Key Points
-
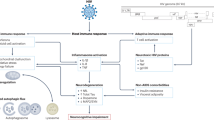
Human immunodeficiency virus (HIV) enters the CNS during the earliest stages of infection. Among neurotropic viruses, many of which cause disease in less than 5% of infected individuals, HIV is distinctly neurovirulent, resulting in neurocognitive impairment in 50% or more of patients.
-
Combination antiretroviral therapy (cART) has markedly prolonged survival and benefited health for many HIV-infected individuals in the United States. At the same time, cART has altered the character of the predominant neurological manifestation of HIV infection from a devastating, severe dementia to a chronic, milder degree of neurocognitive impairment.
-
Clinicians, scientists and the general public have increasingly recognized in recent years the adverse impact of milder degrees of neurocognitive impairment that do not meet the criteria for dementia. Common examples of this include HIV-related neurocognitive disorders, mild cognitive impairment as a precursor to Alzheimer's disease and sports-related post-concussion brain injury.
-
The possible impact of milder degrees of cognitive impairment in HIV-infected individuals who survive for decades is not yet well known. Ongoing studies are characterizing long-term effects on activities of daily living, medication adherence and vocational achievement.
-
Synaptodendritic injury is a specific type of neuronal injury comprising structural and chemical changes that affect the 'business ends' of neurons — the synapses at which they communicate and interact with one another. Synaptodendritic injury correlates closely to the presence and severity of neurocognitive disorders in HIV.
-
By carefully selecting specific antiretrovirals and supplementing them with neuroprotective agents, physicians might be able to facilitate innate CNS repair in HIV infection, promoting enhanced synaptodendritic plasticity, neural functioning and improved clinical neurological status.
Abstract
Approximately 40 million people worldwide are infected with human immunodeficiency virus (HIV). Despite HIV's known propensity to infect the CNS and cause neurological disease, HIV neurocognitive disorders remain under-recognized. Although combination antiretroviral therapy has improved the health of millions of those living with HIV, the penetration into the CNS of many such therapies is limited, and patients' quality of life continues to be diminished by milder, residual neurocognitive impairment. Synaptodendritic neuronal injury is emerging as an important mediator of such deficits in HIV. By carefully selecting specific antiretrovirals and supplementing them with neuroprotective agents, physicians might be able to facilitate innate CNS repair, promoting enhanced synaptodendritic plasticity, neural function and clinical neurological status.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Dore, G. J. et al. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS 13, 1249–1253 (1999).
Tozzi, V. et al. Neurocognitive impairment influences quality of life in HIV-infected patients receiving HAART. Int. J. STD AIDS 15, 254–259 (2004).
Sacktor, N. et al. HIV-associated cognitive impairment before and after the advent of combination therapy. J. Neurovirol. 8, 136–142 (2002).
Woods, S. P. et al. Qualitative aspects of verbal fluency in HIV-associated dementia: a deficit in rule-guided lexical-semantic search processes? Neuropsychologia 42, 801–809 (2004).
Baldewicz, T. T. et al. Changes in neuropsychological functioning with progression of HIV-1 infection: results of an 8-year longitudinal investigation. AIDS Behav. 8, 345–355 (2004).
Heaton, R. K. et al. The impact of HIV-associated neuropsychological impairment on everyday functioning. J. Int. Neuropsychol. Soc. 10, 317–331 (2004).
Morbidity and Mortality Weekly Report. The Global HIV/AIDS pandemic, 2006. MMWR Morb. Mortal. Wkly Rep. 55, 841–844 (2006).
Sotrel, A. & Dal Canto, M. C. HIV-1 and its causal relationship to immunosuppression and nervous system disease in AIDS: a review. Hum. Pathol. 31, 1274–1298 (2000).
Tozzi, V. et al. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res. Hum. Retroviruses 21, 706–713 (2005).
Sacktor, N. et al. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology 67, 311–314 (2006).
Kaul, M. & Lipton, S. A. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc. Natl Acad. Sci. USA 96, 8212–8216 (1999).
Stevenson, M. HIV-1 pathogenesis. Nature Med. 9, 853–60 (2003). Reviews the molecular biology of HIV-1 and the mechanisms by which it causes systemic disease, including AIDS.
Tozzi, V. et al. Prevalence and risk factors for human immunodeficiency virus-associated neurocognitive impairment, 1996 to 2002: results from an urban observational cohort. J. Neurovirol. 11, 265–273 (2005).
