Key Points
-
Leptin plays a key role in the neural regulation of adipose tissues
-
The sympathetic nervous system is crucial in regulating leptin production
-
Fasting differentially modulates the sympathetic outflow to different fat pads
-
The pattern of sympathetic innervation varies across fat pads and animal species
-
Manipulating the sympathetic outflow to adipose tissues may be beneficial to the development of novel anti-obesity and anti-diabetes therapies
Abstract
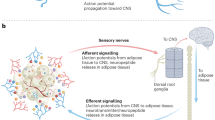
Interactions between the brain and distinct adipose depots have a key role in maintaining energy balance, thereby promoting survival in response to metabolic challenges such as cold exposure and starvation. Recently, there has been renewed interest in the specific central neuronal circuits that regulate adipose depots. Here, we review anatomical, genetic and pharmacological studies on the neural regulation of adipose function, including lipolysis, non-shivering thermogenesis, browning and leptin secretion. In particular, we emphasize the role of leptin-sensitive neurons and the sympathetic nervous system in modulating the activity of brown, white and beige adipose tissues. We provide an overview of advances in the understanding of the heterogeneity of the brain regulation of adipose tissues and offer a perspective on the challenges and paradoxes that the community is facing regarding the actions of leptin on this system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Friedman, J. The long road to leptin. J. Clin. Invest. 126, 4727–4734 (2016).
Friedman, J. 20 years of leptin: leptin at 20: an overview. J. Endocrinol. 223, T1–T8 (2014).
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). This study reports the cloning and sequencing of the mouse Lep gene and has ushered in the molecular era of obesity research.
Ogden, C. L., Carroll, M. D., Fryar, C. D. & Flegal, K. M. Prevalence of obesity among adults and youth: United States, 2011–2014 (US Department of Health and Human Services, 2015).
Chechi, K., Carpentier, A. C. & Richard, D. Understanding the brown adipocyte as a contributor to energy homeostasis. Trends Endocrinol. Metab. 24, 408–420 (2013).
Rosen, E. D. & Spiegelman, B. M. What we talk about when we talk about fat. Cell 156, 20–44 (2014).
Harms, M. & Seale, P. Brown and beige fat: development, function and therapeutic potential. Nat. Med. 19, 1252–1263 (2013).
Bartness, T. J., Liu, Y., Shrestha, Y. B. & Ryu, V. Neural innervation of white adipose tissue and the control of lipolysis. Front. Neuroendocrinol. 35, 473–493 (2014).
Labbe, S. M. et al. Hypothalamic control of brown adipose tissue thermogenesis. Front. Syst. Neurosci. 9, 150 (2015).
Brito, N. A., Brito, M. N. & Bartness, T. J. Differential sympathetic drive to adipose tissues after food deprivation, cold exposure or glucoprivation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R1445–R1452 (2008).
Labbe, S. M. et al. Metabolic activity of brown, “beige,” and white adipose tissues in response to chronic adrenergic stimulation in male mice. Am. J. Physiol. Endocrinol. Metab. 311, E260–E268 (2016).
Morrison, S. F. & Nakamura, K. Central neural pathways for thermoregulation. Front. Biosci. 16, 74–104 (2011).
Fishman, R. B. & Dark, J. Sensory innervation of white adipose tissue. Am. J. Physiol. 253, R942–R944 (1987).
Ahima, R. S. & Flier, J. S. Leptin. Annu. Rev. Physiol. 62, 413–437 (2000).
Flier, J. S. Clinical review 94: what's in a name? In search of leptin's physiologic role. J. Clin. Endocrinol. Metab. 83, 1407–1413 (1998).
Friedman, J. M. & Halaas, J. L. Leptin and the regulation of body weight in mammals. Nature 395, 763–770 (1998).
Munzberg, H., Qualls-Creekmore, E., Berthoud, H. R., Morrison, C. D. & Yu, S. Neural control of energy expenditure. Handb. Exp. Pharmacol. 233, 173–194 (2016).
Morton, G. J., Cummings, D. E., Baskin, D. G., Barsh, G. S. & Schwartz, M. W. Central nervous system control of food intake and body weight. Nature 443, 289–295 (2006).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009). This study demonstrates the presence of functional BAT in humans and has contributed to the recent renewed interest in the role of BAT in regulating energy balance.
Caron, A. & Richard, D. Neuronal systems and circuits involved in the control of food intake and adaptive thermogenesis. Ann. NY Acad. Sci. 1391, 35–53 (2017).
Contreras, C. et al. The brain and brown fat. Ann. Med. 47, 150–168 (2015).
Morrison, S. F. & Madden, C. J. Central nervous system regulation of brown adipose tissue. Compr. Physiol. 4, 1677–1713 (2014). This review summarizes the functional organization and neurochemical influences governing BAT sympathetic nerve activity.
Allison, M. B. & Myers, M. G. Jr. 20 years of leptin: connecting leptin signaling to biological function. J. Endocrinol. 223, T25–35 (2014).
Li, H., Matheny, M. & Scarpace, P. J. β3-adrenergic-mediated suppression of leptin gene expression in rats. Am. J. Physiol. 272, E1031–E1036 (1997).
Trayhurn, P., Duncan, J. S. & Rayner, D. V. Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice: mediation by the sympathetic system. Biochem. J. 311, 729–733 (1995).
