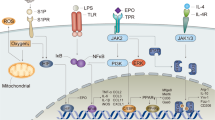
Abstract
Erythropoietin mediates an evolutionarily conserved, ancient immune response that limits damage to the heart, the nervous system and other tissues following injury. New evidence indicates that erythropoietin specifically prevents the destruction of viable tissue surrounding the site of an injury by signalling through a non-haematopoietic receptor. Engineered derivatives of erythropoietin that have a high affinity for this receptor have been developed, and these show robust tissue-protective effects in diverse preclinical models without stimulating erythropoiesis. A recent successful proof-of-concept clinical trial that used erythropoietin to treat human patients who had suffered a stroke encourages the evaluation of both this cytokine and non-erythropoietic derivatives as therapeutic agents to limit tissue injury.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Miyake, T., Kung, C. K. & Goldwasser, E. Purification of human erythropoietin. J. Biol. Chem. 252, 5558–5564 (1977).
Fisher, J. W. Erythropoietin: physiology and pharmacology update. Exp. Biol. Med. (Maywood) 228, 1–14 (2003).
Jelkmann, W. The enigma of the metabolic fate of circulating erythropoietin (Epo) in view of the pharmacokinetics of the recombinant drugs rhEpo and NESP. Eur. J. Haematol. 69, 265–274 (2002).
Tan, C. C., Eckardt, K. U., Firth, J. D. & Ratcliffe, P. J. Feedback modulation of renal and hepatic erythropoietin mRNA in response to graded anemia and hypoxia. Am. J. Physiol. 263, F474–F481 (1992).
Masuda, S. et al. A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J. Biol. Chem. 269, 19488–19493 (1994).
Konishi, Y., Chui, D. H., Hirose, H., Kunishita, T. & Tabira, T. Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 609, 29–35 (1993).
Siren, A. L. et al. Erythropoietin and erythropoietin receptor in human ischemic/hypoxic brain. Acta Neuropathol. (Berl.) 101, 271–276 (2001).
Morishita, E., Masuda, S., Nagao, M., Yasuda, Y. & Sasaki, R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience 76, 105–116 (1997).
Sakanaka, M. et al. In vivo evidence that erythropoietin protects neurons from ischemic damage. Proc. Natl Acad. Sci. USA 95, 4635–4640 (1998).
Brines, M. L. et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc. Natl Acad. Sci. USA 97, 10526–10531 (2000).
Siren, A. L. et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc. Natl Acad. Sci. USA 98, 4044–4049 (2001).
Beleslin-Cokic, B. B. et al. Erythropoietin and hypoxia stimulate erythropoietin receptor and nitric oxide production by endothelial cells. Blood 104, 2073–2080 (2004).
Kawakami, M., Iwasaki, S., Sato, K. & Takahashi, M. Erythropoietin inhibits calcium-induced neurotransmitter release from clonal neuronal cells. Biochem. Biophys. Res. Commun. 279, 293–297 (2000).
Koshimura, K., Murakami, Y., Sohmiya, M., Tanaka, J. & Kato, Y. Effects of erythropoietin on neuronal activity. J. Neurochem. 72, 2565–2572 (1999).
Martinez-Estrada, O. M. et al. Erythropoietin protects the in vitro blood-brain barrier against VEGF-induced permeability. Eur. J. Neurosci. 18, 2538–2544 (2003).
Li, W. et al. Beneficial effect of erythropoietin on experimental allergic encephalomyelitis. Ann. Neurol. 56, 767–777 (2004).
Semenza, G. L. HIF-1 and mechanisms of hypoxia sensing. Curr. Opin. Cell Biol. 13, 167–171 (2001).
Schuster, J. M. & Nelson, P. S. Toll receptors: an expanding role in our understanding of human disease. J. Leukoc. Biol. 67, 767–773 (2000).
Kertesz, N., Wu, J., Chen, T. H., Sucov, H. M. & Wu, H. The role of erythropoietin in regulating angiogenesis. Dev. Biol. 276, 101–110 (2004).
Shingo, T., Sorokan, S. T., Shimazaki, T. & Weiss, S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J. Neurosci. 21, 9733–9743 (2001).
Brines, M. et al. Erythropoietin mediates tissue protection through an erythropoietin and common β-subunit heteroreceptor. Proc. Natl Acad. Sci. USA 101, 14907–14912 (2004).
Chin, K. et al. Production and processing of erythropoietin receptor transcripts in brain. Brain Res. Mol. Brain Res. 81, 29–42 (2000).