Cysique, L. A., Maruff, P. & Brew, B. J. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J. Neurovirol. 10, 350–357 (2004).
Resnick, L., Berger, J. R., Shapshak, P. & Tourtellotte, W. W. Early penetration of the blood-brain-barrier by HIV. Neurology 38, 9–14 (1988).
Haase, A. T. Pathogenesis of lentivirus infections. Nature 322, 130–136 (1986).
Gonzalez-Scarano, F. & Martin-Garcia, J. The neuropathogenesis of AIDS. Nature Rev. Immunol. 5, 69–81 (2005). This comprehensive, authoritative work reviews the cellular and molecular mechanisms by which HIV damages the CNS.
Troyer, R. M. et al. Changes in human immunodeficiency virus type 1 fitness and genetic diversity during disease progression. J. Virol. 79, 9006–9018 (2005).
Xiao, L., Rudolph, D. L., Owen, S. M., Spira, T. J. & Lal, R. B. Adaptation to promiscuous usage of CC and CXC-chemokine coreceptors in vivo correlates with HIV-1 disease progression. AIDS 12, F137–F143 (1998).
Clements, J. E. et al. The central nervous system is a viral reservoir in simian immunodeficiency virus — infected macaques on combined antiretroviral therapy: a model for human immunodeficiency virus patients on highly active antiretroviral therapy. J. Neurovirol. 11, 180–189 (2005). This intriguing study analyses the SIV animal model to test the hypothesis that retroviruses such as SIV and HIV are able to persist in the brain 'sanctuary' even when these viruses are suppressed by antiretroviral therapy and immune responses.
Orenstein, J. M., Meltzer, M. S., Phipps, T. & Gendelman, H. E. Cytoplasmic assembly and accumulation of human immunodeficiency virus types 1 and 2 in recombinant human colony-stimulating factor-1-treated human monocytes: an ultrastructural study. J. Virol. 62, 2578–2586 (1988).
Raposo, G. et al. Human macrophages accumulate HIV-1 particles in MHC II compartments. Traffic 3, 718–729 (2002).
Adle-Biassette, H. et al. Neuronal apoptosis does not correlate with dementia in HIV infection but is related to microglial activation and axonal damage. Neuropathol. Appl. Neurobiol. 25, 123–133 (1999).
Wiley, C. A. & Achim, C. L. Human immunodeficiency virus encephalitis and dementia. Ann. Neurol. 38, 559–560 (1995).
Brew, B. J., Rosenblum, M., Cronin, K. & Price, R. W. AIDS dementia complex and HIV-1 brain infection: clinical-virological correlations. Ann. Neurol. 38, 563–570 (1995).
Cherner, M. et al. Neurocognitive dysfunction predicts postmortem findings of HIV encephalitis. Neurology 59, 1563–1567 (2002).
Everall, I. P. et al. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 9, 209–217 (1999). Carefully analyses associations between markers of neuronal damage, including synaptodendritic injury and clinical brain dysfunction, measured as neurocognitive performance.
Masliah, E. et al. Dendritic injury is a pathological substrate for human immunodeficiency virus-related cognitive disorders. HNRC Group. The HIV Neurobehavioral Research Center. Ann. Neurol. 42, 963–972 (1997).
Masliah, E. Mechanisms of synaptic dysfunction in Alzheimer's disease. Histol. Histopathol. 10, 509–519 (1995).
Masliah, E. et al. Synaptic and neuritic alterations during the progression of Alzheimer's disease. Neurosci. Lett. 174, 67–72 (1994).
Arnold, S. E. Contributions of neuropathology to understanding schizophrenia in late life. Harv. Rev. Psychiatry 9, 69–76 (2001).
Law, A. J., Weickert, C. S., Hyde, T. M., Kleinman, J. E. & Harrison, P. J. Reduced spinophilin but not microtubule-associated protein 2 expression in the hippocampal formation in schizophrenia and mood disorders: molecular evidence for a pathology of dendritic spines. Am. J. Psychiatry 161, 1848–1855 (2004).
Mayer, D., Fischer, H., Schneider, U., Heimrich, B. & Schwemmle, M. Borna disease virus replication in organotypic hippocampal slice cultures from rats results in selective damage of dentate granule cells. J. Virol. 79, 11716–11723 (2005).
Bruce-Keller, A. J. et al. Synaptic transport of human immunodeficiency virus-Tat protein causes neurotoxicity and gliosis in rat brain. J. Neurosci. 23, 8417–8422 (2003).