Trayhurn, P., Duncan, J. S., Rayner, D. V. & Hardie, L. J. Rapid inhibition of ob gene expression and circulating leptin levels in lean mice by the β3-adrenoceptor agonists BRL 35135A and ZD2079. Biochem. Biophys. Res. Commun. 228, 605–610 (1996).
Gettys, T. W., Harkness, P. J. & Watson, P. M. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology 137, 4054–4057 (1996).
Mantzoros, C. S. et al. Activation of β3 adrenergic receptors suppresses leptin expression and mediates a leptin-independent inhibition of food intake in mice. Diabetes 45, 909–914 (1996).
Furness, J. B. The organisation of the autonomic nervous system: peripheral connections. Auton. Neurosci. 130, 1–5 (2006).
McCorry, L. K. Physiology of the autonomic nervous system. Am. J. Pharm. Educ. 71, 78 (2007).
Kreier, F. et al. Selective parasympathetic innervation of subcutaneous and intra-abdominal fat–functional implications. J. Clin. Invest. 110, 1243–1250 (2002).
Giordano, A. et al. White adipose tissue lacks significant vagal innervation and immunohistochemical evidence of parasympathetic innervation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1243–R1255 (2006).
Jiang, H., Ding, X., Cao, Y., Wang, H. & Zeng, W. Dense intra-adipose sympathetic arborizations are essential for cold-induced beiging of mouse white adipose tissue. Cell Metab. 26, 686–692.e3 (2017). This study reveals a dense network of sympathetic fibres in close apposition with adipocytes. It provides evidence that catecholamines deriving from these sympathetic fibres, but not from the adrenal medulla or resident macrophages, are essential for the development of BeAT.
Gilgen, A., Maickel, R. P., Nikodijevic, O. & Brodie, B. B. Essential role of catecholamines in the mobilization of free fatty acids and glucose after exposure to cold. Life Sci. 1, 709–715 (1962).
Young, J. B. & Landsberg, L. Suppression of sympathetic nervous system during fasting. Science 196, 1473–1475 (1977).
Migliorini, R. H., Garofalo, M. A. & Kettelhut, I. C. Increased sympathetic activity in rat white adipose tissue during prolonged fasting. Am. J. Physiol. 272, R656–R661 (1997).
Garofalo, M. A., Kettelhut, I. C., Roselino, J. E. & Migliorini, R. H. Effect of acute cold exposure on norepinephrine turnover rates in rat white adipose tissue. J. Auton. Nerv. Syst. 60, 206–208 (1996).
Zeng, W. et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 (2015).
Trayhurn, P., Thomas, M. E., Duncan, J. S. & Rayner, D. V. Effects of fasting and refeeding on ob gene expression in white adipose tissue of lean and obese (ob/ob) mice. FEBS Lett. 368, 488–490 (1995).
Hube, F. et al. Difference in leptin mRNA levels between omental and subcutaneous abdominal adipose tissue from obese humans. Horm. Metab. Res. 28, 690–693 (1996).
Frederich, R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Koopmans, S. J., Frolich, M., Gribnau, E. H., Westendorp, R. G. & DeFronzo, R. A. Effect of hyperinsulinemia on plasma leptin concentrations and food intake in rats. Am. J. Physiol. 274, E998–E1001 (1998).
Sukumaran, S., Xue, B., Jusko, W. J., Dubois, D. C. & Almon, R. R. Circadian variations in gene expression in rat abdominal adipose tissue and relationship to physiology. Physiol. Genomics 42A, 141–152 (2010).
Boden, G., Chen, X., Mozzoli, M. & Ryan, I. Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metab. 81, 3419–3423 (1996).
Ahima, R. S. et al. Role of leptin in the neuroendocrine response to fasting. Nature 382, 250–252 (1996).
Murakami, T., Iida, M. & Shima, K. Dexamethasone regulates obese expression in isolated rat adipocytes. Biochem. Biophys. Res. Commun. 214, 1260–1267 (1995).
Saladin, R. et al. Transient increase in obese gene expression after food intake or insulin administration. Nature 377, 527–529 (1995).
Moinat, M. et al. Modulation of obese gene expression in rat brown and white adipose tissues. FEBS Lett. 373, 131–134 (1995).
Deng, C. et al. Effects of β-adrenoceptor subtype stimulation on obese gene messenger ribonucleic acid and on leptin secretion in mouse brown adipocytes differentiated in culture. Endocrinology 138, 548–552 (1997).
Trayhurn, P., Duncan, J. S., Hoggard, N. & Rayner, D. V. Regulation of leptin production: a dominant role for the sympathetic nervous system? Proc. Nutr. Soc. 57, 413–419 (1998).
Giacobino, J. P. Role of the β3-adrenoceptor in the control of leptin expression. Horm. Metab. Res. 28, 633–637 (1996).
Slieker, L. J. et al. Regulation of expression of ob mRNA and protein by glucocorticoids and cAMP. J. Biol. Chem. 271, 5301–5304 (1996).
Rayner, D. V., Simon, E., Duncan, J. S. & Trayhurn, P. Hyperleptinaemia in mice induced by administration of the tyrosine hydroxylase inhibitor α-methyl-p-tyrosine. FEBS Lett. 429, 395–398 (1998).
Sivitz, W. I. et al. Sympathetic inhibition, leptin, and uncoupling protein subtype expression in normal fasting rats. Am. J. Physiol. 277, E668–E677 (1999).
