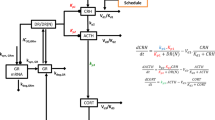
Abstract
The classical concept of hypothalamus–pituitary–adrenal (HPA) homeostasis comprises a feedback system within which circulating levels of glucocorticoid hormones maintain the brain and body in an optimal steady state. However, studies involving new techniques for investigating the real-time dynamics of both glucocorticoid hormones and glucocorticoid receptor function paint a different picture — namely, of continuous dynamic equilibration throughout this neuroendocrine system. This dynamic state is dictated by feedforward and feedback regulatory loops and by stochastic interactions at the level of DNA binding. We propose that this continuous oscillatory activity is crucial for optimal responsiveness of glucocorticoid-sensitive neural processes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bernard, C. Leçons sur les Phénomènes de la Vie Communs aux Animaux et aux Végétaux (J.-B. Baillière, Paris, 1878) (in French).
Cannon, W. Organisation for physiological homeostasis. Physiol. Rev. 9, 399–431 (1929).
Rosenblueth, A., Wiener, N. & Bigelow, J. Behaviour, purpose and teleology. Philos. Sci. 10, 18–24 (1943).
Selye, H. Stress (Acta Medical Publisher, Montreal, 1950).
Sterling, P. & Eyer, J. in Handbook of Life Stress, Cognition and Health (eds Fisher, S. & Reason, J.) 629–639 (Wiley, New York, 1988).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
Keller-Wood, M. E. & Dallman, M. F. Corticosteroid inhibition of ACTH secretion. Endocr. Rev. 5, 1–24 (1984).
Reppert, S. M. & Weaver, D. R. Coordination of circadian timing in mammals. Nature 418, 935–941 (2002).
de Kloet, E. R. & Sarabdjitsingh, R. A. Everything has rhythm: focus on glucocorticoid pulsatility. Endocrinology 149, 3241–3243 (2008).
Sarabdjitsingh, R. A. et al. Disrupted corticosterone pulsatile patterns attenuate responsiveness to glucocorticoid signaling in rat brain. Endocrinology 151, 1177–1186 (2010).
Lightman, S. L. Patterns of exposure to glucocorticoid receptor ligand. Biochem. Soc. Trans. 34, 1117–1118 (2006).
Lightman, S. L. et al. The significance of glucocorticoid pulsatility. Eur. J. Pharmacol. 583, 255–262 (2008).
Veldhuis, J. D., Iranmanesh, A., Lizarralde, G. & Johnson, M. L. Amplitude modulation of a burstlike mode of cortisol secretion subserves the circadian glucocorticoid rhythm. Am. J. Physiol. 257, e6–e14 (1989).
Seale, J. V., Wood, S. A., Atkinson, H. C., Lightman, S. L. & Harbuz, M. S. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology 146, 1973–1982 (2005).
Evuarherhe, O. et al. Organizational role for pubertal androgens on adult hypothalamic-pituitary-adrenal sensitivity to testosterone in the male rat. J. Physiol. 587, 2977–2985 (2009).
Seale, J. V. et al. Gonadectomy reverses the sexually diergic patterns of circadian and stress-induced hypothalamic-pituitary-adrenal axis activity in male and female rats. J. Neuroendocrinol. 16, 516–524 (2004).
Windle, R. J. et al. Increased corticosterone pulse frequency during adjuvant-induced arthritis and its relationship to alterations in stress responsiveness. J. Neuroendocrinol. 13, 905–911 (2001).
Harbuz, M. S. et al. Differential effects of psychological and immunological challenge on the hypothalamo-pituitary-adrenal axis function in adjuvant-induced arthritis. Ann. NY Acad. Sci. 876, 43–52 (1999).
Windle, R. J., Wood, S. A., Lightman, S. L. & Ingram, C. D. The pulsatile characteristics of hypothalamo-pituitary-adrenal activity in female Lewis and Fischer 344 rats and its relationship to differential stress responses. Endocrinology 139, 4044–4052 (1998).
Mershon, J. L., Sehlhorst, C. S., Rebar, R. W. & Liu, J. H. Evidence of a corticotropin-releasing hormone pulse generator in the macaque hypothalamus. Endocrinology 130, 2991–2996 (1992).
Ixart, G., Barbanel, G., Nouguier-Soule, J. & Assenmacher, I. A quantitative study of the pulsatile parameters of CRH-41 secretion in unanesthetized free-moving rats. Exp. Brain Res. 87, 153–158 (1991).