Moore, D. J. et al. Cortical and subcortical neurodegeneration is associated with HIV neurocognitive impairment. AIDS 20, 879–887 (2006).
Wiley, C. A. et al. Distribution of brain HIV load in AIDS. Brain Pathol. 8, 277–284 (1998).
Langford, T. D. et al. Severe, demyelinating leukoencephalopathy in AIDS patients on antiretroviral therapy. AIDS 16, 1019–1029 (2002). Describes severe neuropathological alterations in the brains of patients treated with cART but who experienced virological failure — a resurgence of viral replication due to the development of drug resistance.
Day, M. et al. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nature Neurosci. 9, 251–259 (2006).
Gelbard, H. A. Neuroprotective strategies for HIV-1-associated neurologic disease. Ann. NY Acad. Sci. 890, 312–323 (1999).
Gisslen, M., Rosengren, L., Hagberg, L., Deeks, S. G. & Price, R. W. Cerebrospinal fluid signs of neuronal damage after antiretroviral treatment interruption in HIV-1 infection. AIDS Res. Ther. 2, 6 (2005).
Hagberg, L., Fuchs, D., Rosengren, L. & Gisslen, M. Intrathecal immune activation is associated with cerebrospinal fluid markers of neuronal destruction in AIDS patients. J. Neuroimmunol. 102, 51–55 (2000).
Norgren, N., Rosengren, L. & Stigbrand, T. Elevated neurofilament levels in neurological diseases. Brain Res. 987, 25–31 (2003).
Archibald, S. L. et al. Correlation of in vivo neuroimaging abnormalities with postmortem human immunodeficiency virus encephalitis and dendritic loss. Arch. Neurol. 61, 369–376 (2004).
Grant, I. et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Studies with neuropsychologic testing and magnetic resonance imaging. Ann. Intern. Med. 107, 828–836 (1987).
Fox, H. S., Gold, L. H., Henriksen, S. J. & Bloom, F. E. Simian immunodeficiency virus: a model for neuroAIDS. Neurobiol. Dis. 4, 265–274 (1997).
Roberts, E. S. et al. Host response and dysfunction in the CNS during chronic simian immunodeficiency virus infection. J. Neurosci. 26, 4577–4585 (2006).
Garden, G. A. et al. Caspase cascades in human immunodeficiency virus-associated neurodegeneration. J. Neurosci. 22, 4015–4024 (2002).
Adle-Biassette, H. et al. Neuronal apoptosis in HIV infection in adults. Neuropathol. Appl. Neurobiol. 21, 218–227 (1995).
Gray, F. et al. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin. Neuropathol. 20, 146–155 (2001).
James, H. J. et al. Expression of caspase-3 in brains from paediatric patients with HIV-1 encephalitis. Neuropathol. Appl. Neurobiol. 25, 380–386 (1999).
Gendelman, H. E., Lipton, S. A., Tardieu, M., Bukrinsky, M. I. & Nottet, H. S. The neuropathogenesis of HIV-1 infection. J. Leukoc. Biol. 56, 389–398 (1994).
Gendelman, H. E. & Tardieu, M. Macrophages/microglia and the pathophysiology of CNS injuries in AIDS. J. Leukoc. Biol. 56, 387–388 (1994).
Kaul, M., Garden, G. A. & Lipton, S. A. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410, 988–994 (2001).
Minagar, A. et al. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J. Neurol. Sci. 202, 13–23 (2002).
Pulliam, L., Herndier, B. G., Tang, N. M. & McGrath, M. S. Human immunodeficiency virus-infected macrophages produce soluble factors that cause histological and neurochemical alterations in cultured human brains. J. Clin. Invest. 87, 503–512 (1991).
Celio, M. R. et al. Monoclonal antibodies directed against the calcium binding protein Calbindin D-28k. Cell Calcium 11, 599–602 (1990).
Mattson, M. P., Rychlik, B., Chu, C. & Christakos, S. Evidence for calcium-reducing and excito-protective roles for the calcium-binding protein calbindin-D28k in cultured hippocampal neurons. Neuron 6, 41–51 (1991).
Barbas, H. et al. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cereb. Cortex 15, 1356–1370 (2005).
Blatow, M., Caputi, A., Burnashev, N., Monyer, H. & Rozov, A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron 38, 79–88 (2003).
Dumas, T. C., Powers, E. C., Tarapore, P. E. & Sapolsky, R. M. Overexpression of calbindin D28k in dentate gyrus granule cells alters mossy fiber presynaptic function and impairs hippocampal-dependent memory. Hippocampus 14, 701–709 (2004).