Tieu, K. A guide to neurotoxic animal models of Parkinson's disease. Cold Spring Harb. Perspect. Med. 1, a009316 (2011).
Huang, T. S., Wang, Y. H. & Chen, S. Y. The relation of serum leptin to body mass index and to serum cortisol in men with spinal cord injury. Arch. Phys. Med. Rehabil. 81, 1582–1586 (2000).
Commins, S. P. et al. Norepinephrine is required for leptin effects on gene expression in brown and white adipose tissue. Endocrinology 140, 4772–4778 (1999).
Pereira, M. M. et al. A brain-sparing diphtheria toxin for chemical genetic ablation of peripheral cell lineages. Nat. Commun. 8, 14967 (2017). This study reports the development of a novel tool allowing chemical genetic ablation of genes of interest strictly in peripheral tissues.
Bartolomucci, A. et al. TLQP-21, a VGF-derived peptide, increases energy expenditure and prevents the early phase of diet-induced obesity. Proc. Natl Acad. Sci. USA 103, 14584–14589 (2006).
Cero, C. et al. The neuropeptide TLQP-21 opposes obesity via C3aR1-mediated enhancement of adrenergic-induced lipolysis. Mol. Metab. 6, 148–158 (2017).
Fuke, T. et al. Transcription factor AP-2β inhibits expression and secretion of leptin, an insulin-sensitizing hormone, in 3T3-L1 adipocytes. Int. J. Obes. 34, 670–678 (2010).
Wrann, C. D. et al. FOSL2 promotes leptin gene expression in human and mouse adipocytes. J. Clin. Invest. 122, 1010–1021 (2012).
Lu, Y. H., Dallner, O. S., Birsoy, K., Fayzikhodjaeva, G. & Friedman, J. M. Nuclear factor-Y is an adipogenic factor that regulates leptin gene expression. Mol. Metab. 4, 392–405 (2015).
de la Brousse, F. C., Shan, B. & Chen, J. L. Identification of the promoter of the mouse obese gene. Proc. Natl Acad. Sci. USA 93, 4096–4101 (1996).
Gong, D. W., Bi, S., Pratley, R. E. & Weintraub, B. D. Genomic structure and promoter analysis of the human obese gene. J. Biol. Chem. 271, 3971–3974 (1996).
He, Y., Chen, H., Quon, M. J. & Reitman, M. The mouse obese gene. Genomic organization, promoter activity, and activation by CCAAT/enhancer-binding protein α. J. Biol. Chem. 270, 28887–28891 (1995).
Hwang, C. S., Mandrup, S., MacDougald, O. A., Geiman, D. E. & Lane, M. D. Transcriptional activation of the mouse obese (ob) gene by CCAAT/enhancer binding protein α. Proc. Natl Acad. Sci. USA 93, 873–877 (1996).
Miller, S. G. et al. The adipocyte specific transcription factor C/EBPα modulates human ob gene expression. Proc. Natl Acad. Sci. USA 93, 5507–5511 (1996).
Kim, J. B. et al. Nutritional and insulin regulation of fatty acid synthetase and leptin gene expression through ADD1/SREBP1. J. Clin. Invest. 101, 1–9 (1998).
Chen, X. L., Hartzell, D. L., McGraw, R. A., Hausman, G. J. & Dean, R. G. Analysis of a 762-bp proximal leptin promoter to drive and control regulation of transgene expression of growth hormone receptor in mice. Biochem. Biophys. Res. Commun. 262, 187–192 (1999).
Wrann, C. D. & Rosen, E. D. New insights into adipocyte-specific leptin gene expression. Adipocyte 1, 168–172 (2012). This review highlights the transcriptional mechanisms that regulate adipocyte-specific expression of leptin.
Becker, D. J., Ongemba, L. N., Brichard, V., Henquin, J. C. & Brichard, S. M. Diet- and diabetes-induced changes of ob gene expression in rat adipose tissue. FEBS Lett. 371, 324–328 (1995).
MacDougald, O. A., Hwang, C. S., Fan, H. & Lane, M. D. Regulated expression of the obese gene product (leptin) in white adipose tissue and 3T3-L1 adipocytes. Proc. Natl Acad. Sci. USA 92, 9034–9037 (1995).
Hardie, L. J., Rayner, D. V., Holmes, S. & Trayhurn, P. Circulating leptin levels are modulated by fasting, cold exposure and insulin administration in lean but not Zucker (fa/fa) rats as measured by ELISA. Biochem. Biophys. Res. Commun. 223, 660–665 (1996).
Szkudelski, T. Intracellular mediators in regulation of leptin secretion from adipocytes. Physiol. Res. 56, 503–512 (2007).
Rayner, D. V. The sympathetic nervous system in white adipose tissue regulation. Proc. Nutr. Soc. 60, 357–364 (2001).
Cusin, I., Rohner-Jeanrenaud, F., Stricker-Krongrad, A. & Jeanrenaud, B. The weight-reducing effect of an intracerebroventricular bolus injection of leptin in genetically obese fa/fa rats: reduced sensitivity compared with lean animals. Diabetes 45, 1446–1450 (1996).
Kusminski, C. M., Bickel, P. E. & Scherer, P. E. Targeting adipose tissue in the treatment of obesity-associated diabetes. Nat. Rev. Drug Discov. 15, 639–660 (2016).
Caron, A., Richard, D. & Laplante, M. The roles of mTOR complexes in lipid metabolism. Annu. Rev. Nutr. 35, 321–348 (2015).
