Berlin, 1989).
Vaney, D. I. & Pow, D. V. The dendritic architecture of the cholinergic plexus in the rabbit retina: selective labeling by glycine accumulation in the presence of sarcosine. J. Comp. Neurol. 421, 1–13 (2000).
Dong, W., Sun, W., Zhang, Y., Chen, X. & He, S. Dendritic relationship between starburst amacrine cells and direction-selective ganglion cells in the rabbit retina. J. Physiol. 556, 11–17 (2004).
Famiglietti, E. V. Synaptic organization of starburst amacrine cells in rabbit retina: analysis of serial thin sections by electron microscopy and graphic reconstruction. J. Comp. Neurol. 309, 40–70 (1991).
Vaney, D. I. & Young, H. M. GABA-like immunoreactivity in cholinergic amacrine cells of the rabbit retina. Brain Res. 438, 369–373 (1988).
Kosaka, T., Tauchi, M. & Dahl, J. L. Cholinergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp. Brain Res. 70, 605–617 (1988).
Brecha, N., Johnson, D., Peichl, L. & Wässle, H. Cholinergic amacrine cells of the rabbit retina contain glutamate decarboxylase and γ-aminobutyrate immunoreactivity. Proc. Natl Acad. Sci. USA 85, 6187–6191 (1988).
Masland, R. H., Mills, J. W. & Cassidy, C. The functions of acetylcholine in the rabbit retina. Proc. R. Soc. Lond. B 223, 121–139 (1984).
Vaney, D. I. The mosaic of amacrine cells in the mammalian retina. Prog. Retinal Res. 9, 49–100 (1990).
Miller, R. F. & Bloomfield, S. A. Electroanatomy of a unique amacrine cell in the rabbit retina. Proc. Natl Acad. Sci. USA 80, 3069–3073 (1983).
Velte, T. J. & Miller, R. F. Spiking and nonspiking models of starburst amacrine cells in the rabbit retina. Vis. Neurosci. 14, 1073–1088 (1997).
Euler, T., Detwiler, P. B. & Denk, W. Directionally selective calcium signals in dendrites of starburst amacrine cells. Nature 418, 845–852 (2002). This study showed that the distal dendrites of SACs, where the output synapses are located, respond more strongly to centrifugal than to centripetal image motion.
Ozaita, A. et al. A unique role for Kv3 voltage-gated potassium channels in starburst amacrine cell signaling in mouse retina. J. Neurosci. 24, 7335–7343 (2004).
Fried, S. I., Münch, T. A. & Werblin, F. S. Mechanisms and circuitry underlying directional selectivity in the retina. Nature 420, 411–414 (2002). This study used dual recordings to show that DSGCs receive inhibitory input from SACs located on the null side of the DSGC but not the preferred side.
Lee, S., Kim, K. & Zhou, Z. J. Role of ACh-GABA cotransmission in detecting image motion and motion direction. Neuron 68, 1159–1172 (2010).
Wei, W., Hamby, A. M., Zhou, K. & Feller, M. B. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature 469, 402–406 (2011).
Yonehara, K. et al. Spatially asymmetric reorganization of inhibition establishes a motion-sensitive circuit. Nature 469, 407–410 (2011).
Denk, W. & Horstmann, H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2, e329 (2004).
Briggman, K. L. & Euler, T. Bulk electroporation and population calcium imaging in the adult mammalian retina. J. Neurophysiol. 105, 2601–2609 (2011).
Sterling, P. Microcircuitry of the cat retina. Annu. Rev. Neurosci. 6, 149–185 (1983).
Taylor, W. R., He, S., Levick, W. R. & Vaney, D. I. Dendritic computation of direction selectivity by retinal ganglion cells. Science 289, 2347–2350 (2000).
