Key Points
-
Multiple parallel processing strategies, involving over a dozen retinal ganglion cell types, can be found in the retina. Each ganglion cell type tiles the retina to provide a complete representation across the entire visual field of the visual attributes it conveys to the brain.
-
Three retinal ganglion cell types have been particularly well characterized both anatomically and physiologically and project in parallel from the retina, through the lateral geniculate nucleus of the thalamus to the primary visual cortex.
-
The primary visual cortex receives parallel inputs from the thalamus and uses modularity, defined spatially and by cell type-specific connectivity, to recombine these inputs into new parallel outputs.
-
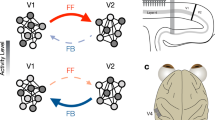
Beyond the primary visual cortex, separate but interacting dorsal and ventral streams perform distinct computations on similar visual information to support distinct behavioural goals.
-
Less is known about the parallel processing strategies that are used in the extrastriate visual cortex. However, there are strong indications that these areas use many of the same strategies that are found in the primary visual cortex.
-
Many of the parallel processing strategies found in the primate visual system are also found in the other sensory processing systems of the mammalian brain.
Abstract
Incoming sensory information is sent to the brain along modality-specific channels corresponding to the five senses. Each of these channels further parses the incoming signals into parallel streams to provide a compact, efficient input to the brain. Ultimately, these parallel input signals must be elaborated on and integrated in the cortex to provide a unified and coherent percept. Recent studies in the primate visual cortex have greatly contributed to our understanding of how this goal is accomplished. Multiple strategies including retinal tiling, hierarchical and parallel processing and modularity, defined spatially and by cell type-specific connectivity, are used by the visual system to recover the intricate detail of our visual surroundings.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gasser, H. S. & Erlanger, J. The role of fiber size in the establishment of a nerve block by pressure or cocaine. Am. J. Physiol. 88, 581–591 (1929).
Bishop, G. H. Fiber groups in the optic nerves. Am. J. Physiol. 106, 460–470 (1933).
Callaway, E. M. Structure and function of parallel pathways in the primate early visual system. J. Physiol. 566, 13–19 (2005).
Goodale, M. A. & Milner, A. D. Separate visual pathways for perception and action. Trends Neurosci. 15, 20–25 (1992).
Hendry, S. H. & Reid, R. C. The koniocellular pathway in primate vision. Annu. Rev. Neurosci. 23, 127–153 (2000).
Livingstone, M. & Hubel, D. Segregation of form, color, movement, and depth: anatomy, physiology, and perception. Science 240, 740–749 (1988).
Ungerleider, L. G. & Mishkin, M. in The Analysis of Visual Behavior (eds Ingle, D. J., Mansfield, R. J. W. & Goodale, M. S.) 549–586 (MIT Press, Cambridge, 1982).
Wassle, H. Parallel processing in the mammalian retina. Nature Rev. Neurosci. 5, 747–757 (2004).
DeYoe, E. A. & Van Essen, D. C. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 11, 219–226 (1988).
Dacey, D. M. in The Cognitive Neurosciences (ed. Gazzaniga, M. S.) 281–301 (MIT Press, Cambridge, 2004).
Rodieck, R. W. The first seps in seeing (Sinauer Associates, 1998).
Field, G. D. & Chichilnisky, E. J. Information processing in the primate retina: circuitry and coding. Annu. Rev. Neurosci. 30, 1–30 (2007).
Watanabe, M. & Rodieck, R. W. Parasol and midget ganglion cells of the primate retina. J. Comp. Neurol. 289, 434–454 (1989).
Dacey, D. M. Parallel pathways for spectral coding in primate retina. Annu. Rev. Neurosci. 23, 743–775 (2000).
Schiller, P. H. & Logothetis, N. K. The color-opponent and broad-band channels of the primate visual system. Trends Neurosci. 13, 392–398 (1990).
Blasdel, G. G. & Lund, J. S. Termination of afferent axons in macaque striate cortex. J. Neurosci. 3, 1389–1413 (1983).
Chatterjee, S. & Callaway, E. M. Parallel colour-opponent pathways to primary visual cortex. Nature 426, 668–671 (2003). This paper showed that colour-opponent LGN neurons with red–green, blue-on or blue-off receptive fields project in parallel to the primate V1 and terminate in anatomically separate compartments.
Hendrickson, A. E., Wilson, J. R. & Ogren, M. P. The neuroanatomical organization of pathways between the dorsal lateral geniculate nucleus and visual cortex in Old World and New World primates. J. Comp. Neurol. 182, 123–136 (1978).