Thomou, T. et al. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455 (2017).
Kim, J. Y. et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Invest. 117, 2621–2637 (2007).
Nishizawa, Y. & Bray, G. A. Ventromedial hypothalamic lesions and the mobilization of fatty acids. J. Clin. Invest. 61, 714–721 (1978).
Takahashi, A. & Shimazu, T. Hypothalamic regulation of lipid metabolism in the rat: effect of hypothalamic stimulation on lipolysis. J. Auton. Nerv. Syst. 4, 195–205 (1981).
Youngstrom, T. G. & Bartness, T. J. White adipose tissue sympathetic nervous system denervation increases fat pad mass and fat cell number. Am. J. Physiol. 275, R1488–R1493 (1998).
Bamshad, M., Aoki, V. T., Adkison, M. G., Warren, W. S. & Bartness, T. J. Central nervous system origins of the sympathetic nervous system outflow to white adipose tissue. Am. J. Physiol. 275, R291–R299 (1998).
Bartness, T. J. & Bamshad, M. Innervation of mammalian white adipose tissue: implications for the regulation of total body fat. Am. J. Physiol. 275, R1399–R1411 (1998).
Sahu, A. Resistance to the satiety action of leptin following chronic central leptin infusion is associated with the development of leptin resistance in neuropeptide Y neurones. J. Neuroendocrinol. 14, 796–804 (2002).
Buettner, C. et al. Leptin controls adipose tissue lipogenesis via central, STAT3-independent mechanisms. Nat. Med. 14, 667–675 (2008).
Shen, J., Tanida, M., Niijima, A. & Nagai, K. In vivo effects of leptin on autonomic nerve activity and lipolysis in rats. Neurosci. Lett. 416, 193–197 (2007).
Rooks, C. R. et al. Sympathetic denervation does not prevent a reduction in fat pad size of rats or mice treated with peripherally administered leptin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R92–R102 (2005).
Penn, D. M., Jordan, L. C., Kelso, E. W., Davenport, J. E. & Harris, R. B. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R1613–R1621 (2006).
Jaubert, A. M., Penot, G., Niang, F., Durant, S. & Forest, C. Rapid nitration of adipocyte phosphoenolpyruvate carboxykinase by leptin reduces glyceroneogenesis and induces fatty acid release. PLoS ONE 7, e40650 (2012).
Rodriguez, V. M., Macarulla, M. T., Echevarria, E. & Portillo, M. P. Lipolysis induced by leptin in rat adipose tissue from different anatomical locations. Eur. J. Nutr. 42, 149–153 (2003).
Siegrist-Kaiser, C. A. et al. Direct effects of leptin on brown and white adipose tissue. J. Clin. Invest. 100, 2858–2864 (1997).
D'Souza, A., M., Neumann, U. H., Glavas, M. M. & Kieffer, T. J. The glucoregulatory actions of leptin. Mol. Metab. 6, 1052–1065 (2017).
Warner, A. et al. Activation of β3-adrenoceptors increases in vivo free fatty acid uptake and utilization in brown but not white fat depots in high-fat-fed rats. Am. J. Physiol. Endocrinol. Metab. 311, E901–E910 (2016).
Plum, L. et al. Enhanced leptin-stimulated Pi3k activation in the CNS promotes white adipose tissue transdifferentiation. Cell Metab. 6, 431–445 (2007).
Chi, J. et al. Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metab. 27, 226–236.e3 (2018).
Zhu, Y. et al. Connexin 43 mediates white adipose tissue beiging by facilitating the propagation of sympathetic neuronal signals. Cell Metab. 24, 420–433 (2016).
Ruan, H. B. et al. O-GlcNAc transferase enables AgRP neurons to suppress browning of white fat. Cell 159, 306–317 (2014).
de Jong, J. M., Larsson, O., Cannon, B. & Nedergaard, J. A stringent validation of mouse adipose tissue identity markers. Am. J. Physiol. Endocrinol. Metab. 308, E1085–E1105 (2015).
Foster, D. O. Quantitative contribution of brown adipose tissue thermogenesis to overall metabolism. Can. J. Biochem. Cell Biol. 62, 618–622 (1984).
Labbe, S. M. et al. In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis. FASEB J. 29, 2046–2058 (2015).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004). This is a comprehensive and detailed review of BAT biology.
Murano, I., Barbatelli, G., Giordano, A. & Cinti, S. Noradrenergic parenchymal nerve fiber branching after cold acclimatisation correlates with brown adipocyte density in mouse adipose organ. J. Anat. 214, 171–178 (2009).
Bartness, T. J. & Song, C. K. Innervation of brown adipose tissue and its role in thermogenesis. Can. J. Diabetes 29, 420–428 (2005).
Bamshad, M., Song, C. K. & Bartness, T. J. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 276, R1569–R1578 (1999).
Nguyen, N. L. et al. Separate and shared sympathetic outflow to white and brown fat coordinately regulates thermoregulation and beige adipocyte recruitment. Am. J. Physiol. Regul. Integr. Comp. Physiol. 312, R132–R145 (2017). This paper provides neuroanatomical and functional evidence of the existence of SNS crosstalk for thermoregulation and BeAT development.
Cano, G. et al. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp. Neurol. 460, 303–326 (2003). This study demonstrates the central circuitry responsible for cold-induced increase in sympathetic outflow to interscapular BAT.
