Abstract
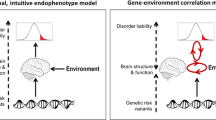
Genes are major contributors to many psychiatric diseases, but their mechanisms of action have long seemed elusive. The intermediate phenotype concept represents a strategy for characterizing the neural systems affected by risk gene variants to elucidate quantitative, mechanistic aspects of brain function implicated in psychiatric disease. Using imaging genetics as an example, we illustrate recent advances, challenges and implications of linking genes to structural and functional variation in brain systems related to cognition and emotion.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sullivan, P. F., Kendler, K. S. & Neale, M. C. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 60, 1187–1192 (2003).
Sullivan, P. F., Neale, M. C. & Kendler, K. S. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry 157, 1552–1562 (2000).
Weinberger, D. R. et al. Prefrontal neurons and the genetics of schizophrenia. Biol. Psychiatry 50, 825–844 (2001).
Meyer-Lindenberg, A. et al. Midbrain dopamine and prefrontal function in humans: interaction and modulation by COMT genotype. Nature Neurosci. 8, 594–596 (2005).
Meyer-Lindenberg, A. et al. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nature Neurosci. 5, 267–271 (2002).
Pezawas, L. et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neurosci. 8, 828–834 (2005).
Waldman, I. D. Statistical approaches to complex phenotypes: evaluating neuropsychological endophenotypes for attention-deficit/hyperactivity disorder. Biol. Psychiatry 57, 1347–1356 (2005).
Goldman, D., Oroszi, G. & Ducci, F. The genetics of addictions: uncovering the genes. Nature Rev. Genet. 6, 521–532 (2005).
Belmonte, M. K. et al. Autism as a disorder of neural information processing: directions for research and targets for therapy. Mol. Psychiatry 9, 646–663 (2004).
Riley, B. P. & McGuffin, P. Linkage and associated studies of schizophrenia. Am. J. Med. Genet. 97, 23–44 (2000).
Menzel, S. Genetic and molecular analyses of complex metabolic disorders: genetic linkage. Ann. NY Acad. Sci. 967, 249–257 (2002).
Harrison, P. J. & Weinberger, D. R. Schizophrenia genes, gene expression, and neuropathology: on the matter of their convergence. Mol. Psychiatry 10, 40–68 (2005).
Risch, N. Genetic linkage and complex diseases, with special reference to psychiatric disorders. Genet. Epidemiol. 7, 3–16; discussion 17–45 (1990).
Gottesman, I. I. & Shields, J. A polygenic theory of schizophrenia. Proc. Natl Acad. Sci. USA 58, 199–205 (1967).
Page, G. P., George, V., Go, R. C., Page, P. Z. & Allison, D. B. 'Are we there yet?': Deciding when one has demonstrated specific genetic causation in complex diseases and quantitative traits. Am. J. Hum. Genet. 73, 711–719 (2003).
Deschepper, C. F., Boutin-Ganache, I., Zahabi, A. & Jiang, Z. In search of cardiovascular candidate genes: interactions between phenotypes and genotypes. Hypertension 39, 332–336 (2002).
Gottesman, I. I. & Gould, T. D. The endophenotype concept in psychiatry: etymology and strategic intentions. Am. J. Psychiatry 160, 636–645 (2003).
Almasy, L. & Blangero, J. Endophenotypes as quantitative risk factors for psychiatric disease: rationale and study design. Am. J. Med. Genet. 105, 42–44 (2001).
Weinberger, D. R. Schizophrenia: new phenes and new genes. Biol. Psychiatry 46, 3–7 (1999).
Alexander, G. E., DeLong, M. R. & Strick, P. L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 9, 357–381 (1986).
Goldman-Rakic, P. S., Selemon, L. D. & Schwartz, M. L. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience 12, 719–743 (1984).
Friston, K. J. The disconnection hypothesis. Schizophr. Res. 30, 115–125 (1998).
Meyer-Lindenberg, A. S. et al. Regionally specific disturbance of dorsolateral prefrontal-hippocampal functional connectivity in schizophrenia. Arch. Gen. Psychiatry 62, 379–386 (2005).