Michael, C. R. Retinal afferent arborization patterns, dendritic field orientations, and the segregation of function in the lateral geniculate nucleus of the monkey. Proc. Natl Acad. Sci. USA 85, 4914–4918 (1988).
Dacey, D. M. & Lee, B. B. The 'blue-on' opponent pathway in primate retina originates from a distinct bistratified ganglion cell type. Nature 367, 731–735 (1994).
Dacey, D. M., Peterson, B. B., Robinson, F. R. & Gamlin, P. D. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron 37, 15–27 (2003). This paper showed that there are at least a dozen anatomically distinct cell types that project in parallel from the retina to the primate LGN.
Hendry, S. H. & Yoshioka, T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science 264, 575–577 (1994). This paper demonstrated that staining for calbindin or α-calcium/calmodulin-dependent protein kinase reveals a population of neurons in the primate LGN that are distinct from the magnocellular and parvocellular neurons. These are termed koniocellular neurons.
Livingstone, M. S. & Hubel, D. H. Thalamic inputs to cytochrome oxidase-rich regions in monkey visual cortex. Proc. Natl Acad. Sci. USA 79, 6098–6101 (1982).
Kaplan, E. & Shapley, R. M. X and Y cells in the lateral geniculate nucleus of macaque monkeys. J. Physiol. 330, 125–143 (1982).
Xu, X. et al. A comparison of koniocellular, magnocellular and parvocellular receptive field properties in the lateral geniculate nucleus of the owl monkey (Aotus trivirgatus). J. Physiol. 531, 203–218 (2001).
Dacey, D. M. et al. Melanopsin-expressing ganglion cells in primate retina signal colour and irradiance and project to the LGN. Nature 433, 749–754 (2005).
Merigan, W. H., Byrne, C. E. & Maunsell, J. H. Does primate motion perception depend on the magnocellular pathway? J. Neurosci. 11, 3422–3429 (1991). This paper demonstrated that motion perception can persist following inactivation of the magnocellular LGN. However, there was some impairment in motion perception at high temporal frequencies and low spatial frequencies.
Merigan, W. H., Katz, L. M. & Maunsell, J. H. The effects of parvocellular lateral geniculate lesions on the acuity and contrast sensitivity of macaque monkeys. J. Neurosci. 11, 994–1001 (1991).
Merigan, W. H. Chromatic and achromatic vision of macaques: role of the P pathway. J. Neurosci. 9, 776–783 (1989).
Schiller, P. H., Logothetis, N. K. & Charles, E. R. Role of the color-opponent and broad-band channels in vision. Vis. Neurosci. 5, 321–346 (1990).
Merigan, W. H. & Eskin, T. A. Spatio-temporal vision of macaques with severe loss of Pβ retinal ganglion cells. Vision Res. 26, 1751–1761 (1986).
Schiller, P. H., Logothetis, N. K. & Charles, E. R. Functions of the colour-opponent and broad-band channels of the visual system. Nature 343, 68–70 (1990).
Zeki, S. & Shipp, S. The functional logic of cortical connections. Nature 335, 311–317 (1988).
Bartfeld, E. & Grinvald, A. Relationships between orientation-preference pinwheels, cytochrome oxidase blobs, and ocular-dominance columns in primate striate cortex. Proc. Natl Acad. Sci. USA 89, 11905–11909 (1992).
Obermayer, K. & Blasdel, G. G. Geometry of orientation and ocular dominance columns in monkey striate cortex. J. Neurosci. 13, 4114–4129 (1993).
Orban, G. A. Higher order visual processing in macaque extrastriate cortex. Physiol. Rev. 88, 59–89 (2008).
Bullier, J., Hupe, J. M., James, A. C. & Girard, P. The role of feedback connections in shaping the responses of visual cortical neurons. Prog. Brain Res. 134, 193–204 (2001).
Maunsell, J. H. R. in Matters of Intelligence (ed. Vaina, L. M.) 59–87 (Reidel, Dordrecht, Holland, 1987).
Horton, J. C. & Hubel, D. H. Regular patchy distribution of cytochrome oxidase staining in primary visual cortex of macaque monkey. Nature 292, 762–764 (1981). This paper demonstrated a modular anatomical feature in the primate V1 that led the way for later studies correlating function and/or connectivity with V1 modularity.
Fitzpatrick, D., Lund, J. S. & Blasdel, G. G. Intrinsic connections of macaque striate cortex: afferent and efferent connections of lamina 4C. J. Neurosci. 5, 3329–3349 (1985).
Hawken, M. J., Parker, A. J. & Lund, J. S. Laminar organization and contrast sensitivity of direction-selective cells in the striate cortex of the Old World monkey. J. Neurosci. 8, 3541–3548 (1988).