Morrison, S. F., Ramamurthy, S. & Young, J. B. Reduced rearing temperature augments responses in sympathetic outflow to brown adipose tissue. J. Neurosci. 20, 9264–9271 (2000).
Yu, S. et al. Glutamatergic preoptic area neurons that express leptin receptors drive temperature-dependent body weight homeostasis. J. Neurosci. 36, 5034–5046 (2016).
Fischer, A. W. et al. Leptin raises defended body temperature without activating thermogenesis. Cell Rep. 14, 1621–1631 (2016). This recent study suggests that leptin is pyrexic rather than thermogenic.
Campfield, L. A., Smith, F. J., Guisez, Y., Devos, R. & Burn, P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 (1995).
Pelleymounter, M. A. et al. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269, 540–543 (1995).
Satoh, N. et al. Sympathetic activation of leptin via the ventromedial hypothalamus: leptin-induced increase in catecholamine secretion. Diabetes 48, 1787–1793 (1999).
Haque, M. S. et al. Role of the sympathetic nervous system and insulin in enhancing glucose uptake in peripheral tissues after intrahypothalamic injection of leptin in rats. Diabetes 48, 1706–1712 (1999).
De Fanti, B. A., Milagro, F. I., Lamas, O., Martinez-Anso, E. & Martinez, J. A. Immunomanipulation of appetite and body temperature through the functional mimicry of leptin. Obes. Res. 10, 833–837 (2002).
Luheshi, G. N., Gardner, J. D., Rushforth, D. A., Loudon, A. S. & Rothwell, N. J. Leptin actions on food intake and body temperature are mediated by IL-1. Proc. Natl Acad. Sci. USA 96, 7047–7052 (1999).
Dodd, G. T. et al. The thermogenic effect of leptin is dependent on a distinct population of prolactin-releasing peptide neurons in the dorsomedial hypothalamus. Cell Metab. 20, 639–649 (2014).
Collins, S. et al. Role of leptin in fat regulation. Nature 380, 677 (1996).
Morrison, S. F. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 286, R832–837 (2004).
Haynes, W. G., Morgan, D. A., Walsh, S. A., Mark, A. L. & Sivitz, W. I. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 100, 270–278 (1997).
Kamohara, S., Burcelin, R., Halaas, J. L., Friedman, J. M. & Charron, M. J. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature 389, 374–377 (1997).
Harlan, S. M., Guo, D. F., Morgan, D. A., Fernandes-Santos, C. & Rahmouni, K. Hypothalamic mTORC1 signaling controls sympathetic nerve activity and arterial pressure and mediates leptin effects. Cell Metab. 17, 599–606 (2013).
Rahmouni, K. & Morgan, D. A. Hypothalamic arcuate nucleus mediates the sympathetic and arterial pressure responses to leptin. Hypertension 49, 647–652 (2007).
do Carmo, J. M. et al. Control of blood pressure, appetite, and glucose by leptin in mice lacking leptin receptors in proopiomelanocortin neurons. Hypertension 57, 918–926 (2011).
Scarpace, P. J. & Matheny, M. Leptin induction of UCP1 gene expression is dependent on sympathetic innervation. Am. J. Physiol. 275, E259–E264 (1998).
Haynes, W. G., Sivitz, W. I., Morgan, D. A., Walsh, S. A. & Mark, A. L. Sympathetic and cardiorenal actions of leptin. Hypertension 30, 619–623 (1997).
Sarmiento, U. et al. Morphologic and molecular changes induced by recombinant human leptin in the white and brown adipose tissues of C57BL/6 mice. Lab. Invest. 77, 243–256 (1997).
Harris, R. B. et al. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology 139, 8–19 (1998).
Commins, S. P. et al. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology 140, 292–300 (1999).
Kaiyala, K. J., Ogimoto, K., Nelson, J. T., Muta, K. & Morton, G. J. Physiological role for leptin in the control of thermal conductance. Mol. Metab. 5, 892–902 (2016).
Ottaway, N. et al. Diet-induced obese mice retain endogenous leptin action. Cell Metab. 21, 877–882 (2015). This study shows that the anorexigenic effects of leptin are maintained in hyperleptinaemic diet-induced obesity mice through the use of a novel leptin receptor antagonist.
Ryu, V. & Bartness, T. J. Short and long sympathetic-sensory feedback loops in white fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R886–R900 (2014).
Shi, H. & Bartness, T. J. Neurochemical phenotype of sympathetic nervous system outflow from brain to white fat. Brain Res. Bull. 54, 375–385 (2001).
Song, C. K., Jackson, R. M., Harris, R. B., Richard, D. & Bartness, T. J. Melanocortin-4 receptor mRNA is expressed in sympathetic nervous system outflow neurons to white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 289, R1467–R1476 (2005).
Song, C. K. et al. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: neuroanatomical and functional evidence. Am. J. Physiol. Regul. Integr. Comp. Physiol. 295, R417–R428 (2008).
Mercer, J. G. et al. Localization of leptin receptor mRNA and the long form splice variant (Ob-Rb) in mouse hypothalamus and adjacent brain regions by in situ hybridization. FEBS Lett. 387, 113–116 (1996).
Elmquist, J. K., Bjorbaek, C., Ahima, R. S., Flier, J. S. & Saper, C. B. Distributions of leptin receptor mRNA isoforms in the rat brain. J. Comp. Neurol. 395, 535–547 (1998).