Taylor, W. R. & Vaney, D. I. Diverse synaptic mechanisms generate direction selectivity in the rabbit retina. J. Neurosci. 22, 7712–7720 (2002).Together with reference 79, this study reported that somatic voltage-clamp recordings show that both the inhibitory and excitatory inputs to DSGCs are direction selective.
Borg-Graham, L. J. The computation of directional selectivity in the retina occurs presynaptic to the ganglion cell. Nature Neurosci. 4, 176–183 (2001).
Lee, S. & Zhou, Z. J. The synaptic mechanism of direction selectivity in distal processes of starburst amacrine cells. Neuron 51, 787–799 (2006).
Yoshida, K. et al. A key role of starburst amacrine cells in originating retinal directional selectivity and optokinetic eye movement. Neuron 30, 771–780 (2001).
Amthor, F. R., Keyser, K. T. & Dmitrieva, N. A. Effects of the destruction of starburst-cholinergic amacrine cells by the toxin AF64A on rabbit retinal directional selectivity. Vis. Neurosci. 19, 495–509 (2002).
Rall, W. in Neural Theory and Modeling (ed. Reis, R. F.) 73–97 (Stanford Univ. Press, Stanford, 1964).
Borg-Graham, L. J. & Grzywacz, N. in Single Neuron Computation (eds McKenna, T., Davis, J. & Zornetzer, S. F.) 347–375 (Academic Press, San Diego, 1992).
Poznanski, R. R. Modelling the electrotonic structure of starburst amacrine cells in the rabbit retina: a functional interpretation of dendritic morphology. Bull. Math. Biol. 54, 905–928 (1992).
Tukker, J. J., Taylor, W. R. & Smith, R. G. Direction selectivity in a model of the starburst amacrine cell. Vis. Neurosci. 21, 611–625 (2004).
Oesch, N. W. & Taylor, W. R. Tetrodotoxin-resistant sodium channels contribute to directional responses in starburst amacrine cells. PLoS ONE 5, e12447 (2010).
Hausselt, S. E., Euler, T., Detwiler, P. B. & Denk, W. A dendrite-autonomous mechanism for direction selectivity in retinal starburst amacrine cells. PLoS Biol. 5, e185 (2007).
Taylor, W. R. & Wässle, H. Receptive field properties of starburst cholinergic amacrine cells in the rabbit retina. Eur. J. Neurosci. 7, 2308–2321 (1995).
Peters, B. N. & Masland, R. H. Responses to light of starburst amacrine cells. J. Neurophysiol. 75, 469–480 (1996).
Münch, T. A. & Werblin, F. S. Symmetric interactions within a homogeneous starburst cell network can lead to robust asymmetries in dendrites of starburst amacrine cells. J. Neurophysiol. 96, 471–477 (2006).
Gavrikov, K. E., Dmitriev, A. V., Keyser, K. T. & Mangel, S. C. Cation-chloride cotransporters mediate neural computation in the retina. Proc. Natl Acad. Sci. USA 100, 16047–16052 (2003).
Gavrikov, K. E., Nilson, J. E., Dmitriev, A. V., Zucker, C. L. & Mangel, S. C. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc. Natl Acad. Sci. USA 103, 18793–18798 (2006).
Enciso, G. A. et al. A model of direction selectivity in the starburst amacrine cell network. J. Comput. Neurosci. 28, 567–578 (2010).
Euler, T. et al. Eyecup scope: optical recordings of light stimulus-evoked fluorescence signals in the retina. Pflügers Arch. 457, 1393–1414 (2009).
Fried, S. I., Münch, T. A. & Werblin, F. S. Directional selectivity is formed at multiple levels by laterally offset inhibition in the rabbit retina. Neuron 46, 117–127 (2005).
Chiao, C. C. & Masland, R. H. Starburst cells nondirectionally facilitate the responses of direction-selective retinal ganglion cells. J. Neurosci. 22, 10509–10513 (2002).