Livingstone, M. S. & Hubel, D. H. Anatomy and physiology of a color system in the primate visual cortex. J. Neurosci. 4, 309–356 (1984). This paper demonstrated the functional specialization of CO patches in V1.
Ts'o, D. Y. & Gilbert, C. D. The organization of chromatic and spatial interactions in the primate striate cortex. J. Neurosci. 8, 1712–1727 (1988).
Sincich, L. C. & Horton, J. C. The circuitry of V1 and V2: integration of color, form, and motion. Annu. Rev. Neurosci. 28, 303–326 (2004).
Callaway, E. M. & Wiser, A. K. Contributions of individual layer 2–5 spiny neurons to local circuits in macaque primary visual cortex. Vis. Neurosci. 13, 907–922 (1996).
Lachica, E. A., Beck, P. D. & Casagrande, V. A. Parallel pathways in macaque monkey striate cortex: anatomically defined columns in layer III. Proc. Natl Acad. Sci. USA 89, 3566–3570 (1992). This paper showed that neurons in the CO patches of V1 receive convergent input from the magnocellular and parvocellular recipient layers of V1.
Yabuta, N. H. & Callaway, E. M. Functional streams and local connections of layer 4C neurons in primary visual cortex of the macaque monkey. J. Neurosci. 18, 9489–9499 (1998). This paper described anatomical substrates for the convergence of information from both magnocellular and parvocellular pathways in both CO patches and interpatches of V1.
Yoshioka, T., Levitt, J. B. & Lund, J. S. Independence and merger of thalamocortical channels within macaque monkey primary visual cortex: anatomy of interlaminar projections. Vis. Neurosci. 11, 467–489 (1994).
Malpeli, J. G., Schiller, P. H. & Colby, C. L. Response properties of single cells in monkey striate cortex during reversible inactivation of individual lateral geniculate laminae. J. Neurophysiol. 46, 1102–1119 (1981).
Nealey, T. A. & Maunsell, J. H. Magnocellular and parvocellular contributions to the responses of neurons in macaque striate cortex. J. Neurosci. 14, 2069–2079 (1994). This paper demonstrated that, following inactivation of either the magnocellular or the parvocellular pathway, visual responsivity can persist for neurons in both CO patches and interpatches of V1.
Edwards, D. P., Purpura, K. P. & Kaplan, E. Contrast sensitivity and spatial frequency response of primate cortical neurons in and around the cytochrome oxidase blobs. Vision Res. 35, 1501–1523 (1995).
Leventhal, A. G., Thompson, K. G., Liu, D., Zhou, Y. & Ault, S. J. Concomitant sensitivity to orientation, direction, and color of cells in layers 2, 3, and 4 of monkey striate cortex. J. Neurosci. 15, 1808–1818 (1995). This paper challenged the tripartite scheme introduced in reference 42. It demonstrated that there is not a strict segregation of V1 neurons selective for orientation, direction and colour.
O'Keefe, L. P., Levitt, J. B., Kiper, D. C., Shapley, R. M. & Movshon, J. A. Functional organization of owl monkey lateral geniculate nucleus and visual cortex. J. Neurophysiol. 80, 594–609 (1998).
Johnson, E. N., Hawken, M. J. & Shapley, R. The spatial transformation of color in the primary visual cortex of the macaque monkey. Nature Neurosci. 4, 409–416 (2001).
Lennie, P., Krauskopf, J. & Sclar, G. Chromatic mechanisms in striate cortex of macaque. J. Neurosci. 10, 649–669 (1990).
Anderson, J. C., Martin, K. A. & Whitteridge, D. Form, function, and intracortical projections of neurons in the striate cortex of the monkey Macacus nemestrinus. Cereb. Cortex 3, 412–420 (1993).
Yabuta, N. H., Sawatari, A. & Callaway, E. M. Two functional channels from primary visual cortex to dorsal visual cortical areas. Science 292, 297–300 (2001). This paper demonstrated that local inputs to layer 4B neurons in V1 depend on cell type. Spiny stellate neurons receive input only from the magnocellular-recipient layer 4Cα, whereas pyramidal neurons receive input from both layer 4Cα and the parvocellular-recipient layer 4Cβ.
Sawatari, A. & Callaway, E. M. Diversity and cell type specificity of local excitatory connections to neurons in layer 3B of monkey primary visual cortex. Neuron 25, 459–471 (2000).
Briggs, F. & Callaway, E. M. Layer-specific input to distinct cell types in layer 6 of monkey primary visual cortex. J. Neurosci. 21, 3600–3608 (2001).
DeYoe, E. A. & Van Essen, D. C. Segregation of efferent connections and receptive field properties in visual area V2 of the macaque. Nature 317, 58–61 (1985).