Dunnett, S. B., Meldrum, A. & Muir, J. L. Frontal-striatal disconnection disrupts cognitive performance of the frontal-type in the rat. Neuroscience 135, 1055–1065 (2005).
Pantelis, C. et al. Frontal-striatal cognitive deficits in patients with chronic schizophrenia. Brain 120, 1823–1843 (1997).
Swerdlow, N. R., Geyer, M. A. & Braff, D. L. Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacology (Berl.) 156, 194–215 (2001).
Carlsson, A. A paradigm shift in brain research. Science 294, 1021–1024 (2001).
Jaskiw, G. E., Karoum, F. K. & Weinberger, D. R. Persistent elevations in dopamine and its metabolites in the nucleus accumbens after mild subchronic stress in rats with ibotenic acid lesions of the medial prefrontal cortex. Brain Res. 534, 321–323 (1990).
Williams, G. V. & Goldman-Rakic, P. S. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376, 572–575 (1995).
Schultz, W. Predictive reward signal of dopamine neurons. J. Neurophysiol. 80, 1–27 (1998).
Squire, L. R., Stark, C. E. & Clark, R. E. The medial temporal lobe. Annu. Rev. Neurosci. 27, 279–306 (2004).
Harrison, P. J. & Eastwood, S. L. Neuropathological studies of synaptic connectivity in the hippocampal formation in schizophrenia. Hippocampus 11, 508–519 (2001).
Heckers, S. Neuroimaging studies of the hippocampus in schizophrenia. Hippocampus 11, 520–528 (2001).
Heckers, S. et al. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nature Neurosci. 1, 318–323 (1998).
Simons, J. S. & Spiers, H. J. Prefrontal and medial temporal lobe interactions in long-term memory. Nature Rev. Neurosci. 4, 637–648 (2003).
Phillips, M. L., Drevets, W. C., Rauch, S. L. & Lane, R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol. Psychiatry 54, 504–514 (2003).
Weinberger, D. R., Berman, K. F., Suddath, R. & Torrey, E. F. Evidence of dysfunction of a prefrontal-limbic network in schizophrenia: a magnetic resonance imaging and regional cerebral blood flow study of discordant monozygotic twins. Am. J. Psychiatry 149, 890–897 (1992).
Bertolino, A. et al. Altered development of prefrontal neurons in rhesus monkeys with neonatal mesial temporo-limbic lesions: a proton magnetic resonance spectroscopic imaging study. Cereb. Cortex 7, 740–748 (1997).
Weinberger, D. R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 44, 660–669 (1987).
Lewis, D. A. et al. Dopamine transporter immunoreactivity in monkey cerebral cortex: regional, laminar, and ultrastructural localization. J. Comp. Neurol. 432, 119–136 (2001).
Tunbridge, E. M., Bannerman, D. M., Sharp, T. & Harrison, P. J. Catechol-o-methyltransferase inhibition improves set-shifting performance and elevates stimulated dopamine release in the rat prefrontal cortex. J. Neurosci. 24, 5331–5335 (2004).
Tunbridge, E. M., Harrison, P. J. & Weinberger, D. R. Catechol-O-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol. Psychiatry 60, 141–151 (2006).
Owen, M. J., Williams, N. M. & O'Donovan, M. C. The molecular genetics of schizophrenia: new findings promise new insights. Mol. Psychiatry 9, 14–27 (2004).
Murphy, K. C. Schizophrenia and velo-cardio-facial syndrome. Lancet 359, 426–430 (2002).
Chen, J. et al. Functional analysis of genetic variation in catechol-O-methyltransferase (COMT): effects on mRNA, protein, and enzyme activity in postmortem human brain. Am. J. Hum. Genet. 75, 807–821 (2004).
Egan, M. F. et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc. Natl Acad. Sci. USA 98, 6917–6922 (2001).
Goldberg, T. E. et al. Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Arch. Gen. Psychiatry 60, 889–896 (2003).
Mattay, V. S. et al. Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc. Natl Acad. Sci. USA 100, 6186–6191 (2003).
Akil, M. et al. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J. Neurosci. 23, 2008–2013 (2003).
Callicott, J. H. et al. Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl Acad. Sci. USA 102, 8627–8632 (2005).