Chikuma, M., Masuda, S., Kobayashi, T., Nagao, M. & Sasaki, R. Tissue-specific regulation of erythropoietin production in the murine kidney, brain, and uterus. Am. J. Physiol. Endocrinol. Metab. 279, E1242–E1248 (2000).
Nagai, A. et al. Erythropoietin and erythropoietin receptors in human CNS neurons, astrocytes, microglia, and oligodendrocytes grown in culture. J. Neuropathol. Exp. Neurol. 60, 386–392 (2001).
Masuda, S. et al. Functional erythropoietin receptor of the cells with neural characteristics. Comparison with receptor properties of erythroid cells. J. Biol. Chem. 268, 11208–11216 (1993).
Livnah, O. et al. Crystallographic evidence for preformed dimers of erythropoietin receptor before ligand activation. Science 283, 987–990 (1999).
Eid, T. et al. Increased expression of erythropoietin receptor on blood vessels in the human epileptogenic hippocampus with sclerosis. J. Neuropathol. Exp. Neurol. 63, 73–83 (2004).
Juul, S. E., Anderson, D. K., Li, Y. & Christensen, R. D. Erythropoietin and erythropoietin receptor in the developing human central nervous system. Pediatr. Res. 43, 40–49 (1998).
Suzuki, N. et al. Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood 100, 2279–2288 (2002).
Masuda, S., Chikuma, M. & Sasaki, R. Insulin-like growth factors and insulin stimulate erythropoietin production in primary cultured astrocytes. Brain Res. 746, 63–70 (1997).
Johns, L., Sinclair, A. J. & Davies, J. A. Hypoxia/hypoglycemia-induced amino acid release is decreased in vitro by preconditioning. Biochem. Biophys. Res. Commun. 276, 134–136 (2000).
Blondeau, N., Plamondon, H., Richelme, C., Heurteaux, C. & Lazdunski, M. K(ATP) channel openers, adenosine agonists and epileptic preconditioning are stress signals inducing hippocampal neuroprotection. Neuroscience 100, 465–474 (2000).
Ho, V., Acquaviva, A., Duh, E. & Bunn, H. F. Use of a marked erythropoietin gene for investigation of its cis-acting elements. J. Biol. Chem. 270, 10084–10090 (1995).
Bernaudin, M. et al. A potential role for erythropoietin in focal permanent cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 19, 643–651 (1999).
Mori, M. et al. Activation of extracellular signal-regulated kinases ERK1 and ERK2 induces Bcl-xL up-regulation via inhibition of caspase activities in erythropoietin signaling. J. Cell Physiol. 195, 290–297 (2003).
Ruscher, K. et al. Erythropoietin is a paracrine mediator of ischemic tolerance in the brain: evidence from an in vitro model. J. Neurosci. 22, 10291–10301 (2002).
Kilic, U. et al. Erythropoietin protects from axotomy-induced degeneration of retinal ganglion cells by activating ERK-1/-2. FASEB J. 19, 249–251 (2004).
Digicaylioglu, M. & Lipton, S. A. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature 412, 641–647 (2001).
Marrero, M. B., Venema, R. C., Ma, H., Ling, B. N. & Eaton, D. C. Erythropoietin receptor-operated Ca2+ channels: activation by phospholipase C-γ 1. Kidney Int. 53, 1259–1268 (1998).
Yamamoto, M., Koshimura, K., Sohmiya, M., Murakami, Y. & Kato, Y. Effect of erythropoietin on nitric oxide production in the rat hippocampus using in vivo brain microdialysis. Neuroscience 128, 163–168 (2004).
Yun, H. Y., Dawson, V. L. & Dawson, T. M. Neurobiology of nitric oxide. Crit. Rev. Neurobiol. 10, 291–316 (1996).
Villa, P. et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 198, 971–975 (2003).
Agnello, D. et al. Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res. 952, 128–134 (2002).
Cuzzocrea, S. et al. Erythropoietin reduces the degree of arthritis caused by type II collagen in the mouse. Arthritis Rheum. 52, 940–950 (2005).
Sela, S. et al. The polymorphonuclear leukocyte — a new target for erythropoietin. Nephron 88, 205–210 (2001).
Grasso, G. et al. Beneficial effects of systemic administration of recombinant human erythropoietin in rabbits subjected to subarachnoid hemorrhage. Proc. Natl Acad. Sci. USA 99, 5627–5631 (2002).