Roe, A. W. & Ts'o, D. Y. Visual topography in primate V2: multiple representation across functional stripes. J. Neurosci. 15, 3689–3715 (1995).
Shipp, S. & Zeki, S. Segregation of pathways leading from area V2 to areas V4 and V5 of macaque monkey visual cortex. Nature 315, 322–325 (1985).
Shipp, S. & Zeki, S. The functional organization of area V2, I: specialization across stripes and layers. Vis. Neurosci. 19, 187–210 (2002).
Livingstone, M. S. & Hubel, D. H. Specificity of cortico-cortical connections in monkey visual system. Nature 304, 531–534 (1983).
Livingstone, M. S. & Hubel, D. H. Connections between layer 4B of area 17 and the thick cytochrome oxidase stripes of area 18 in the squirrel monkey. J. Neurosci. 7, 3371–3377 (1987).
Sincich, L. C. & Horton, J. C. Divided by cytochrome oxidase: a map of the projections from V1 to V2 in macaques. Science 295, 1734–1737 (2002). This paper demonstrated the connectivity between CO-defined modules in primate area V1 and those in area V2.
Xiao, Y. & Felleman, D. J. Projections from primary visual cortex to cytochrome oxidase thin stripes and interstripes of macaque visual area 2. Proc. Natl Acad. Sci. USA 101, 7147–7151 (2004).
Chen, G., Lu, H. D. & Roe, A. W. A map for horizontal disparity in monkey V2. Neuron 58, 442–450 (2008).
Xiao, Y., Wang, Y. & Felleman, D. J. A spatially organized representation of colour in macaque cortical area V2. Nature 421, 535–539 (2003).
Burkhalter, A., Felleman, D. J., Newsome, W. T. & Van Essen, D. C. Anatomical and physiological asymmetries related to visual areas V3 and VP in macaque extrastriate cortex. Vision Res. 26, 63–80 (1986).
Shipp, S. & Zeki, S. The organization of connections between areas v5 and v1 in macaque monkey visual cortex. Eur. J. Neurosci. 1, 309–332 (1989).
Sincich, L. C. & Horton, J. C. Independent projection streams from macaque striate cortex to the second visual area and middle temporal area. J. Neurosci. 23, 5684–5692 (2003).
Nassi, J. J. & Callaway, E. M. Specialized circuits from primary visual cortex to V2 and area MT. Neuron 55, 799–808 (2007).
Raiguel, S. E., Lagae, L., Gulyas, B. & Orban, G. A. Response latencies of visual cells in macaque areas V1, V2 and V5. Brain Res. 493, 155–159 (1989).
Schmolesky, M. T. et al. Signal timing across the macaque visual system. J. Neurophysiol. 79, 3272–3278 (1998).
Maunsell, J. H. & Newsome, W. T. Visual processing in monkey extrastriate cortex. Annu. Rev. Neurosci. 10, 363–401 (1987).
Conway, B. R., Moeller, S. & Tsao, D. Y. Specialized color modules in macaque extrastriate cortex. Neuron 56, 560–573 (2007).
Pasupathy, A. & Connor, C. E. Responses to contour features in macaque area V4. J. Neurophysiol. 82, 2490–2502 (1999).
Schein, S. J. & Desimone, R. Spectral properties of V4 neurons in the macaque. J. Neurosci. 10, 3369–3389 (1990).
Felleman, D. J., Burkhalter, A. & Van Essen, D. C. Cortical connections of areas V3 and VP of macaque monkey extrastriate visual cortex. J. Comp. Neurol. 379, 21–47 (1997).
Andersen, R. A., Asanuma, C., Essick, G. & Siegel, R. M. Corticocortical connections of anatomically and physiologically defined subdivisions within the inferior parietal lobule. J. Comp. Neurol. 296, 65–113 (1990).
Blatt, G. J., Andersen, R. A. & Stoner, G. R. Visual receptive field organization and cortico-cortical connections of the lateral intraparietal area (area LIP) in the macaque. J. Comp. Neurol. 299, 421–445 (1990).
Boussaoud, D., Ungerleider, L. G. & Desimone, R. Pathways for motion analysis: cortical connections of the medial superior temporal and fundus of the superior temporal visual areas in the macaque. J. Comp. Neurol. 296, 462–495 (1990).
Lewis, J. W. & Van Essen, D. C. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J. Comp. Neurol. 428, 112–137 (2000).
Maunsell, J. H. & Van Essen, D. C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 3, 2563–2586 (1983).
Rizzolatti, G. & Matelli, M. Two different streams form the dorsal visual system: anatomy and functions. Exp. Brain Res. 153, 146–157 (2003).