Engler, D. et al. Studies of the regulation of the hypothalamic-pituitary-adrenal axis in sheep with hypothalamic-pituitary disconnection. II. Evidence for in vivo ultradian hypersecretion of proopiomelanocortin peptides by the isolated anterior and intermediate pituitary. Endocrinology 127, 1956–1966 (1990).
Mindell, D. Between Human and Machine: Feedback, Control and Computing Before Cybernetics (Johns Hopkins Univ. Press, Baltimore, 2002).
Papaikonomou, E. Rat adrenocortical dynamics. J. Physiol. 265, 119–131 (1977).
Dallman, M. F. et al. Regulation of ACTH secretion: variations on a theme of B. Recent Prog. Horm. Res. 43, 113–173 (1987).
Henley, D. E. et al. Hypothalamic-pituitary-adrenal axis activation in obstructive sleep apnea: the effect of continuous positive airway pressure therapy. J. Clin. Endocrinol. Metab. 94, 4234–4242 (2009).
Russell, G. M. et al. Rapid glucocorticoid receptor-mediated inhibition of hypothalamic-pituitary-adrenal ultradian activity in healthy males. J. Neurosci. 30, 6106–6115 (2010).
Walker, J. J., Terry, J. R. & Lightman, S. L. Origin of ultradian pulsatility in the hypothalamic-pituitary-adrenal axis. Proc. Biol. Sci. 277, 1627–1633 (2010).
Windle, R. J., Wood, S. A., Shanks, N., Lightman, S. L. & Ingram, C. D. Ultradian rhythm of basal corticosterone release in the female rat: dynamic interaction with the response to acute stress. Endocrinology 139, 443–450 (1998).
Ulrich-Lai, Y. M., Arnhold, M. M. & Engeland, W. C. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am. J. Physiol. Regul. Integr. Comp. Physiol. 290, R1128–R1135 (2006).
Son, G. H. et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc. Natl Acad. Sci. USA 105, 20970–20975 (2008).
Cameron, A. et al. Temperature-responsive release of cortisol from its binding globulin: a protein thermocouple. J. Clin. Endocrinol. Metab. 14 July 2010 (doi:10.1210/jc.2010–0942).
Ballard, P. L. Delivery and transport of glucocorticoids to target cells. Monogr. Endocrinol. 12, 25–48 (1979).
Sarabdjitsingh, R. A. et al. Variations in stress responsiveness over the ultradian glucocorticoid cycle. Endocrinology (in the press).
Haller, J., Halasz, J., Mikics, E., Kruk, M. R. & Makara, G. B. Ultradian corticosterone rhythm and the propensity to behave aggressively in male rats. J. Neuroendocrinol. 12, 937–940 (2000).
Young, E. A., Abelson, J. & Lightman, S. L. Cortisol pulsatility and its role in stress regulation and health. Front. Neuroendocrinol. 25, 69–76 (2004).
Pariante, C. M. & Lightman, S. L. The HPA axis in major depression: classical theories and new developments. Trends Neurosci. 31, 464–468 (2008).
Pariante, C. M. & Miller, A. H. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol. Psychiatry 49, 391–404 (2001).
Young, E. A., Carlson, N. E. & Brown, M. B. Twenty-four-hour ACTH and cortisol pulsatility in depressed women. Neuropsychopharmacology 25, 267–276 (2001).
Sonino, N. & Fava, G. A. Psychiatric disorders associated with Cushing's syndrome. Epidemiology, pathophysiology and treatment. CNS Drugs 15, 361–373 (2001).
Koob, G. F. & Le Moal, M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology 24, 97–129 (2001).
Goldbeter, A., Dupont, G. & Halloy, J. The frequency encoding of pulsatility. Novartis Found. Symp. 227, 19–36 (2000).
Goldbeter, A. Biochemical Oscillations and Cellular Rhythms: The Molecular Basis of Periodic and Chaotic Behaviour (Cambridge Univ. Press, Cambridge, UK, 1996).
Darmon, M., Brachet, P. & Da Silva, L. H. Chemotactic signals induce cell differentiation in Dictyostelium discoideum. Proc. Natl Acad. Sci. USA 72, 3163–3166 (1975).
Cai, L., Dalal, C. K. & Elowitz, M. B. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature 455, 485–490 (2008).
Matthews, D. R., Naylor, B. A. & Jones, R. G. Pulsatile insulin has greater hypoglycemic effect than continuous delivery. Diabetes 37, 617–621 (1983).
Wildt, L. et al. Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 109, 376–385 (1981).