Laque, A. et al. Leptin receptor neurons in the mouse hypothalamus are colocalized with the neuropeptide galanin and mediate anorexigenic leptin action. Am. J. Physiol. Endocrinol. Metab. 304, E999–E1011 (2013).
Cone, R. D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 8, 571–578 (2005).
Ollmann, M. M. et al. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278, 135–138 (1997).
Cowley, M. A. et al. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411, 480–484 (2001).
Elias, C. F. et al. Leptin activates hypothalamic CART neurons projecting to the spinal cord. Neuron 21, 1375–1385 (1998).
Kishi, T. et al. Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J. Comp. Neurol. 457, 213–235 (2003).
Mountjoy, K. G., Mortrud, M. T., Low, M. J., Simerly, R. B. & Cone, R. D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 8, 1298–1308 (1994).
Begriche, K., Sutton, G. M. & Butler, A. A. Homeostastic and non-homeostatic functions of melanocortin-3 receptors in the control of energy balance and metabolism. Physiol. Behav. 104, 546–554 (2011).
Huszar, D. et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88, 131–141 (1997).
Fan, W., Boston, B. A., Kesterson, R. A., Hruby, V. J. & Cone, R. D. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385, 165–168 (1997).
Begriche, K., Girardet, C., McDonald, P. & Butler, A. A. Melanocortin-3 receptors and metabolic homeostasis. Prog. Mol. Biol. Transl Sci. 114, 109–146 (2013).
Satoh, N. et al. Satiety effect and sympathetic activation of leptin are mediated by hypothalamic melanocortin system. Neurosci. Lett. 249, 107–110 (1998).
Haynes, W. G., Morgan, D. A., Djalali, A., Sivitz, W. I. & Mark, A. L. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension 33, 542–547 (1999).
Williams, D. L., Bowers, R. R., Bartness, T. J., Kaplan, J. M. & Grill, H. J. Brainstem melanocortin 3/4 receptor stimulation increases uncoupling protein gene expression in brown fat. Endocrinology 144, 4692–4697 (2003).
Voss-Andreae, A. et al. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology 148, 1550–1560 (2007).
Balthasar, N. et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123, 493–505 (2005).
Berglund, E. D. et al. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat. Neurosci. 17, 911–913 (2014).
Henry, F. E., Sugino, K., Tozer, A., Branco, T. & Sternson, S. M. Cell type-specific transcriptomics of hypothalamic energy-sensing neuron responses to weight-loss. eLife 4, e09800 (2015).
Lam, B. Y. H. et al. Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol. Metab. 6, 383–392 (2017).
Williams, K. W. et al. Xbp1s in Pomc neurons connects ER stress with energy balance and glucose homeostasis. Cell Metab. 20, 471–482 (2014).
Dodd, G. T. et al. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell 160, 88–104 (2015).
Kong, D. et al. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151, 645–657 (2012).
Dodd, G. T. et al. A hypothalamic phosphatase switch coordinates energy expenditure with feeding. Cell Metab. 26, 375–393.e7 (2017).
Thompson, R. H. & Swanson, L. W. Organization of inputs to the dorsomedial nucleus of the hypothalamus: a reexamination with Fluorogold and PHAL in the rat. Brain Res. Brain Res. Rev. 27, 89–118 (1998).
Yoshida, K. et al. Neurons of the rat preoptic area and the raphe pallidus nucleus innervating the brown adipose tissue express the prostaglandin E receptor subtype EP3. Eur. J. Neurosci. 18, 1848–1860 (2003).
Trayhurn, P., Thurlby, P. L. & James, W. P. A defective response to cold in the obese (obob) mouse and the obese Zucker (fafa) rat [proceedings]. Proc. Nutr. Soc. 35, 133A (1976).
Trayhurn, P., Thurlby, P. L. & James, W. P. Thermogenic defect in pre-obese ob/ob mice. Nature 266, 60–62 (1977).
Zhang, Y. et al. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 31, 1873–1884 (2011).
Rezai-Zadeh, K. et al. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 3, 681–693 (2014).
Steiner, A. A. et al. A new function of the leptin receptor: mediation of the recovery from lipopolysaccharide-induced hypothermia. FASEB J. 18, 1949–1951 (2004).
Xu, Y. et al. Glutamate release mediates leptin action on energy expenditure. Mol. Metab. 2, 109–115 (2013).
Zaretskaia, M. V., Zaretsky, D. V., Shekhar, A. & DiMicco, J. A. Chemical stimulation of the dorsomedial hypothalamus evokes non-shivering thermogenesis in anesthetized rats. Brain Res. 928, 113–125 (2002).
Cao, W. H., Fan, W. & Morrison, S. F. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience 126, 229–240 (2004).
Dimicco, J. A. & Zaretsky, D. V. The dorsomedial hypothalamus: a new player in thermoregulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R47–R63 (2007).
Morrison, S. F., Nakamura, K. & Madden, C. J. Central control of thermogenesis in mammals. Exp. Physiol. 93, 773–797 (2008).
Gautron, L., Lazarus, M., Scott, M. M., Saper, C. B. & Elmquist, J. K. Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach. J. Comp. Neurol. 518, 2090–2108 (2010).
Elmquist, J. K., Ahima, R. S., Elias, C. F., Flier, J. S. & Saper, C. B. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc. Natl Acad. Sci. USA 95, 741–746 (1998).