Jouvenceau, A. et al. Glutamatergic synaptic responses and long-term potentiation are impaired in the CA1 hippocampal area of calbindin D28k-deficient mice. Synapse 33, 172–180 (1999).
Molinari, S. et al. Deficits in memory and hippocampal long-term potentiation in mice with reduced calbindin D28K expression. Proc. Natl Acad. Sci. USA 93, 8028–8033 (1996).
Wang, G. J. et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain 127, 2452–2458 (2004).
Quasney, M. W. et al. Increased frequency of the tumor necrosis factor-α-308 A allele in adults with human immunodeficiency virus dementia. Ann. Neurol. 50, 157–162 (2001).
McGuire, W., Hill, A. V., Allsopp, C. E., Greenwood, B. M. & Kwiatkowski, D. Variation in the TNF-α promoter region associated with susceptibility to cerebral malaria. Nature 371, 508–510 (1994).
Iskander, S., Walsh, K. A. & Hammond, R. R. Human CNS cultures exposed to HIV-1 gp120 reproduce dendritic injuries of HIV-1-associated dementia. J. Neuroinflammation 1, 7 (2004).
Tenneti, L. & Lipton, S. A. Involvement of activated caspase-3-like proteases in N-methyl-D-aspartate-induced apoptosis in cerebrocortical neurons. J. Neurochem. 74, 134–142 (2000).
Toggas, S. M. et al. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature 367, 188–193 (1994).
Kaul, M. & Lipton, S. A. Experimental and potential future therapeutic approaches for HIV-1 associated dementia targeting receptors for chemokines, glutamate and erythropoietin. Neurotox. Res. 8, 167–186 (2005).
Fontana, G., Valenti, L. & Raiteri, M. Gp120 can revert antagonism at the glycine site of NMDA receptors mediating GABA release from cultured hippocampal neurons. J. Neurosci. Res. 49, 732–738 (1997).
Griffin, W. C., Middaugh, L. D., Cook, J. E. & Tyor, W. R. The severe combined immunodeficient (SCID) mouse model of human immunodeficiency virus encephalitis: deficits in cognitive function. J. Neurovirol. 10, 109–115 (2004).
Cook, J. E. et al. Highly active antiretroviral therapy and human immunodeficiency virus encephalitis. Ann. Neurol. 57, 795–803 (2005).
Persidsky, Y. et al. Human immunodeficiency virus encephalitis in SCID mice. Am. J. Pathol. 149, 1027–1053 (1996).
Nath, A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J. Infect. Dis. 186, S193–S198 (2002). Succinctly and cogently summarizes toxic effects of proteins deriving from HIV on brain structure and function.
Chauhan, A. et al. Intracellular human immunodeficiency virus Tat expression in astrocytes promotes astrocyte survival but induces potent neurotoxicity at distant sites via axonal transport. J. Biol. Chem. 278, 13512–13519 (2003).
Cartier, L., Hartley, O., Dubois-Dauphin, M. & Krause, K. H. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res. Brain Res. Rev. 48, 16–42 (2005).
Kaul, M. & Lipton, S. Mechanisms of neuroimmunity and neurodegeneration associated with HIV-1 infection and AIDS. J. Neuroimmune Pharmacol. 1, 138–151 (2006). Describes the role of host factors, including microglia, macrophages and cytokines in neuronal injury in HIV infection.
Brenneman, D. E. et al. Neuronal cell killing by the envelope protein of HIV and its prevention by vasoactive intestinal peptide. Nature 335, 639–642 (1988).
Garden, G. A. et al. HIV associated neurodegeneration requires p53 in neurons and microglia. FASEB J. 18, 1141–1143 (2004).
Giulian, D., Vaca, K. & Noonan, C. A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science 250, 1593–1596 (1990).
Makrigeorgi-Butera, M., Hagel, C., Laas, R., Puschel, K. & Stavrou, D. Comparative brain pathology of HIV-seronegative and HIV-infected drug addicts. Clin. Neuropathol. 15, 324–329 (1996).
Tomlinson, G. S., Simmonds, P., Busuttil, A., Chiswick, A. & Bell, J. E. Upregulation of microglia in drug users with and without pre-symptomatic HIV infection. Neuropathol. Appl. Neurobiol. 25, 369–379 (1999).
Langford, D. et al. Patterns of selective neuronal damage in methamphetamine-user AIDS patients. J. Acquir. Immune Defic. Syndr. 34, 467–474 (2003).