Belchetz, P. E., Plant, T. M., Nakai, Y., Keogh, E. J. & Knobil, E. Hypophysial responses to continuous and intermittent delivery of hypopthalamic gonadotropin-releasing hormone. Science 202, 631–633 (1978).
Tannenbaum, G. S. & Martin, J. B. Evidence for an endogenous ultradian rhythm governing growth hormone secretion in the rat. Endocrinology 98, 562–570 (1976).
Waxman, D. J., Ram, P. A., Pampori, N. A. & Shapiro, B. H. Growth hormone regulation of male-specific rat liver P450s 2A2 and 3A2: induction by intermittent growth hormone pulses in male but not female rats rendered growth hormone deficient by neonatal monosodium glutamate. Mol. Pharmacol. 48, 790–797 (1995).
Norstedt, G. & Palmiter, R. Secretory rhythm of growth hormone regulates sexual differentiation of mouse liver. Cell 36, 805–812 (1984).
Kitchener, P., Di Blasi, F., Borrelli, E. & Piazza, P. V. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur. J. Neurosci. 19, 1837–1846 (2004).
Revest, J. M. et al. The MAPK pathway and Egr-1 mediate stress-related behavioral effects of glucocorticoids. Nature Neurosci. 8, 664–672 (2005).
Conway-Campbell, B. L., Knight, D. M., Pooley, J. R., Hager, G. L. & Lightman, S. L. Intranuclear activation of glucocorticoid receptors defines a novel mechanism for continuous cellular responsiveness during glucocorticoid pulsatility. 90th Meeting of the Endocrine Society (San Francisco, California) Abstract 1–67 (2008).
Stavreva, D. A. et al. Ultradian hormone stimulation induces glucocorticoid receptor-mediated pulses of gene transcription. Nature Cell Biol. 11, 1093–1102 (2009).
Stavreva, D. A., Muller, W. G., Hager, G. L., Smith, C. L. & McNally, J. G. Rapid glucocorticoid receptor exchange at a promoter is coupled to transcription and regulated by chaperones and proteasomes. Mol. Cell. Biol. 24, 2682–2697 (2004).
Yang, J., Liu, J. & DeFranco, D. B. Subnuclear trafficking of glucocorticoid receptors in vitro: chromatin recycling and nuclear export. J. Cell Biol. 137, 523–538 (1997).
Carrigan, A. et al. An active nuclear retention signal in the glucocorticoid receptor functions as a strong inducer of transcriptional activation. J. Biol. Chem. 282, 10963–10971 (2007).
Wang, X., Pongrac, J. L. & DeFranco, D. B. Glucocorticoid receptors in hippocampal neurons that do not engage proteasomes escape from hormone-dependent down-regulation but maintain transactivation activity. Mol. Endocrinol. 16, 1987–1998 (2002).
Deroo, B. J. et al. Proteasomal inhibition enhances glucocorticoid receptor transactivation and alters its subnuclear trafficking. Mol. Cell. Biol. 22, 4113–4123 (2002).
Kinyamu, H. K. & Archer, T. K. Proteasome activity modulates chromatin modifications and RNA polymerase II phosphorylation to enhance glucocorticoid receptor-mediated transcription. Mol. Cell. Biol. 27, 4891–4904 (2007).
Conway-Campbell, B. L. et al. Proteasome-dependent down-regulation of activated nuclear hippocampal glucocorticoid receptors determines dynamic responses to corticosterone. Endocrinology 148, 5470–5477 (2007).
Breuner, C. W. & Orchinik, M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J. Endocrinol. 175, 99–112 (2002).
Droste, S. K. et al. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology 149, 3244–3253 (2008).
Reul, J. M. & de Kloet, E. R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology 117, 2505–2511 (1985).
de Kloet, E. R., Vreugdenhil, E., Oitzl, M. S. & Joels, M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 19, 269–301 (1998).
Yamamoto, S. et al. Expression of the Per1 gene in the hamster: brain atlas and circadian characteristics in the suprachiasmatic nucleus. J. Comp. Neurol. 430, 518–532 (2001).
Hastings, M. H. et al. Expression of clock gene products in the suprachiasmatic nucleus in relation to circadian behaviour. Novartis Found. Symp. 253, 203–217; discussion 102–109, 218–222, 281–284 (2003).
Conway-Campbell, B. L. et al. Glucocorticoid ultradian rhythmicity directs cyclical gene pulsing of the hippocampal CLOCK gene Period 1. J. Neuroendocrinol. 23 Jul 2010 (doi:10.1111/j.1365–28262010.02051.x).
Koyanagi, S. et al. Chronic treatment with prednisolone represses the circadian oscillation of clock gene expression in mouse peripheral tissues. Mol. Endocrinol. 20, 573–583 (2006).