Dacheux, R. F., Chimento, M. F. & Amthor, F. R. Synaptic input to the on-off directionally selective ganglion cell in the rabbit retina. J. Comp. Neurol. 456, 267–278 (2003).
Yamada, E. S. et al. Synaptic connections of starburst amacrine cells and localization of acetylcholine receptors in primate retinas. J. Comp. Neurol. 461, 76–90 (2003).
Koch, C., Douglas, R. & Wehmeier, U. Visibility of synaptically induced conductance changes: theory and simulations of anatomically characterized cortical pyramidal cells. J. Neurosci. 10, 1728–1744 (1990).
Spruston, N., Jaffe, D. B., Williams, S. H. & Johnston, D. Voltage- and space-clamp errors associated with the measurement of electrotonically remote synaptic events. J. Neurophysiol. 70, 781–802 (1993).
Williams, S. R. & Mitchell, S. J. Direct measurement of somatic voltage clamp errors in central neurons. Nature Neurosci. 11, 790–798 (2008).
Poleg-Polsky, A. & Diamond, J. S. Imperfect space clamp permits electrotonic interactions between inhibitory and excitatory synaptic conductances, distorting voltage clamp recordings. PLoS ONE 6, e19463 (2011).
Grzywacz, N. M., Amthor, F. R. & Merwine, D. K. Necessity of acetylcholine for retinal directionally selective responses to drifting gratings in rabbit. J. Physiol. 512, 575–581 (1998).
He, S. & Levick, W. R. Spatial-temporal response characteristics of the ON-OFF direction selective ganglion cells in the rabbit retina. Neurosci. Lett. 285, 25–28 (2000).
Sivyer, B., van Wyk, M., Vaney, D. I. & Taylor, W. R. Synaptic inputs and timing underlying the velocity tuning of direction-selective ganglion cells in rabbit retina. J. Physiol. 588, 3243–3253 (2010).
Torre, V. & Poggio, T. A synaptic mechanism possibly underlying directional selectivity to motion. Proc. R. Soc. Lond. B 202, 409–416 (1978).
Koch, C., Poggio, T. & Torre, V. Nonlinear interactions in a dendritic tree: localization, timing, and role in information processing. Proc. Natl Acad. Sci. USA 80, 2799–2802 (1983).
Oesch, N., Euler, T. & Taylor, W. R. Direction-selective dendritic action potentials in rabbit retina. Neuron 47, 739–750 (2005). This study showed that DSGCs produce dendritic spikes, and the authors proposed that dendritic spiking provides a mechanism for the direction-selective subunits to function independently.
Schachter, M. J., Oesch, N., Smith, R. G. & Taylor, W. R. Dendritic spikes amplify the synaptic signal to enhance detection of motion in a simulation of the direction-selective ganglion cell. PLoS Comput. Biol. 6, e1000899 (2010).
Brandstätter, J. H., Greferath, U., Euler, T. & Wässle, H. Co-stratification of GABAA receptors with the directionally selective circuitry of the rat retina. Vis. Neurosci. 6, 345–358 (1995).
O'Malley, D. M. & Masland, R. H. Co-release of acetylcholine and γ-aminobutyric acid by a retinal neuron. Proc. Natl Acad. Sci. USA 86, 3414–3418 (1989).
Grzywacz, N. M., Merwine, D. K. & Amthor, F. R. Complementary roles of two excitatory pathways in retinal directional selectivity. Vis. Neurosci. 15, 1119–1127 (1998).
MacNeil, M. A., Heussy, J. K., Dacheux, R. F., Raviola, E. & Masland, R. H. The shapes and numbers of amacrine cells: matching of photofilled with Golgi-stained cells in the rabbit retina and comparison with other mammalian species. J. Comp. Neurol. 413, 305–326 (1999).
Daw, N. W. & Wyatt, H. J. Raising rabbits in a moving visual environment: an attempt to modify directional sensitivity in the retina. J. Physiol. 240, 309–330 (1974).