Gorio, A. et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc. Natl Acad. Sci. USA 99, 9450–9455 (2002).
Wang, L., Zhang, Z., Wang, Y., Zhang, R. & Chopp, M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 35, 1732–1737 (2004).
Nagelhus, E. A., Mathiisen, T. M. & Ottersen, O. P. Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1. Neuroscience 129, 905–913 (2004).
Dirnagl, U., Simon, R. P. & Hallenbeck, J. M. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 26, 248–254 (2003).
Dawson, T. M. Preconditioning-mediated neuroprotection through erythropoietin? Lancet 359, 96–97 (2002).
Bernaudin, M. et al. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J. Cereb. Blood Flow Metab. 22, 393–403 (2002).
Lee, S. M. et al. EPO receptor-mediated ERK kinase and NF-κB activation in erythropoietin-promoted differentiation of astrocytes. Biochem. Biophys. Res. Commun. 320, 1087–1095 (2004).
Juul, S. E. et al. Erytropoietin concentrations in cerebrospinal fluid of nonhuman primates and fetal sheep following high-dose recombinant erythropoietin. Biol. Neonate 85, 138–144 (2004).
Dame, C., Juul, S. E. & Christensen, R. D. The biology of erythropoietin in the central nervous system and its neurotrophic and neuroprotective potential. Biol. Neonate 79, 228–235 (2001).
Ehrenreich, H. et al. Erythropoietin: a candidate compound for neuroprotection in schizophrenia. Mol. Psychiatry 9, 42–54 (2004).
Grimm, C. et al. HIF-1-induced erythropoietin in the hypoxic retina protects against light-induced retinal degeneration. Nature Med. 8, 718–724 (2002).
Foresta, C. et al. Erythropoietin stimulates testosterone production in man. J. Clin. Endocrinol. Metab. 78, 753–756 (1994).
Banks, W. A., Jumbe, N. L., Farrell, C. L., Niehoff, M. L. & Heatherington, A. C. Passage of erythropoietic agents across the blood-brain barrier: a comparison of human and murine erythropoietin and the analog darbepoetin alfa. Eur. J. Pharmacol. 505, 93–101 (2004).
Yamaji, R. et al. Brain capillary endothelial cells express two forms of erythropoietin receptor mRNA. Eur. J. Biochem. 239, 494–500 (1996).
Harris, K. W. & Winkelmann, J. C. Enzyme-linked immunosorbent assay detects a potential soluble form of the erythropoietin receptor in human plasma. Am. J. Hematol. 52, 8–13 (1996).
Juul, S. Recombinant erythropoietin as a neuroprotective treatment: in vitro and in vivo models. Clin. Perinatol. 31, 129–142 (2004).
Gassmann, M. et al. Non-erythroid functions of erythropoietin. Adv. Exp. Med. Biol. 543, 323–330 (2003).
Ghezzi, P. & Brines, M. Erythropoietin as an antiapoptotic, tissue-protective cytokine. Cell Death Differ. 11 (Suppl. 1), S37–S44 (2004).
Tsuchiya, D., Hong, S., Rajdev, S., Panter, S. & Weinstein, P. Evaluation of the neuroprotective effect of erythropoietin in cerebral ischemia produced by the intraluminal suture model. Soc. Neurosci. Abstr. 764.17 (2001).
Sadamoto, Y. et al. Erythropoietin prevents place navigation disability and cortical infarction in rats with permanent occlusion of the middle cerebral artery. Biochem. Biophys. Res. Commun. 253, 26–32 (1998).
Celik, M. et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc. Natl Acad. Sci. USA 99, 2258–2263 (2002).
Junk, A. K. et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA 99, 10659–10664 (2002).
Kumral, A. et al. Neuroprotective effect of erythropoietin on hypoxic-ischemic brain injury in neonatal rats. Biol. Neonate 83, 224–228 (2003).
Rex, T. S. et al. Systemic but not intraocular Epo gene transfer protects the retina from light-and genetic-induced degeneration. Mol. Ther. 10, 855–861 (2004).
Sattler, M. B. et al. Neuroprotective effects and intracellular signaling pathways of erythropoietin in a rat model of multiple sclerosis. Cell Death Differ. 11 (suppl. 2), S181–S192 (2004).
Adembri, C. et al. Erythropoietin attenuates post-traumatic injury in organotypic hippocampal slices. J. Neurotrauma 21, 1103–1112 (2004).