Gothelf, D. et al. COMT genotype predicts longitudinal cognitive decline and psychosis in 22q11.2 deletion syndrome. Nature Neurosci. 8, 1500–1502 (2005).
Craddock, N., Owen, M. J. & O'Donovan, M. C. The catechol-O-methyl transferase (COMT) gene as a candidate for psychiatric phenotypes: evidence and lessons. Mol. Psychiatry 11, 446–458 (2006).
Fan, J. B. et al. Catechol-O-methyltransferase gene Val/Met functional polymorphism and risk of schizophrenia: a large-scale association study plus meta-analysis. Biol. Psychiatry 57, 139–144 (2005).
Munafo, M. R., Bowes, L., Clark, T. G. & Flint, J. Lack of association of the COMT (Val158/108 Met) gene and schizophrenia: a meta-analysis of case-control studies. Mol. Psychiatry 10, 765–770 (2005).
Schott, B. H. et al. The dopaminergic midbrain participates in human episodic memory formation: evidence from genetic imaging. J. Neurosci. 26, 1407–1417 (2006).
Cohen, M. X., Young, J., Baek, J. M., Kessler, C. & Ranganath, C. Individual differences in extraversion and dopamine genetics predict neural reward responses. Brain Res. Cogn Brain Res. 25, 851–861 (2005).
Egan, M. F. et al. Variation in GRM3 affects cognition, prefrontal glutamate, and risk for schizophrenia. Proc. Natl Acad. Sci. USA 101, 12604–12609 (2004).
de Quervain, D. J. & Papassotiropoulos, A. Identification of a genetic cluster influencing memory performance and hippocampal activity in humans. Proc. Natl Acad. Sci. USA 103, 4270–4274 (2006).
Callicott, J. H. et al. Abnormal fMRI response of the dorsolateral prefrontal cortex in cognitively intact siblings of patients with schizophrenia. Am. J. Psychiatry 160, 709–719 (2003).
Cannon, T. D. et al. Association of DISC1/TRAX haplotypes with schizophrenia, reduced prefrontal gray matter, and impaired short- and long-term memory. Arch. Gen. Psychiatry 62, 1205–1213 (2005).
Bertolino, A. et al. Prefrontal-hippocampal coupling during declarative memory is modulated by COMT Val158Met genotype. Biol. Psychiatry 4 Sept 2006 (doi: 10.1016/j.biopsych.2006.03.078).
Shifman, S. et al. A highly significant association between a COMT haplotype and schizophrenia. Am. J. Hum. Genet. 71, 1296–1302 (2002).
Bray, N. J. et al. A haplotype implicated in schizophrenia susceptibility is associated with reduced COMT expression in human brain. Am. J. Hum. Genet. 73, 152–161 (2003).
Palmatier, M. A. et al. COMT haplotypes suggest P2 promoter region relevance for schizophrenia. Mol. Psychiatry 9, 859–870 (2004).
Meyer-Lindenberg, A. et al. Impact of complex genetic variation in COMT on human brain function. Mol. Psychiatry 20 June 2006 (doi: 10.1038/sj.mp.4001860).
Diaz-Asper, C. M. Weinberger, D. R. & Goldberg, T. E. Catechol-O-methyltransferase polymorphisms and some implications for cognitive therapeutics. NeuroRX 3, 97–105 (2006).
Devlin, B., Roeder, K. & Wasserman, L. Genomic control, a new approach to genetic-based association studies. Theor. Popul. Biol. 60, 155–166 (2001).
Laurienti, P. J., Burdette, J. H. & Maldjian, J. A. Separating neural processes using mixed event-related and epoch-based fMRI paradigms. J. Neurosci. Methods 131, 41–50 (2003).
Friedman, L. & Glover, G. H. Report on a multicenter fMRI quality assurance protocol. J. Magn. Reson. Imaging 23, 827–839 (2006).
Hariri, A. R. et al. Serotonin transporter genetic variation and the response of the human amygdala. Science 297, 400–403 (2002).
Friston, K. J. et al. Classical and Bayesian inference in neuroimaging: theory. Neuroimage 16, 465–483 (2002).
Manji, H. K., Drevets, W. C. & Charney, D. S. The cellular neurobiology of depression. Nature Med. 7, 541–547 (2001).