Letendre, S. L. et al. The effects of hepatitis C, HIV, and methamphetamine dependence on neuropsychological performance: biological correlates of disease. AIDS 19, S72–S78 (2005).
Everall, I. et al. Methamphetamine stimulates interferon inducible genes in HIV infected brain. J. Neuroimmunol. 170, 158–171 (2005).
Chana, G. et al. Cognitive deficits and degeneration of interneurons in HIV+ methamphetamine users. Neurology 67, 1486–1489 (2006).
Rippeth, J. D. et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. J. Int. Neuropsychol. Soc. 10, 1–14 (2004).
Cherner, M. et al. Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS 18, S27–S34 (2004).
Morgello, S. et al. Effects of hepatic function and hepatitis C virus on the nervous system assessment of advanced-stage HIV-infected individuals. AIDS 19, S116–S122 (2005).
Bohotin, C. R., Badescu, M., Popescu, D. N. & Bohotin, V. Motor cortex plasticity — from physiology to clinical neurology. Rom. J. Physiol. 41, 99–108 (2004).
Mazurova, Y., Rudolf, E., Latr, I. & Osterreicher, J. Proliferation and differentiation of adult endogenous neural stem cells in response to neurodegenerative process within the striatum. Neurodegener. Dis. 3, 12–18 (2006).
Nithianantharajah, J. & Hannan, A. J. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nature Rev. Neurosci. 7, 697–709 (2006).
Bellizzi, M. J., Lu, S. M., Masliah, E. & Gelbard, H. A. Synaptic activity becomes excitotoxic in neurons exposed to elevated levels of platelet-activating factor. J. Clin. Invest. 115, 3185–3192 (2005).
Hansson, E. & Ronnback, L. Glial neuronal signaling in the central nervous system. Faseb J. 17, 341–348 (2003).
Turchan, J., Sacktor, N., Wojna, V., Conant, K. & Nath, A. Neuroprotective therapy for HIV dementia. Curr. HIV Res. 1, 373–383 (2003).
Isackson, P. J. Trophic factor response to neuronal stimuli or injury. Curr. Opin. Neurobiol. 5, 350–357 (1995).
Pezet, S. & Malcangio, M. Brain-derived neurotrophic factor as a drug target for CNS disorders. Expert Opin. Ther. Targets 8, 391–399 (2004).
Shanley, L. J., Irving, A. J. & Harvey, J. Leptin enhances NMDA receptor function and modulates hippocampal synaptic plasticity. J. Neurosci. 21, RC186 (2001).
Heaton, R. K. et al. The HNRC 500 — neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. J. Int. Neuropsychol. Soc. 1, 231–251 (1995).
Deutsch, R. et al. AIDS-associated mild neurocognitive impairment is delayed in the era of highly active antiretroviral therapy. AIDS 15, 1898–1899 (2001).
McArthur, J. C. HIV dementia: an evolving disease. J. Neuroimmunol. 157, 3–10 (2004). Describes recent changes in the clinical manifestations of HIV brain disease relating to the introduction of cART.
Basso, M. R. & Bornstein, R. A. Effects of immunosuppression and disease severity upon neuropsychological function in HIV infection. J. Clin. Exp. Neuropsychol. 22, 104–114 (2000).
Ellis, R. J. et al. Progression to neuropsychological impairment in human immunodeficiency virus infection predicted by elevated cerebrospinal fluid levels of human immunodeficiency virus RNA. Arch. Neurol. 59, 923–928 (2002).
Kaplan, R. M. et al. Validity of the quality of well-being scale for persons with human immunodeficiency virus infection. HNRC Group. HIV Neurobehavioral Research Center. Psychosom. Med. 57, 138–147 (1995).
Albert, S. M. et al. Neuropsychologic impairment in early HIV infection. A risk factor for work disability. Arch. Neurol. 52, 525–530 (1995).
Heaton, R. K. et al. Neuropsychological impairment in human immunodeficiency virus-infection: implications for employment. HNRC Group. HIV Neurobehavioral Research Center. Psychosom. Med. 56, 8–17 (1994).
van Gorp, W. G., Baerwald, J. P., Ferrando, S. J., McElhiney, M. C. & Rabkin, J. G. The relationship between employment and neuropsychological impairment in HIV infection. J. Int. Neuropsychol. Soc. 5, 534–539 (1999).
Marcotte, T. D. et al. A multimodal assessment of driving performance in HIV infection. Neurology 63, 1417–1422 (2004).