Elias, C. F. et al. Chemical characterization of leptin-activated neurons in the rat brain. J. Comp. Neurol. 423, 261–281 (2000).
Marsh, A. J. et al. Cardiovascular responses evoked by leptin acting on neurons in the ventromedial and dorsomedial hypothalamus. Hypertension 42, 488–493 (2003).
Enriori, P. J., Sinnayah, P., Simonds, S. E., Garcia Rudaz, C. & Cowley, M. A. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 31, 12189–12197 (2011).
Iijima, N. et al. Cytochemical study of prolactin-releasing peptide (PrRP) in the rat brain. Neuroreport 10, 1713–1716 (1999).
Lam, D. D. et al. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 13, 584–591 (2011).
Yoshida, T. & Bray, G. A. Catecholamine turnover in rats with ventromedial hypothalamic lesions. Am. J. Physiol. 246, R558–R565 (1984).
Saito, M., Minokoshi, Y. & Shimazu, T. Ventromedial hypothalamic stimulation accelerates norepinephrine turnover in brown adipose tissue of rats. Life Sci. 41, 193–197 (1987).
Perkins, M. N., Rothwell, N. J., Stock, M. J. & Stone, T. W. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature 289, 401–402 (1981).
Holt, S. J., Wheal, H. V. & York, D. A. Hypothalamic control of brown adipose tissue in Zucker lean and obese rats. Effect of electrical stimulation of the ventromedial nucleus and other hypothalamic centres. Brain Res. 405, 227–233 (1987).
Hugie, T., Halvorson, I. & Thornhill, J. Brown adipose tissue temperature responses following electrical stimulation of ventromedial hypothalamic and lateral preoptic areas or after norepinephrine infusion to Long Evans or Sprague-Dawley rats. Brain Res. 575, 57–62 (1992).
Ruffin, M. & Nicolaidis, S. Electrical stimulation of the ventromedial hypothalamus enhances both fat utilization and metabolic rate that precede and parallel the inhibition of feeding behavior. Brain Res. 846, 23–29 (1999).
Minokoshi, Y., Haque, M. S. & Shimazu, T. Microinjection of leptin into the ventromedial hypothalamus increases glucose uptake in peripheral tissues in rats. Diabetes 48, 287–291 (1999).
Toda, C. et al. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 58, 2757–2765 (2009).
Ikeda, Y., Luo, X., Abbud, R., Nilson, J. H. & Parker, K. L. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol. Endocrinol. 9, 478–486 (1995).
Bingham, N. C., Anderson, K. K., Reuter, A. L., Stallings, N. R. & Parker, K. L. Selective loss of leptin receptors in the ventromedial hypothalamic nucleus results in increased adiposity and a metabolic syndrome. Endocrinology 149, 2138–2148 (2008).
Dhillon, H. et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49, 191–203 (2006).
Kim, K. W. et al. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc. Natl Acad. Sci. USA 108, 10673–10678 (2011).
Hill, C. E., Gould, D. J., Strigas, J., Burcher, E. & Vidovic, M. Sensory nerves play an efferent role in the function of the arterioles, but not the dilator muscle, of the rat iris. J. Auton. Nerv. Syst. 58, 89–100 (1996).
Norman, D., Mukherjee, S., Symons, D., Jung, R. T. & Lever, J. D. Neuropeptides in interscapular and perirenal brown adipose tissue in the rat: a plurality of innervation. J. Neurocytol. 17, 305–311 (1988).
De Matteis, R., Ricquier, D. & Cinti, S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J. Neurocytol. 27, 877–886 (1998).
Giordano, A., Morroni, M., Santone, G., Marchesi, G. F. & Cinti, S. Tyrosine hydroxylase, neuropeptide Y, substance P, calcitonin gene-related peptide and vasoactive intestinal peptide in nerves of rat periovarian adipose tissue: an immunohistochemical and ultrastructural investigation. J. Neurocytol. 25, 125–136 (1996).
Song, C. K., Schwartz, G. J. & Bartness, T. J. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R501–R511 (2009).
Niijima, A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. J. Auton. Nerv. Syst. 73, 19–25 (1998).
Niijima, A. Reflex effects from leptin sensors in the white adipose tissue of the epididymis to the efferent activity of the sympathetic and vagus nerve in the rat. Neurosci. Lett. 262, 125–128 (1999).
Murphy, K. T. et al. Leptin-sensitive sensory nerves innervate white fat. Am. J. Physiol. Endocrinol. Metab. 304, E1338–E1347 (2013).
Shi, Z. et al. Sympathetic activation by chemical stimulation of white adipose tissues in rats. J. Appl. Physiol. 112, 1008–1014 (2012).
Lafontan, M. & Berlan, M. Fat cell adrenergic receptors and the control of white and brown fat cell function. J. Lipid Res. 34, 1057–1091 (1993).
Montague, C. T., Prins, J. B., Sanders, L., Digby, J. E. & O'Rahilly, S. Depot- and sex-specific differences in human leptin mRNA expression: implications for the control of regional fat distribution. Diabetes 46, 342–347 (1997).
Tchkonia, T. et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 17, 644–656 (2013).
Flier, J. S. & Maratos-Flier, E. Leptin's physiologic role: does the emperor of energy balance have no clothes? Cell Metab. 26, 24–26 (2017).
Nguyen, N. L., Randall, J., Banfield, B. W. & Bartness, T. J. Central sympathetic innervations to visceral and subcutaneous white adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 306, R375–R386 (2014).