Orhan, B., Yalcin, S., Nurlu, G., Zeybek, D. & Muftuoglu, S. Erythropoietin against cisplatin-induced peripheral neurotoxicity in rats. Med. Oncol. 21, 197–203 (2004).
Keswani, S. C. et al. A novel endogenous erythropoietin mediated pathway prevents axonal degeneration. Ann. Neurol. 56, 815–826 (2004).
Campana, W. M. & Myers, R. R. Erythropoietin and erythropoietin receptors in the peripheral nervous system: changes after nerve injury. FASEB J. 15, 1804–1806 (2001).
Campana, W. M. & Myers, R. R. Exogenous erythropoietin protects against dorsal root ganglion apoptosis and pain following peripheral nerve injury. Eur. J. Neurosci. 18, 1497–1506 (2003).
Erbayraktar, S. et al. Asialoerythropoietin is a nonerythropoietic cytokine with broad neuroprotective activity in vivo. Proc. Natl Acad. Sci. USA 100, 6741–6746 (2003).
Leist, M. et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305, 239–242 (2004).
Springborg, J. B. et al. A single subcutaneous bolus of erythropoietin normalizes cerebral blood flow autoregulation after subarachnoid haemorrhage in rats. Br. J. Pharmacol. 135, 823–829 (2002).
Genc, S. et al. Erythropoietin exerts neuroprotection in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated C57/BL mice via increasing nitric oxide production. Neurosci. Lett. 298, 139–141 (2001).
Bianchi, R. et al. Erythropoietin both protects from and reverses experimental diabetic neuropathy. Proc. Natl Acad. Sci. USA 101, 823–828 (2004).
Lieberman, J. et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol. Psychiatry 49, 487–499 (2001).
Anagnostou, A., Lee, E. S., Kessimian, N., Levinson, R. & Steiner, M. Erythropoietin has a mitogenic and positive chemotactic effect on endothelial cells. Proc. Natl Acad. Sci. USA 87, 5978–5982 (1990).
Tojo, A., Fukamachi, H., Kasuga, M., Urabe, A. & Takaku, F. Identification of erythropoietin receptors on fetal liver erythroid cells. Biochem. Biophys. Res. Commun. 148, 443–448 (1987).
Campana, W. M., Misasi, R. & O'Brien, J. S. Identification of a neurotrophic sequence in erythropoietin. Int. J. Mol. Med. 1, 235–241 (1998).
Jubinsky, P. T., Krijanovski, O. I., Nathan, D. G., Tavernier, J. & Sieff, C. A. The beta chain of the interleukin-3 receptor functionally associates with the erythropoietin receptor. Blood 90, 1867–1873 (1997).
Scott, C. L. et al. Reassessment of interactions between hematopoietic receptors using common beta-chain and interleukin-3-specific receptor beta-chain-null cells: no evidence of functional interactions with receptors for erythropoietin, granulocyte colony-stimulating factor, or stem cell factor. Blood 96, 1588–1590 (2000).
Wang, X. et al. The nonerythropoietic asialoerythropoietin protects against neonatal hypoxia-ischemia as potently as erythropoietin. J. Neurochem. 91, 900–910 (2004).
Dong, Y. J., Kung, C. & Goldwasser, E. Receptor binding of asialoerythropoietin. J. Cell Biochem. 48, 269–276 (1992).
Elliott, S., Lorenzini, T., Chang, D., Barzilay, J. & Delorme, E. Mapping of the active site of recombinant human erythropoietin. Blood 89, 493–502 (1997).
Miyashita, K. et al. Blood pressure response to erythropoietin injection in hemodialysis and predialysis patients. Hypertens. Res. 27, 79–84 (2004).
Vaziri, N. D. et al. In vivo and in vitro pressor effects of erythropoietin in rats. Am. J. Physiol. 269, F838–F845 (1995).
Carlini, R., Obialo, C. I. & Rothstein, M. Intravenous erythropoietin (rHuEPO) administration increases plasma endothelin and blood pressure in hemodialysis patients. Am. J. Hypertens. 6, 103–107 (1993).
Carlini, R. G., Dusso, A. S., Obialo, C. I., Alvarez, U. M. & Rothstein, M. Recombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cells. Kidney Int. 43, 1010–1014 (1993).
Quaschning, T. et al. Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. FASEB J. 17, 259–261 (2003).