Marcotte, T. D. et al. Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Arch. Neurol. 60, 1406–1412 (2003).
Tozzi, V. et al. Changes in neurocognitive performance in a cohort of patients treated with HAART for 3 years. J. Acquir. Immune Defic. Syndr. 28, 19–27 (2001).
Stuss, D. T. & Alexander, M. P. Executive functions and the frontal lobes: a conceptual view. Psychol. Res. 63, 289–298 (2000).
Grant, I. et al. HIV-1 associated neurocognitive disorder. The HNRC Group. Clin. Neuropharmacol. 15, 364A–365A (1992).
Martin, E. M. et al. Cognitive impulsivity and HIV serostatus in substance dependent males. J. Int. Neuropsychol. Soc. 10, 931–938 (2004).
Ernst, T., Chang, L. & Arnold, S. Increased glial metabolites predict increased working memory network activation in HIV brain injury. Neuroimage 19, 1686–1693 (2003).
Martin, E. M. et al. Performance of patients with early HIV-1 infection on the Stroop Task. J. Clin. Exp. Neuropsychol. 14, 857–868 (1992).
Martin, E. M. et al. Delayed nonmatch-to-sample performance in HIV-seropositive and HIV-seronegative polydrug abusers. Neuropsychology 17, 283–288 (2003).
Stout, J. C. et al. Decline in working memory associated with HIV infection. HNRC Group. Psychol. Med. 25, 1221–1232 (1995).
Peavy, G. et al. Verbal memory performance of patients with human immunodeficiency virus infection: evidence of subcortical dysfunction. The HNRC Group. J. Clin. Exp. Neuropsychol. 16, 508–523 (1994).
Carey, C. L., Woods, S. P., Rippeth, J. D., Heaton, R. K. & Grant, I. Prospective memory in HIV-1 infection. J. Clin. Exp. Neuropsychol. 28, 536–548 (2006).
Letendre, S. L. et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann. Neurol. 56, 416–423 (2004). Showed that cART has salutary effects in individuals with HIV-associated neurocognitive impairment, and that optimizing the CNS penetration of antiretroviral drugs benefits neurocognitive outcomes.
Chang, L. et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann. Neurol. 56, 259–272 (2004).
Chang, L. et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology 57, 1001–1007 (2001).
Ernst, T., Chang, L., Jovicich, J., Ames, N. & Arnold, S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology 59, 1343–1349 (2002).
Ances, B. M. et al. Caudate blood flow and volume are reduced in HIV+ neurocognitively impaired patients. Neurology 66, 862–866 (2006).
Tuszynski, M. H. et al. A phase 1 clinical trial of nerve growth factor gene therapy for Alzheimer disease. Nature Med 11, 551–555 (2005).
Everall, I. P. et al. Lithium ameliorates HIV-gp120-mediated neurotoxicity. Mol. Cell. Neurosci. 21, 493–501 (2002).
Letendre, S. L. et al. Lithium improves HIV-associated neurocognitive impairment. AIDS 20, 1885–1888 (2006).
Zink, M. C. et al. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. JAMA 293, 2003–2011 (2005).
Lipton, S. A. & Chen, H. S. Paradigm shift in neuroprotective drug development: clinically tolerated NMDA receptor inhibition by memantine. Cell Death Differ. 11, 18–20 (2004).
Toggas, S. M., Masliah, E. & Mucke, L. Prevention of HIV-1 gp120-induced neuronal damage in the central nervous system of transgenic mice by the NMDA receptor antagonist memantine. Brain Res 706, 303–307 (1996).
Chen, H. S. et al. Neuroprotective concentrations of the N-methyl-D-aspartate open-channel blocker memantine are effective without cytoplasmic vacuolation following post-ischemic administration and do not block maze learning or long-term potentiation. Neuroscience 86, 1121–1132 (1998).
Nath, A. et al. Synergistic neurotoxicity by human immunodeficiency virus proteins Tat and gp120: protection by memantine. Ann. Neurol. 47, 186–194 (2000).
Langford, T. D., Letendre, S. L., Larrea, G. J. & Masliah, E. Changing patterns in the neuropathogenesis of HIV during the HAART era. Brain Pathol. 13, 195–210 (2003). This up-to-date review summarizes neuropathological manifestations of HIV in the brain and recent changes related to antiretroviral therapy.
Demakis, G. J. Frontal lobe damage and tests of executive processing: a meta-analysis of the category test, stroop test, and trail-making test. J. Clin. Exp. Neuropsychol. 26, 441–450 (2004).