Distler, C., Boussaoud, D., Desimone, R. & Ungerleider, L. G. Cortical connections of inferior temporal area TEO in macaque monkeys. J. Comp. Neurol. 334, 125–150 (1993).
Nakamura, H., Gattass, R., Desimone, R. & Ungerleider, L. G. The modular organization of projections from areas V1 and V2 to areas V4 and TEO in macaques. J. Neurosci. 13, 3681–3691 (1993).
Ungerleider, L. G., Galkin, T. W., Desimone, R. & Gattass, R. Cortical connections of area V4 in the macaque. Cereb. Cortex 18, 477–499 (2008).
Bullier, J., Schall, J. D. & Morel, A. Functional streams in occipito-frontal connections in the monkey. Behav. Brain Res. 76, 89–97 (1996).
Tanne-Gariepy, J., Rouiller, E. M. & Boussaoud, D. Parietal inputs to dorsal versus ventral premotor areas in the macaque monkey: evidence for largely segregated visuomotor pathways. Exp. Brain Res. 145, 91–103 (2002).
Saleem, K. S., Suzuki, W., Tanaka, K. & Hashikawa, T. Connections between anterior inferotemporal cortex and superior temporal sulcus regions in the macaque monkey. J. Neurosci. 20, 5083–5101 (2000).
Janssen, P., Vogels, R. & Orban, G. A. Selectivity for 3D shape that reveals distinct areas within macaque inferior temporal cortex. Science 288, 2054–2056 (2000).
Luppino, G., Hamed, S. B., Gamberini, M., Matelli, M. & Galletti, C. Occipital (V6) and parietal (V6A) areas in the anterior wall of the parieto-occipital sulcus of the macaque: a cytoarchitectonic study. Eur. J. Neurosci. 21, 3056–3076 (2005).
Rozzi, S. et al. Cortical connections of the inferior parietal cortical convexity of the macaque monkey. Cereb. Cortex 16, 1389–1417 (2006).
Borra, E. et al. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex 18, 1094–1111 (2008).
Galletti, C., Gamberini, M., Kutz, D. F., Baldinotti, I. & Fattori, P. The relationship between V6 and PO in macaque extrastriate cortex. Eur. J. Neurosci. 21, 959–970 (2005).
Albright, T. D. Direction and orientation selectivity of neurons in visual area MT of the macaque. J. Neurophysiol. 52, 1106–1130 (1984).
DeAngelis, G. C., Cumming, B. G. & Newsome, W. T. Cortical area MT and the perception of stereoscopic depth. Nature 394, 677–680 (1998).
Lappe, M., Bremmer, F., Pekel, M., Thiele, A. & Hoffmann, K. P. Optic flow processing in monkey STS: a theoretical and experimental approach. J. Neurosci. 16, 6265–6285 (1996).
Siegel, R. M. & Read, H. L. Analysis of optic flow in the monkey parietal area 7a. Cereb. Cortex 7, 327–346 (1997).
Desimone, R., Schein, S. J., Moran, J. & Ungerleider, L. G. Contour, color and shape analysis beyond the striate cortex. Vision Res. 25, 441–452 (1985).
Desimone, R., Albright, T. D., Gross, C. G. & Bruce, C. Stimulus-selective properties of inferior temporal neurons in the macaque. J. Neurosci. 4, 2051–2062 (1984).
Komatsu, H. & Ideura, Y. Relationships between color, shape, and pattern selectivities of neurons in the inferior temporal cortex of the monkey. J. Neurophysiol. 70, 677–694 (1993).
Latto, R. The role of inferior parietal cortex and the frontal eye-fields in visuospatial discriminations in the macaque monkey. Behav. Brain Res. 22, 41–52 (1986).
Newsome, W. T. & Pare, E. B. A selective impairment of motion perception following lesions of the middle temporal visual area (MT). J. Neurosci. 8, 2201–2211 (1988). This paper showed that lesions of MT result in the impairment of motion perception.
Orban, G. A., Saunders, R. C. & Vandenbussche, E. Lesions of the superior temporal cortical motion areas impair speed discrimination in the macaque monkey. Eur. J. Neurosci. 7, 2261–2276 (1995).
Pasternak, T. & Merigan, W. H. Motion perception following lesions of the superior temporal sulcus in the monkey. Cereb. Cortex 4, 247–259 (1994).
Quintana, J. & Fuster, J. M. Spatial and temporal factors in the role of prefrontal and parietal cortex in visuomotor integration. Cereb. Cortex 3, 122–132 (1993).
De Weerd, P., Peralta, M. R., Desimone, R. & Ungerleider, L. G. Loss of attentional stimulus selection after extrastriate cortical lesions in macaques. Nature Neurosci. 2, 753–758 (1999).
