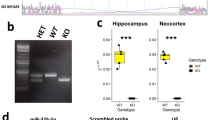
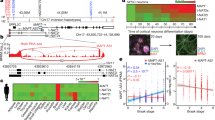
Abstract
Interest in the functions of microRNAs (miRNAs) in the nervous system has recently expanded to include their roles in neurodegeneration. Investigations have begun to reveal the influence of miRNAs on both neuronal survival and the accumulation of toxic proteins that are associated with neurodegeneration, and are providing clues as to how these toxic proteins can influence miRNA expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Kim, V. N., Han, J. & Siomi, M. C. Biogenesis of small RNAs in animals. Nature Rev. Mol. Cell Biol. 10, 126–139 (2009).
Vasudevan, S., Tong, Y. & Steitz, J. A. Cell-cycle control of microRNA-mediated translation regulation. Cell Cycle 7, 1545–1549 (2008).
Filipowicz, W., Bhattacharyya, S. N. & Sonenberg, N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nature Rev. Genet. 9, 102–114 (2008).
Visvanathan, J., Lee, S., Lee, B., Lee, J. W. & Lee, S. K. The microRNA miR-124 antagonizes the anti-neural REST/SCP1 pathway during embryonic CNS development. Genes Dev. 21, 744–749 (2007).
De Pietri Tonelli, D. et al. miRNAs are essential for survival and differentiation of newborn neurons but not for expansion of neural progenitors during early neurogenesis in the mouse embryonic neocortex. Development 135, 3911–3921 (2008).
Cheng, L. C., Pastrana, E., Tavazoie, M. & Doetsch, F. miR-124 regulates adult neurogenesis in the subventricular zone stem cell niche. Nature Neurosci. 12, 399–408 (2009).
Fiore, R. et al. Mef2-mediated transcription of the miR379–410 cluster regulates activity-dependent dendritogenesis by fine-tuning Pumilio2 protein levels. EMBO J. 28, 697–710 (2009).
Vo, N. et al. A cAMP-response element binding protein-induced microRNA regulates neuronal morphogenesis. Proc. Natl Acad. Sci. USA 102, 16426–16431 (2005).
Siegel, G. et al. A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nature Cell Biol. 11, 705–716 (2009).
Schratt, G. M. et al. A brain-specific microRNA regulates dendritic spine development. Nature 439, 283–289 (2006).
Chartier-Harlin, M. C. et al. α-synuclein locus duplication as a cause of familial Parkinson's disease. Lancet 364, 1167–1169 (2004).
Rovelet-Lecrux, A. et al. APP locus duplication causes autosomal dominant early-onset Alzheimer disease with cerebral amyloid angiopathy. Nature Genet. 38, 24–26 (2006).
Sleegers, K. et al. APP duplication is sufficient to cause early onset Alzheimer's dementia with cerebral amyloid angiopathy. Brain 129, 2977–2983 (2006).
Theuns, J. et al. Promoter mutations that increase amyloid precursor-protein expression are associated with Alzheimer disease. Am. J. Hum. Genet. 78, 936–946 (2006).
Brouwers, N. et al. Genetic risk and transcriptional variability of amyloid precursor protein in Alzheimer's disease. Brain 129, 2984–2991 (2006).
Kim, J. et al. A microRNA feedback circuit in midbrain dopamine neurons. Science 317, 1220–1224 (2007).
Schaefer, A. et al. Cerebellar neurodegeneration in the absence of microRNAs. J. Exp. Med. 204, 1553–1558 (2007).
Cuellar, T. L. et al. Dicer loss in striatal neurons produces behavioral and neuroanatomical phenotypes in the absence of neurodegeneration. Proc. Natl Acad. Sci. USA 105, 5614–5619 (2008).
Davis, T. H. et al. Conditional loss of Dicer disrupts cellular and tissue morphogenesis in the cortex and hippocampus. J. Neurosci. 28, 4322–4330 (2008).
Choi, P. S. et al. Members of the miRNA-200 family regulate olfactory neurogenesis. Neuron 57, 41–55 (2008).
Bateman, A. & Bennett, H. P. The granulin gene family: from cancer to dementia. Bioessays 30 Sep 2009 (doi:10.1002/bies.200900086).
Cruts, M. et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920–924 (2006).
Baker, M. et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916–919 (2006).
Gass, J. et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum. Mol. Genet. 15, 2988–3001 (2006).
Rademakers, R. et al. Common variation in the miR-659 binding-site of GRN is a major risk factor for TDP43-positive frontotemporal dementia. Hum. Mol. Genet. 17, 3631–3642 (2008).