Robertson, K. R. et al. Highly active antiretroviral therapy improves neurocognitive functioning. J. Acquir. Immune Defic. Syndr. 36, 562–566 (2004).
Starace, F. et al. Cognitive and affective disorders associated to HIV infection in the HAART era: findings from the NeuroICONA study. Cognitive impairment and depression in HIV/AIDS. The NeuroICONA study. Acta Psychiatr. Scand. 106, 20–26 (2002).
Suarez, S. et al. Outcome of patients with HIV-1-related cognitive impairment on highly active antiretroviral therapy. AIDS 15, 195–200 (2001).
Maschke, M. et al. Incidence and prevalence of neurological disorders associated with HIV since the introduction of highly active antiretroviral therapy (HAART). J. Neurol. Neurosurg. Psychiatry 69, 376–380 (2000).
Langford, D. et al. Relationship of antiretroviral treatment to postmortem brain tissue viral load in human immunodeficiency virus-infected patients. J. Neurovirol. 12, 100–107 (2006).
Wynn, H. E., Brundage, R. C. & Fletcher, C. V. Clinical implications of CNS penetration of antiretroviral drugs. CNS Drugs 16, 595–609 (2002).
Nickle, D. C., Shriner, D., Mittler, J. E., Frenkel, L. M. & Mullins, J. I. Importance and detection of virus reservoirs and compartments of HIV infection. Curr. Opin. Microbiol. 6, 410–416 (2003).
Ellis, R. J. et al. Neurocognitive impairment is an independent risk factor for death in HIV infection. San Diego HIV Neurobehavioral Research Center Group. Arch. Neurol. 54, 416–424 (1997).
Soontornniyomkij, V., Wang, G., Pittman, C. A., Wiley, C. A. & Achim, C. L. Expression of brain-derived neurotrophic factor protein in activated microglia of human immunodeficiency virus type 1 encephalitis. Neuropathol. Appl. Neurobiol. 24, 453–460 (1998).
Mocchetti, I. & Bachis, A. Brain-derived neurotrophic factor activation of TrkB protects neurons from HIV-1/gp120-induced cell death. Crit. Rev. Neurobiol. 16, 51–57 (2004).
Wang, J. Y. et al. Involvement of α1β1 integrin in insulin-like growth factor-1-mediated protection of PC12 neuronal processes from tumor necrosis factor-α-induced injury. J. Neurosci. Res. 83, 7–18 (2006).
Everall, I. P. et al. Amelioration of neurotoxic effects of HIV envelope protein gp120 by fibroblast growth factor: a strategy for neuroprotection. J. Neuropathol. Exp. Neurol. 60, 293–301 (2001).
Hashimoto, M. et al. Fibroblast growth factor 1 regulates signaling via the glycogen synthase kinase-3β pathway. Implications for neuroprotection. J. Biol. Chem. 277, 32985–32991 (2002).
Kalehua, A. N. et al. Monocyte chemoattractant protein-1 and macrophage inflammatory protein-2 are involved in both excitotoxin-induced neurodegeneration and regeneration. Exp. Cell Res. 297, 197–211 (2004).
Langford, D., Sanders, V. J., Mallory, M., Kaul, M. & Masliah, E. Expression of stromal cell-derived factor 1α protein in HIV encephalitis. J. Neuroimmunol. 127, 115–126 (2002).
Udagawa, J. et al. The role of leptin in the development of the cerebral cortex in mouse embryos. Endocrinology 147, 647–658 (2006).
Acknowledgements
The authors wish to thank A. Bhatt and F. Duitch for their assistance with preparing the manuscript. The authors also wish to acknowledge the contributions of our colleagues in the HIV Neurobehavioral Research Center (HNRC) Group whose insights have contributed to the concepts in this review. The San Diego HNRC group is affiliated with the University of California, San Diego, USA, the Naval Hospital, San Diego, and the San Diego Veterans Affairs Healthcare System, and includes: I. Grant, J. H. Atkinson, J. A. McCutchan and T. D. Marcotte; Naval Hospital San Diego: M. R. Wallace; Neuromedical Component: J. A. McCutchan, R. J. Ellis, S. Letendre, R. Schrier; Neurobehavioural Component: R. K. Heaton, M. Cherner, S. P. Woods; Imaging Component: T. Jernigan, J. Hesselink, M. J. Taylor; Neurobiology Component: E. Masliah, I. Everall, D. Langford; Clinical Trials Component: J. A. McCutchan, J. H. Atkinson, R. J. Ellis, S. Letendre; Data Management Unit: A. C. Gamst, C. Cushman, D. R. Masys; Statistics Unit: I. Abramson, D. Lazzaretto and T. Wolfson. The views expressed in this article are those of the authors and do not reflect the official policy or position of the Department of the Navy, Department of Defense, or the United States Government. The HNRC is supported by a Center award from the National Institute of Mental Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
OMIM
FURTHER INFORMATION
Glossary
- Combination antiretroviral therapy
-
(cART). A strategy by which multiple anti-human immuno-deficiency virus drugs are used in specific combinations to suppress viral replication and thereby reverse and prevent progressive immune deficiency and restore health.