Bremner, J. D. et al. Hippocampal volume reduction in major depression. Am. J. Psychiatry 157, 115–118 (2000).
Quirin, M., Gillath, O., Pruessner, J. C. & Eggert, L. D. Adult attachment insecurity and hippocampal cell density. Soc. Cogn. Affect Neurosci. 5, 39–47 (2010).
Deuschle, M. et al. Diurnal activity and pulsatility of the hypothalamus-pituitary-adrenal system in male depressed patients and healthy controls. J. Clin. Endocrinol. Metab. 82, 234–238 (1997).
Joëls, M. & Baram, T. Z. The neuro-symphony of stress. Nature Rev. Neurosci. 10, 459–466 (2009).
Spiga, F. & Lightman, S. L. Dose-dependent effects of corticosterone on nuclear glucocorticoid receptors and their binding to DNA in the brain and pituitary of the rat. Brain Res. 1293, 101–107 (2009).
John, S. et al. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol. Cell 29, 611–624 (2008).
Reul, J. M. & Chandramohan, Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology 32, S21–S25 (2007).
Reul, J. M., Hesketh, S. A., Collins, A. & Mecinas, M. G. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory formation. Epigenetics 4, 434–439 (2009).
George, C. L. et al. The pattern dependent effects of glucocorticoid exposure on prefrontal cortex transcriptional output. 92nd annual meeting of the Endocrine Society (Abstr.) P1–626 (San Diego, California, 2010).
Meijer, O. C. Coregulator proteins and corticosteroid action in the brain. J. Neuroendocrinol. 14, 499–505 (2002).
Meijer, O. C., van der, L. S., Lachize, S., Steenbergen, P. J. & de Kloet, E. R. Steroid receptor coregulator diversity: what can it mean for the stressed brain? Neuroscience 138, 891–899 (2005).
Krauskopf, B., Osinga, H. M. & Galan-vioque, J. Numerical Continuation Methods For Dynamic Systems: Path Following and Boundary Value Problems (Springer, Berlin, 2007).
Henley, D. E. et al. Development of an automated blood sampling system for use in humans. J. Med. Eng. Technol. 33, 199–208 (2009).
Acknowledgements
We gratefully acknowledge funding from the Wellcome Trust (Programme Grant 089647/Z/09/Z), the Biotechnology and Biological Sciences Research Council and the Neuroendocrine Charitable Trust.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (figure)
Schematic of the automated infusion and ABS system. (PDF 295 kb)
Related links
Glossary
- Allostatic load
-
The physiological costs of chronic exposure to a fluctuating or heightened neural or neuroendocrine response that results from repeated or chronic stress.
- Circadian cycle
-
The regular cycling of biological processes in an organism over a 24-hour period that occurs regardless of the zeitgeber.
- Frequency encoding
-
The encoding of the information in a system by the frequency of the input.
- Heteronuclear RNA
-
The immediate transcript of a gene, which is subsequently processed into mRNA.
- Numerical continuation
-
A method of computing approximate solutions of a system of parameterized nonlinear equations.
- Portal blood
-
The blood in the portal venous system that connects the hypothalamus and the anterior pituitary.
- Stress
-
A condition that disturbs the physiological (or psychological) homeostasis of an animal.
- Thermocouple
-
A temperature sensor.
- Ultradian rhythm
-
A rhythm with a shorter period than a circadian rhythm — that is, with a frequency greater than one cycle in 24 hours.
Rights and permissions
About this article
Cite this article
Lightman, S., Conway-Campbell, B. The crucial role of pulsatile activity of the HPA axis for continuous dynamic equilibration. Nat Rev Neurosci 11, 710–718 (2010). https://doi.org/10.1038/nrn2914
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2914
This article is cited by
-
Iatrogenic adrenal insufficiency in adults
Nature Reviews Endocrinology (2024)
-
Glucocorticoid Receptor (GR) antagonism as disease-modifying treatment for MDD with childhood trauma: protocol of the RESET-medication randomized controlled trial
BMC Psychiatry (2023)
-
Phase-shifting the circadian glucocorticoid profile induces disordered feeding behaviour by dysregulating hypothalamic neuropeptide gene expression
Communications Biology (2023)
-
Effects of very low-calorie ketogenic diet on hypothalamic–pituitary–adrenal axis and renin–angiotensin–aldosterone system
Journal of Endocrinological Investigation (2023)
-
A mathematical representation of the reactive scope model
Journal of Mathematical Biology (2023)