Elstrott, J. & Feller, M. B. Vision and the establishment of direction-selectivity: a tale of two circuits. Curr. Opin. Neurobiol. 19, 293–297 (2009).
Masland, R. H. Maturation of function in the developing rabbit retina. J. Comp. Neurol. 175, 275–286 (1977).
Chan, Y. C. & Chiao, C. C. Effect of visual experience on the maturation of ON-OFF direction selective ganglion cells in the rabbit retina. Vision Res. 48, 2466–2475 (2008).
Chen, M., Weng, S., Deng, Q., Xu, Z. & He, S. Physiological properties of direction-selective ganglion cells in early postnatal and adult mouse retina. J. Physiol. 587, 819–828 (2009).
Wong, R. O. Differential growth and remodelling of ganglion cell dendrites in the postnatal rabbit retina. J. Comp. Neurol. 294, 109–132 (1990).
Diao, L., Sun, W., Deng, Q. & He, S. Development of the mouse retina: emerging morphological diversity of the ganglion cells. J. Neurobiol. 61, 236–249 (2004).
Wong, R. O. Retinal waves and visual system development. Annu. Rev. Neurosci. 22, 29–47 (1999).
Torborg, C. L. & Feller, M. B. Spontaneous patterned retinal activity and the refinement of retinal projections. Prog. Neurobiol. 76, 213–235 (2005).
Elstrott, J. & Feller, M. B. Direction-selective ganglion cells show symmetric participation in retinal waves during development. J. Neurosci. 30, 11197–11201 (2010).
Amthor, F. R., Takahashi, E. S. & Oyster, C. W. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J. Comp. Neurol. 280, 97–121 (1989).
Acknowledgements
This paper is dedicated to Horace Barlow on the occasion of his ninetieth birthday and Bill Levick on the occasion of his eightieth birthday, in recognition of their outstanding pioneering studies on direction selectivity in the retina, which laid the foundation for the research described in this Review. W.R.T. is supported by a grant from the National Eye Institute (EY014888) and a Lew R. Wasserman Award from Research to Prevent Blindness.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Microcircuit
-
An assembly of neural elements that are smaller than whole neurons but can independently perform computations.
- Accessory optic system
-
(AOS). The AOS is the fourth primary visual system, after the thalamic, tectal and pretectal systems, and comprises the medial, lateral and dorsal terminal nuclei.
- Varicosities
-
Swellings along neuronal processes that are the sites of en passant synapses.
- Channelrhodopsin 2
-
A light-gated ion channel that can be genetically expressed in individual neurons or populations of neurons, enabling them to be depolarized selectively by photostimulation.
- Serial block-face scanning electron microscopy
-
A technique for obtaining unbroken aligned series of images at sub-micron resolution by successively scanning then sectioning the face of the specimen block on the same apparatus.
- Reversal potential
-
The membrane potential at which the net ion current flow becomes zero.
- Inhibitory shunt
-
Suppression of excitatory postsynaptic potentials resulting from an increase in neuronal membrane conductance.
Rights and permissions
About this article
Cite this article
Vaney, D., Sivyer, B. & Taylor, W. Direction selectivity in the retina: symmetry and asymmetry in structure and function. Nat Rev Neurosci 13, 194–208 (2012). https://doi.org/10.1038/nrn3165
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn3165
This article is cited by
-
Starburst amacrine cells, involved in visual motion perception, lose their synaptic input from dopaminergic amacrine cells and degenerate in Parkinson’s disease patients
Translational Neurodegeneration (2023)
-
Spatial organization of the mouse retina at single cell resolution by MERFISH
Nature Communications (2023)
-
An ON-type direction-selective ganglion cell in primate retina
Nature (2023)
-
A sign-inverted receptive field of inhibitory interneurons provides a pathway for ON-OFF interactions in the retina
Nature Communications (2023)
-
Origins of direction selectivity in the primate retina
Nature Communications (2022)