Merigan, W. H. Basic visual capacities and shape discrimination after lesions of extrastriate area V4 in macaques. Vis. Neurosci. 13, 51–60 (1996).
Vogels, R., Saunders, R. C. & Orban, G. A. Effects of inferior temporal lesions on two types of orientation discrimination in the macaque monkey. Eur. J. Neurosci. 9, 229–245 (1997).
Milner, A. D. & Goodale, M. A. Two visual systems re-viewed. Neuropsychologia 46, 774–785 (2008).
Maunsell, J. H. & Van Essen, D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. II. Binocular interactions and sensitivity to binocular disparity. J. Neurophysiol. 49, 1148–1167 (1983).
Roy, J. P., Komatsu, H. & Wurtz, R. H. Disparity sensitivity of neurons in monkey extrastriate area MST. J. Neurosci. 12, 2478–2492 (1992).
Janssen, P., Vogels, R. & Orban, G. A. Macaque inferior temporal neurons are selective for disparity-defined three-dimensional shapes. Proc. Natl Acad. Sci. USA 96, 8217–8222 (1999).
Uka, T., Tanaka, H., Yoshiyama, K., Kato, M. & Fujita, I. Disparity selectivity of neurons in monkey inferior temporal cortex. J. Neurophysiol. 84, 120–132 (2000).
Watanabe, M., Tanaka, H., Uka, T. & Fujita, I. Disparity-selective neurons in area V4 of macaque monkeys. J. Neurophysiol. 87, 1960–1973 (2002).
Krug, K., Cumming, B. G. & Parker, A. J. Comparing perceptual signals of single V5/MT neurons in two binocular depth tasks. J. Neurophysiol. 92, 1586–1596 (2004).
Uka, T. & DeAngelis, G. C. Linking neural representation to function in stereoscopic depth perception: roles of the middle temporal area in coarse versus fine disparity discrimination. J. Neurosci. 26, 6791–6802 (2006).
Janssen, P., Vogels, R., Liu, Y. & Orban, G. A. At least at the level of inferior temporal cortex, the stereo correspondence problem is solved. Neuron 37, 693–701 (2003).
Umeda, K., Tanabe, S. & Fujita, I. Representation of stereoscopic depth based on relative disparity in macaque area V4. J. Neurophysiol. 98, 241–252 (2007).
Durand, J. B. et al. Anterior regions of monkey parietal cortex process visual 3D shape. Neuron 55, 493–505 (2007).
Ferrera, V. P., Rudolph, K. K. & Maunsell, J. H. Responses of neurons in the parietal and temporal visual pathways during a motion task. J. Neurosci. 14, 6171–6186 (1994).
Sereno, A. B. & Maunsell, J. H. Shape selectivity in primate lateral intraparietal cortex. Nature 395, 500–503 (1998).
Sereno, M. E., Trinath, T., Augath, M. & Logothetis, N. K. Three-dimensional shape representation in monkey cortex. Neuron 33, 635–652 (2002).
Tolias, A. S., Keliris, G. A., Smirnakis, S. M. & Logothetis, N. K. Neurons in macaque area V4 acquire directional tuning after adaptation to motion stimuli. Nature Neurosci. 8, 591–593 (2005).
Lehky, S. R. & Sereno, A. B. Comparison of shape encoding in primate dorsal and ventral visual pathways. J. Neurophysiol. 97, 307–319 (2007).
Murata, A., Gallese, V., Luppino, G., Kaseda, M. & Sakata, H. Selectivity for the shape, size, and orientation of objects for grasping in neurons of monkey parietal area AIP. J. Neurophysiol. 83, 2580–2601 (2000).
Janssen, P., Srivastava, S., Ombelet, S. & Orban, G. A. Coding of shape and position in macaque lateral intraparietal area. J. Neurosci. 28, 6679–6690 (2008).
Schlack, A. & Albright, T. D. Remembering visual motion: neural correlates of associative plasticity and motion recall in cortical area MT. Neuron 53, 881–890 (2007).
Sincich, L. C., Park, K. F., Wohlgemuth, M. J. & Horton, J. C. Bypassing V1: a direct geniculate input to area MT. Nature Neurosci. 7, 1123–1128 (2004).
Nassi, J. J., Lyon, D. C. & Callaway, E. M. The parvocellular LGN provides a robust disynaptic input to the visual motion area MT. Neuron 50, 319–327 (2006).
Nassi, J. J. & Callaway, E. M. Multiple circuits relaying primate parallel visual pathways to the middle temporal area. J. Neurosci. 26, 12789–12798 (2006). This paper showed that the different pathways by which visual information is carried from V1 to MT have different relationships to the magnocellular and parvocellular pathways.