- Neurotropism
-
The capacity of a pathogen, such as a virus, to invade the CNS and infect its cells.
- Neurovirulence
-
The capacity of a pathogen to cause disease of the nervous system.
- Efflux transporter
-
A cellular protein that pumps substrate (such as a drug) out of the cell.
- HIV encephalitis
-
Brain inflammation (infiltration of leukocytes and activation of CNS glia) accompanied by the expression of viral nucleic acids and proteins.
- Synaptodendritic injury
-
Anatomical and functional damage to pre- and postsynaptic structures that occurs without neuron death, and is potentially reversible.
- Borna disease
-
A newly classified non-segmented negative-strand RNA virus causing a sporadic, transmissible, progressive neurological disease in many species. Infection causes movement and behavioural disturbances reminiscent of some neuropsychiatric syndromes. Evidence in recent years indicates that Borna disease virus infects humans; it might be associated with various neuropsychiatric disorders, including schizophrenia.
- Hippocampal dentate gyrus
-
Grey matter composed of three layers situated above the gyrus hippocampi. The molecular layer is continuous with the hippocampus in the hippocampal fissure. The granular layer consists of closely arranged spherical or oval neurons, called granule cells, the axons of which pass through the polymorphic layer and end on the dendrites of pyramidal cells in the hippocampus.
- Dendritic beading
-
Bulging or knotted appearance of dendrites, indicative of excitotoxic stress or ischaemic injury.
- Axoplasmic flow
-
Directed transport of organelles and molecules along a nerve cell axon. Transport can be from the cell body or towards the cell body.
- Envelope protein
-
Glycoprotein expressed on the surface of the human immunodeficiency virus particle.
- Activation state
-
Intercellular signalling through chemical mediators such as cytokines can result in changes in the activation state such that target cells (for example, immune cells and glia) change their metabolic activity, gene transcription, protein expression, morphology and other characteristics.
- Morris water maze
-
A behavioural assessment procedure designed to assess spatial memory in mice or rats.
- HIV-associated dementia
-
(HAD). Documented moderate to severe impairment in two or more cognitive areas (usually the impairment is severe and pervasive) with marked decline in everyday functioning.
- Asymptomatic neurocognitive impairment
-
(ANI). Documented mild impairment in two or more cognitive areas without a clear effect on everyday functioning.
- Mild neurocognitive disorder
-
(MND). Documented mild to moderate impairment in two or more cognitive areas with mild to moderate decline in everyday functioning.
- FSTC circuits
-
Frontal-striato-thalamo-cortical brain circuits. Reciprocal loops that interconnect neurons in the prefrontal cortex, striatum and thalamus, and that subserve complex cognitive abilities such as planning and execution of multi-step tasks in the context of ongoing distractions and new information.
Rights and permissions
About this article
Cite this article
Ellis, R., Langford, D. & Masliah, E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8, 33–44 (2007). https://doi.org/10.1038/nrn2040
Issue Date:
DOI: https://doi.org/10.1038/nrn2040
This article is cited by
-
Effects of Morphine on Gp120-induced Neuroinflammation Under Immunocompetent Vs. Immunodeficient Conditions
Journal of Neuroimmune Pharmacology (2023)
-
Progressive Degeneration and Adaptive Excitability in Dopamine D1 and D2 Receptor-Expressing Striatal Neurons Exposed to HIV-1 Tat and Morphine
Cellular and Molecular Neurobiology (2023)
-
Update on Central Nervous System Effects of HIV in Adolescents and Young Adults
Current HIV/AIDS Reports (2023)
-
Verbal Memory Performance and Depressive Symptoms in Persons with Treated HIV
AIDS and Behavior (2023)
-
CCR5 closes the temporal window for memory linking
Nature (2022)