Mayberg, H. S. Limbic-cortical dysregulation: a proposed model of depression. J. Neuropsychiatry Clin. Neurosci. 9, 471–481 (1997).
Drevets, W. C. et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 386, 824–827 (1997).
Mayberg, H. S. et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am. J. Psychiatry 156, 675–682 (1999).
Paus, T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Rev. Neurosci. 2, 417–424 (2001).
Stefanacci, L. & Amaral, D. G. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J. Comp. Neurol. 451, 301–323 (2002).
Sotres-Bayon, F., Bush, D. E. & LeDoux, J. E. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn. Mem. 11, 525–535 (2004).
Nemeroff, C. B. & Owens, M. J. Treatment of mood disorders. Nature Neurosci. 5, S1068–S1070 (2002).
Lesch, K. P. et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531 (1996).
Caspi, A. et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 301, 386–389 (2003).
Hariri, A. R. et al. A susceptibility gene for affective disorders and the response of the human amygdala. Arch. Gen. Psychiatry 62, 146–152 (2005).
Heinz, A. et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neurosci. 8, 20–21 (2005).
Brown, S. M. et al. A regulatory variant of the human tryptophan hydroxylase-2 gene biases amygdala reactivity. Mol. Psychiatry 10, 884–888, 805 (2005).
Canli, T., Congdon, E., Gutknecht, L., Constable, R. T. & Lesch, K. P. Amygdala responsiveness is modulated by tryptophan hydroxylase-2 gene variation. J. Neural Transm. 112, 1479–1485 (2005).
Sabol, S. Z., Hu, S. & Hamer, D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum. Genet. 103, 273–279 (1998).
Caspi, A. et al. Role of genotype in the cycle of violence in maltreated children. Science 297, 851–854 (2002).
Meyer-Lindenberg, A. et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl Acad. Sci. USA 103, 6269–6274 (2006).
Fan, J., Fossella, J., Sommer, T., Wu, Y. & Posner, M. I. Mapping the genetic variation of executive attention onto brain activity. Proc. Natl Acad. Sci. USA 100, 7406–7411 (2003).
Svenningsson, P. et al. DARPP-32: an integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 44, 269–296 (2004).
Weinberger, D. R. et al. Variation in PPP1R1B predicts risk for schizophrenia, cognitive function, and gene expression in brain. Am. J. Med. Genet. B Neuropsychiatr. Genet. 138B, 139–140 (2005).
Meyer-Lindenberg, A. et al. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc. Natl Acad. Sci. USA 103, 6269–6274 (2006).
Freedman, R. et al. Linkage of a neurophysiological deficit in schizophrenia to a chromosome 15 locus. Proc. Natl Acad. Sci. USA 94, 587–592 (1997).
Goldman-Rakic, P. S., Muly, E. C. & Williams, G. V. D1 receptors in prefrontal cells and circuits. Brain Res. Brain Res. Rev. 31, 295–301 (2000).
Seamans, J. K., Gorelova, N., Durstewitz, D. & Yang, C. R. Bidirectional dopamine modulation of GABAergic inhibition in prefrontal cortical pyramidal neurons. J. Neurosci. 21, 3628–3638 (2001).
Acknowledgements
This work was supported by the National Institute of Mental Health's Intramural Research Program. We thank C. Rainey for help with figure preparation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Meyer-Lindenberg, A., Weinberger, D. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci 7, 818–827 (2006). https://doi.org/10.1038/nrn1993
Issue Date:
DOI: https://doi.org/10.1038/nrn1993
This article is cited by
-
Exploring Imaging Genetic Markers of Alzheimer’s Disease Based on a Novel Nonlinear Correlation Analysis Algorithm
Journal of Molecular Neuroscience (2024)
-
Progressive trajectories of schizophrenia across symptoms, genes, and the brain
BMC Medicine (2023)
-
The brain structure, immunometabolic and genetic mechanisms underlying the association between lifestyle and depression
Nature Mental Health (2023)
-
Machine Learning for Brain Imaging Genomics Methods: A Review
Machine Intelligence Research (2023)
-
Rare CNVs and phenome-wide profiling highlight brain structural divergence and phenotypical convergence
Nature Human Behaviour (2023)