Movshon, J. A. & Newsome, W. T. Visual response properties of striate cortical neurons projecting to area MT in macaque monkeys. J. Neurosci. 16, 7733–7741 (1996).
Ponce, C. R., Lomber, S. G. & Born, R. T. Integrating motion and depth via parallel pathways. Nature Neurosci. 11, 216–223 (2008). This paper showed that selective inactivation of indirect pathways from V1 (through V2 and/or V3) to MT results in a selective reduction in disparity tuning but not direction tuning of MT neurons. This suggests that the direct pathway from V1 to MT is sufficient for direction but not disparity tuning.
Albright, T. D., Desimone, R. & Gross, C. G. Columnar organization of directionally selective cells in visual area MT of the macaque. J. Neurophysiol. 51, 16–31 (1984).
DeAngelis, G. C. & Newsome, W. T. Organization of disparity-selective neurons in macaque area MT. J. Neurosci. 19, 1398–1415 (1999).
Liu, J. & Newsome, W. T. Functional organization of speed tuned neurons in visual area MT. J. Neurophysiol. 89, 246–256 (2003).
Adams, D. L. & Zeki, S. Functional organization of macaque V3 for stereoscopic depth. J. Neurophysiol. 86, 2195–2203 (2001).
Zeki, S. M. The third visual complex of rhesus monkey prestriate cortex. J. Physiol. 277, 245–272 (1978).
DeYoe, E. A., Felleman, D. J., Van Essen, D. C. & McClendon, E. Multiple processing streams in occipitotemporal visual cortex. Nature 371, 151–154 (1994).
Xiao, Y., Zych, A. & Felleman, D. J. Segregation and convergence of functionally defined V2 thin stripe and interstripe compartment projections to area V4 of macaques. Cereb. Cortex 9, 792–804 (1999).
Zeki, S. & Shipp, S. Modular connections between areas V2 and V4 of macaque monkey visual cortex. Eur. J. Neurosci. 1, 494–506 (1989).
Gegenfurtner, K. R., Kiper, D. C. & Levitt, J. B. Functional properties of neurons in macaque area V3. J. Neurophysiol. 77, 1906–1923 (1997).
Lagae, L., Gulyas, B., Raiguel, S. & Orban, G. A. Laminar analysis of motion information processing in macaque V5. Brain Res. 496, 361–367 (1989).
Berezovskii, V. K. & Born, R. T. Specificity of projections from wide-field and local motion-processing regions within the middle temporal visual area of the owl monkey. J. Neurosci. 20, 1157–1169 (2000).
Ohki, K., Chung, S., Ch'ng, Y. H., Kara, P. & Reid, R. C. Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 433, 597–603 (2005).
Tan, E. M. et al. Selective and quickly reversible inactivation of mammalian neurons in vivo using the Drosophila allatostatin receptor. Neuron 51, 157–170 (2006).
Wickersham, I. R. et al. Monosynaptic restriction of transsynaptic tracing from single, genetically targeted neurons. Neuron 53, 639–647 (2007).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature Neurosci. 8, 1263–1268 (2005).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Blackshaw, S. E., Nicholls, J. G. & Parnas, I. Expanded receptive fields of cutaneous mechanoreceptor cells after single neurone deletion in leech central nervous system. J. Physiol. 326, 261–268 (1982).
Grueber, W. B., Graubard, K. & Truman, J. W. Tiling of the body wall by multidendritic sensory neurons in Manduca sexta. J. Comp. Neurol. 440, 271–283 (2001).
Grueber, W. B., Jan, L. Y. & Jan, Y. N. Tiling of the Drosophila epidermis by multidendritic sensory neurons. Development 129, 2867–2878 (2002).
Ma, M. & Shepherd, G. M. Functional mosaic organization of mouse olfactory receptor neurons. Proc. Natl Acad. Sci. USA 97, 12869–12874 (2000).
Schoppa, N. E. & Urban, N. N. Dendritic processing within olfactory bulb circuits. Trends Neurosci. 26, 501–506 (2003).
Schreiner, C. E., Read, H. L. & Sutter, M. L. Modular organization of frequency integration in primary auditory cortex. Annu. Rev. Neurosci. 23, 501–529 (2000).
Sur, M., Wall, J. T. & Kaas, J. H. Modular segregation of functional cell classes within the postcentral somatosensory cortex of monkeys. Science 212, 1059–1061 (1981).
Yu, J. et al. Local-circuit phenotypes of layer 5 neurons in motor-frontal cortex of YFP-H mice. Front. Neural Circuits 2, 6 (2008).