Wolf, R. F. et al. Erythropoietin potentiates thrombus development in a canine arterio-venous shunt model. Thromb. Haemost. 77, 1020–1024 (1997).
Leyland-Jones, B. Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol. 4, 459–460 (2003).
Rosenzweig, M. Q., Bender, C. M., Lucke, J. P., Yasko, J. M. & Brufsky, A. M. The decision to prematurely terminate a trial of R-HuEPO due to thrombotic events. J. Pain Symptom Manage. 27, 185–190 (2004).
Peterson, L. FDA oncologic drugs advisory committee (ODAC) meeting on the safety of erythropoietin in oncology. Trends Med. 1–4 (2004).
Cotter, D. et al. Hematocrit was not validated as a surrogate end point for survival among epoetin-treated hemodialysis patients. J. Clin. Epidemiol. 57, 1086–1095 (2004).
Yasuda, Y. et al. Erythropoietin regulates tumour growth of human malignancies. Carcinogenesis 24, 1021–1029 (2003).
Pajonk, F., Weil, A., Sommer, A., Suwinski, R. & Henke, M. The erythropoietin-receptor pathway modulates survival of cancer cells. Oncogene 23, 8987–8991 (2004).
Henke, M. et al. Erythropoietin to treat head and neck cancer patients with anaemia undergoing radiotherapy: randomised, double-blind, placebo-controlled trial. Lancet 362, 1255–1260 (2003).
Glezer, I. & Rivest, S. Glucocorticoids: protectors of the brain during innate immune responses. Neuroscientist 10, 538–552 (2004).
McClure, B. J. et al. Molecular assembly of the ternary granulocyte-macrophage colony-stimulating factor receptor complex. Blood 101, 1308–1315 (2003).
Juhaszova, M. et al. Glycogen synthase kinase-3β mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J. Clin. Invest. 113, 1535–1549 (2004).
Digicaylioglu, M., Garden, G., Timberlake, S., Fletcher, L. & Lipton, S. A. Acute neuroprotective synergy of erythropoietin and insulin-like growth factor I. Proc. Natl Acad. Sci. USA 101, 9855–9860 (2004).
Acknowledgements
Recently, the number and diversity of publications concerning non-erythroid actions of erythropoietin has grown in an exponential manner. We apologize to our many colleagues for omission of their important work due to stringent space limitations in this brief perspective. We especially thank our principal colleagues, T. Coleman, Q.-w. Xie and M. Yamin at Warren Pharmaceuticals; P. Ghezzi, R. Latini and colleagues at the Mario Negri Pharmacological Institute in Milan, Italy; G. Grasso and A. Sfacteria, University of Messina, Sicily, Italy; M. Leist, T. Sager, L. Torup and co-workers at H. Lundbeck A/S, Copenhagen, Denmark; O. Yilmaz, S. Erbayraktar, Z. Erbayraktar and N. Gokmen at Dokuz Eylul University, Izmir, Turkey; and H. Ehrenreich and A. Siren, Max Planck Institute for Experimental Medicine, Gottingen, Germany. M.B. and A.C. are supported by the Kenneth S. Warren Institute and Warren Pharmaceuticals. We are employed by Warren Pharmaceuticals, which is engaged in developing non-erythropoietic tissue protective cytokines for clinical use.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M. B. and A. C. are employees of Warren Pharmaceuticals, Inc., which is engaged in developing tissue-protective erythropoietin analogues.
Rights and permissions
About this article
Cite this article
Brines, M., Cerami, A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci 6, 484–494 (2005). https://doi.org/10.1038/nrn1687
Issue Date:
DOI: https://doi.org/10.1038/nrn1687
This article is cited by
-
Intellectual disability and autism in propionic acidemia: a biomarker-behavioral investigation implicating dysregulated mitochondrial biology
Molecular Psychiatry (2024)
-
Nanoengineered injectable hydrogels derived from layered double hydroxides and alginate for sustained release of protein therapeutics in tissue engineering applications
Journal of Nanobiotechnology (2023)
-
Introducing the brain erythropoietin circle to explain adaptive brain hardware upgrade and improved performance
Molecular Psychiatry (2022)
-
A Novel Plant-Produced Asialo-rhuEPO Protects Brain from Ischemic Damage Without Erythropoietic Action
Translational Stroke Research (2022)
-
Recombinant human erythropoietin and interferon-β-1b protect against 3-nitropropionic acid-induced neurotoxicity in rats: possible role of JAK/STAT signaling pathway
Inflammopharmacology (2022)