Hebert, S. S. et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/β-secretase expression. Proc. Natl Acad. Sci. USA 105, 6415–6420 (2008).
Bettens, K. et al. APP and BACE1 miRNA genetic variability has no major role in risk for Alzheimer disease. Hum. Mutat. 30, 1207–1213 (2009).
Wang, G. et al. Variation in the miRNA-433 binding site of FGF20 confers risk for Parkinson disease by overexpression of α-synuclein. Am. J. Hum. Genet. 82, 283–289 (2008).
Rideout, H. J., Dietrich, P., Savalle, M., Dauer, W. T. & Stefanis, L. Regulation of α-synuclein by bFGF in cultured ventral midbrain dopaminergic neurons. J. Neurochem. 84, 803–813 (2003).
Skovronsky, D. M., Lee, V. M. & Trojanowski, J. Q. Neurodegenerative diseases: new concepts of pathogenesis and their therapeutic implications. Annu. Rev. Pathol. 1, 151–170 (2006).
Wider, C. et al. FGF20 and Parkinson's disease: no evidence of association or pathogenicity via α-synuclein expression. Mov. Disord. 24, 455–459 (2009).
Bilen, J., Liu, N., Burnett, B. G., Pittman, R. N. & Bonini, N. M. MicroRNA pathways modulate polyglutamine-induced neurodegeneration. Mol. Cell 24, 157–163 (2006).
Lee, Y. et al. miR-19, miR-101 and miR-130 co-regulate ATXN1 levels to potentially modulate SCA1 pathogenesis. Nature Neurosci. 11, 1137–1139 (2008).
Packer, A. N., Xing, Y., Harper, S. Q., Jones, L. & Davidson, B. L. The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington's disease. J. Neurosci. 28, 14341–14346 (2008).
Zuccato, C. et al. Huntingtin interacts with REST/NRSF to modulate the transcription of NRSE-controlled neuronal genes. Nature Genet. 35, 76–83 (2003).
Conaco, C., Otto, S., Han, J. J. & Mandel, G. Reciprocal actions of REST and a microRNA promote neuronal identity. Proc. Natl Acad. Sci. USA 103, 2422–2427 (2006).
Johnson, R. et al. A microRNA-based gene dysregulation pathway in Huntington's disease. Neurobiol. Dis. 29, 438–445 (2008).
Midoux, P., Pichon, C., Yaouanc, J. J. & Jaffres, P. A. Chemical vectors for gene delivery: a current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J. Pharmacol. 157, 166–178 (2009).
Pena, J. T. et al. miRNA in situ hybridization in formaldehyde and EDC-fixed tissues. Nature Methods 6, 139–141 (2009).
Chi, S. W., Zang, J. B., Mele, A. & Darnell, R. B. Argonaute HITS-CLIP decodes microRNA–mRNA interaction maps. Nature 460, 479–486 (2009).
Baek, D. et al. The impact of microRNAs on protein output. Nature 455, 64–71 (2008).
Selbach, M. et al. Widespread changes in protein synthesis induced by microRNAs. Nature 455, 58–63 (2008).
Bartel, D. P. MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233 (2009).
Acknowledgements
We apologize to authors whose papers were not discussed here owing to the short format. This work was funded by United States Public Health Service grant DA00266. T.M.D. is the Leonard and Madlyn Abramson Professor in Neurodegenerative Diseases at the Johns Hopkins University School of Medicine.
Author information
Authors and Affiliations
Corresponding authors
Related links
Related links
DATABASES
mirBase
OMIM
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Eacker, S., Dawson, T. & Dawson, V. Understanding microRNAs in neurodegeneration. Nat Rev Neurosci 10, 837–841 (2009). https://doi.org/10.1038/nrn2726
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2726
This article is cited by
-
MicroRNA-650 Regulates the Pathogenesis of Alzheimer’s Disease Through Targeting Cyclin-Dependent Kinase 5
Molecular Neurobiology (2023)
-
Lewy bodies, iron, inflammation and neuromelanin: pathological aspects underlying Parkinson’s disease
Journal of Neural Transmission (2023)
-
Potentials of miR-9-5p in promoting epileptic seizure and improving survival of glioma patients
Acta Epileptologica (2022)
-
A Review of Molecular Interplay between Neurotrophins and miRNAs in Neuropsychological Disorders
Molecular Neurobiology (2022)
-
The Transcription Factor TFCP2L1 is Associated with Myelination via miR708-5p Regulation in the Peripheral Nerve System
Neurochemical Research (2022)