Barbour, D. L. & Callaway, E. M. Excitatory local connections of superficial neurons in rat auditory cortex. J. Neurosci. 28, 11174–11185 (2008).
Bureau, I., von Saint Paul, F. & Svoboda, K. Interdigitated paralemniscal and lemniscal pathways in the mouse barrel cortex. PLoS Biol. 4, e382 (2006).
Xu, X. & Callaway, E. M. Laminar specificity of functional input to distinct types of inhibitory cortical neurons. J. Neurosci. 29, 70–85 (2009).
Belin, P. & Zatorre, R. J. 'What', 'where' and 'how' in auditory cortex. Nature Neurosci. 3, 965–966 (2000).
Dijkerman, H. C. & de Haan, E. H. Somatosensory processes subserving perception and action. Behav. Brain Sci. 30, 189–201; discussion 201–39 (2007).
Kaas, J. H. & Hackett, T. A. 'What' and 'where' processing in auditory cortex. Nature Neurosci. 2, 1045–1047 (1999).
Savic, I., Gulyas, B., Larsson, M. & Roland, P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron 26, 735–745 (2000).
Tian, B., Reser, D., Durham, A., Kustov, A. & Rauschecker, J. P. Functional specialization in rhesus monkey auditory cortex. Science 292, 290–293 (2001).
Acknowledgements
We thank R. Born and J. Maunsell for helpful comments and the US National Institutes of Health for their support (RO1-EY010742 and F32-EY018982).
Author information
Authors and Affiliations
Corresponding author
Glossary
- Percept
-
The perception that arises internally, in the mind, based on an external stimulus, such as a visual stimulus.
- Parallel processing
-
Simultaneous processing of information through independent circuits.
- Photoreceptor
-
A specialized cell in the retina that detects light and responds with a change in membrane potential and a change in neurotransmitter release.
- Tiling
-
Relatively uniform and complete coverage of space.
- Hierarchical processing
-
Processing that takes place in serial order, with more sophisticated properties emerging at higher levels through the build-up of simpler properties at lower levels.
- Modularity
-
When repeating modules are used to conduct similar operations. Typically, in the visual cortex each module will perform an operation related to visual information from a portion of the visual space. Together the modules cover the space so that the operation is conducted over the entire visual scene.
- Eccentricity
-
Distance from the centre. It is typically used to describe the distance of a visual receptive field from the centre of gaze and is expressed as an angle, in degrees.
- Colour-opponent signal
-
The signal that results when a visual receptive field is excited in response to one colour and inhibited in response to another.
- Receptive field
-
The location in visual space where a change in light can cause a change in neuronal activity.
- Broadband
-
Typically used to describe visual receptive fields that are not colour-opponent. Whether a broadband cell is excited or inhibited by a stimulus at a particular part of its receptive field is relatively independent of the wavelength of the light.
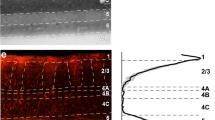
- Cytochrome oxidase blobs
-
Patches in the upper layers of the primate primary visual cortex with high concentrations of the metabolic enzyme cytochrome oxidase.
- Random-dot stereogram
-
A pair of random-dot images that generate the sensation of depth when the eyes are positioned so that they focus at a location in front of or behind the images.
- Disynaptic
-
Traversing two synaptic contacts. If a trans-synaptic anatomical tracer were to spread from one neuron across synaptic contacts to a second neuron, the spread would be monosynaptic. If the tracer continued to spread from the second neuron across more synaptic contacts to a third neuron, the spread would be disynaptic.
- Meynert cell
-
A large projection neuron in the deep layers of the visual cortex.
- Antidromic stimulation
-
Stimulation that is used to determine whether a neuron recorded in one location projects to another, distant location. Antidromic stimulation at the distant location generates action potentials that propagate back to, and are detected at, the recorded neuron.
- Fixating
-
Maintaining the eye position at a particular location.
Rights and permissions
About this article
Cite this article
Nassi, J., Callaway, E. Parallel processing strategies of the primate visual system. Nat Rev Neurosci 10, 360–372 (2009). https://doi.org/10.1038/nrn2619
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn2619
This article is cited by
-
Rat superior colliculus encodes the transition between static and dynamic vision modes
Nature Communications (2024)
-
Assessing perceptual chromatic equiluminance using a reflexive pupillary response
Scientific Reports (2024)
-
Recent advances in bioinspired vision systems with curved imaging structures
Rare Metals (2024)
-
Brain activity characteristics of RGB stimulus: an EEG study
Scientific Reports (2023)
-
A neural machine code and programming framework for the reservoir computer
Nature Machine Intelligence (2023)