Brito, M. N., Brito, N. A., Baro, D. J., Song, C. K. & Bartness, T. J. Differential activation of the sympathetic innervation of adipose tissues by melanocortin receptor stimulation. Endocrinology 148, 5339–5347 (2007).
Youngstrom, T. G. & Bartness, T. J. Catecholaminergic innervation of white adipose tissue in Siberian hamsters. Am. J. Physiol. 268, R744–R751 (1995).
Pirzgalska, R. M. et al. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med. 23, 1309–1318 (2017). This recent study demonstrates that sympathetic-neuron-associated macrophages can metabolize catecholamines, thus identifying a new molecular target for the treatment of obesity.
Zhang, F. et al. An adipose tissue atlas: an image-guided identification of human-like BAT and beige depots in rodents. Cell Metab. 27, 252–262.e3 (2018).
Acknowledgements
The authors apologize to all colleagues whose work could not be cited owing to space limitations. This work was supported by US National Institutes of Health grants DK053301, R01 DK088423 and R01 DK100659 to J.K.E. A.C. is a Canadian Diabetes Association postdoctoral fellow.
Author information
Authors and Affiliations
Contributions
A.C. wrote the manuscript and prepared the figures. J.K.E., A.C., L.G. and S.L. researched, discussed and reviewed and/or edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- SNS outflow
-
The release of neurotransmitters derived from sympathetic neurons in peripheral tissues.
- Fat mobilization
-
A process, in response to signals for energy, in which fatty acids are released from triglyceride stores in fat cells and delivered to organs.
- Dermomyotome
-
The epithelial cell layer constituting the dorsal part of the somite lying under the ectoderm. It gives rise to the dorsal dermis and to the skeletal muscle of the myotome.
- Bilateral chain
-
A symmetric chain of sympathetic ganglia located just ventral and lateral to the spinal cord.
- Terminal ganglia
-
Parasympathetic ganglia situated within or close to an innervated organ and the site where preganglionic nerve fibres terminate.
- Noradrenaline turnover
-
A surrogate index of changes in sympathetic activation, based on the assumption that tissue levels of noradrenaline decline at a rate proportional to initial noradrenaline concentrations. Increased noradrenaline turnover implies increased sympathetic activation.
- Zeitgeber time
-
A quantification of time based on the period of a zeitgeber. Under standard light–dark cycles, the time at lights-on is usually defined as zeitgeber time 0 for diurnal organisms, and the time at lights-off is defined as zeitgeber time 12 for nocturnal animals.
- β-Blockers
-
β-Adrenergic blocking agents that prevent the effects of the hormone adrenaline.
- Ectopic lipid accumulation
-
An accumulation of lipids in lean tissues such as kidneys, liver, muscle and heart that results in lipotoxicity and metabolic diseases.
- Lipotoxicity
-
The accumulation of lipid intermediates in non-adipose tissue, leading to cellular dysfunction and death.
- Multilocularity
-
The appearance of cytoplasmic lipids arranged in numerous small droplets.
- Thoracolumbar ganglia
-
Ganglia arising from the thoracolumbar (T1–L2) regions of the spinal cord.
- Fluoro-gold
-
A fluorescent retrograde axonal tracer.
- Thermoneutrality
-
The state of thermal balance at which an organism does not need to generate heat in order to maintain a normal core temperature.
- Pyrexic
-
Relating to factors that raise body temperature above normal.
- Leptin resistance
-
Reduced sensitivity with respect to the metabolic responses to exogenously administered leptin.
- Calorimetry
-
The process of measuring the amount of energy as heat released, or absorbed, by an organism.
- O-GlcNAc transferase
-
(OGT). An enzyme that catalyses the addition of an N-acetylglucosamine group in O-glycosidic linkage to serine or threonine residues of intracellular proteins.
- Hypothermic
-
A condition that leads to a decrease in body core temperature — be that regulated, accidental or forced — when the body dissipates more heat than it generates.
- Anapyrexic
-
Relating to decreases in body temperature below normal.
- Lipopolysaccharide
-
Large molecules found in the outer membrane of Gram-negative bacteria. They elicit strong immune responses.
- Omental
-
Adipose tissue located in the omentum, a layer of peritoneum that surrounds abdominal organs.
- Cachexia
-
A syndrome characterized by uncontrolled weight loss, muscle atrophy, fatigue and weakness.
Rights and permissions
About this article
Cite this article
Caron, A., Lee, S., Elmquist, J. et al. Leptin and brain–adipose crosstalks. Nat Rev Neurosci 19, 153–165 (2018). https://doi.org/10.1038/nrn.2018.7
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2018.7
This article is cited by
-
Peripheral extracellular vesicles in neurodegeneration: pathogenic influencers and therapeutic vehicles
Journal of Nanobiotechnology (2024)
-
Inter-organ crosstalk during development and progression of type 2 diabetes mellitus
Nature Reviews Endocrinology (2024)
-
Associations between chronic widespread pain, pressure pain thresholds, leptin, and metabolic factors in individuals with knee pain
BMC Musculoskeletal Disorders (2023)
-
How much obesity and diabetes do impair male fertility?
Reproductive Biology and Endocrinology (2023)
-
Correlation analysis between polymorphism of leptin and IGFI genes and body measurements in Barki and Farafra sheep
Beni-Suef University Journal of Basic and Applied Sciences (2023)