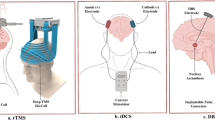
Abstract
Substance use disorders (SUDs) are one of the leading causes of morbidity and mortality worldwide. In spite of considerable advances in understanding the neural underpinnings of SUDs, therapeutic options remain limited. Recent studies have highlighted the potential of transcranial magnetic stimulation (TMS) as an innovative, safe and cost-effective treatment for some SUDs. Repetitive TMS (rTMS) influences neural activity in the short and long term by mechanisms involving neuroplasticity both locally, under the stimulating coil, and at the network level, throughout the brain. The long-term neurophysiological changes induced by rTMS have the potential to affect behaviours relating to drug craving, intake and relapse. Here, we review TMS mechanisms and evidence that rTMS is opening new avenues in addiction treatments.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Koob, G. & Volkow, N. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010).
Chen, B. et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496, 359–362 (2013).
Levy, D. et al. Repeated electrical stimulation of reward-related brain regions affects cocaine but not “natural” reinforcement. J. Neurosci. 27, 14179–14189 (2007).
Melis, M., Spiga, S. & Diana, M. The dopamine hypothesis of drug addiction: hypodopaminergic state. Int. Rev. Neurobiol. 63, 101–154 (2005).
Strafella, A., Paus, T., Barrett, J. & Dagher, A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 21, RC157 (2001).
Barker, A., Jalinous, R. & Freeston, I. Non-invasive magnetic stimulation of human motor cortex. Lancet 1, 1106–1107 (1985).
Terao, Y. & Ugawa, Y. Basic mechanisms of TMS. J. Clin. Neurophysiol. 19, 322–343 (2002).
Di Lazzaro, V., Ziemann, U. & Lemon, R. State of the art: physiology of transcranial motor cortex stimulation. Brain Stimul. 1, 345–362 (2008).
Rossi, S., Hallett, M., Rossini, P. M., & Pascual-Leone, A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin. Neurophysiol. 120, 2008–2039 (2009).
Huang, Y.-Z., Edwards, M., Rounis, E., Bhatia, K. & Rothwell, J. Theta burst stimulation of the human motor cortex. Neuron 45, 201–206 (2005).
Hamada, M. et al. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clin. Neurophysiol. 118, 2672–2682 (2007).
Pascual-Leone, A., Valls-Sole, J., Wassermann, E. & Hallett, M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 117, 847–858 (1994).
Chen, R. et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48, 1398–1403 (1997).
Silvanto, J. & Pascual-Leone, A. State-dependency of transcranial magnetic stimulation. Brain Topogr. 21, 1–10 (2008).
Silvanto, J., Cattaneo, Z., Battelli, L. & Pascual-Leone, A. Baseline cortical excitability determines whether TMS disrupts or facilitates behavior. J. Neurophysiol. 99, 2725–2730 (2008).
Ilmoniemi, R. et al. Neuronal responses to magnetic stimulation reveal cortical reactivity and connectivity. Neuroreport 8, 3537–3540 (1997).
Paus, T. et al. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J. Neurosci. 17, 3178–3184 (1997).
Bohning, D. et al. Echoplanar BOLD fMRI of brain activation induced by concurrent transcranial magnetic stimulation. Invest. Radiol. 33, 336–340 (1998).
Bestmann, S., Baudewig, J., Siebner, H., Rothwell, J. & Frahm, J. Functional MRI of the immediate impact of transcranial magnetic stimulation on cortical and subcortical motor circuits. Eur. J. Neurosci. 19, 1950–1962 (2004).
Radman, T., Ramos, R., Brumberg, J. & Bikson, M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2, 215–228 (2009).
Pashut, T. et al. Patch-clamp recordings of rat neurons from acute brain slices of the somatosensory cortex during magnetic stimulation. Front. Cell Neurosci. 8, 145 (2014).
Edgley, S., Eyre, J., Lemon, R. & Miller, S. Comparison of activation of corticospinal neurons and spinal motor neurons by magnetic and electrical transcranial stimulation in the lumbosacral cord of the anaesthetized monkey. Brain 120, 839–853 (1997).
Mueller, J. et al. Simultaneous transcranial magnetic stimulation and single-neuron recording in alert non-human primates. Nat. Neurosci. 17, 1130–1136 (2014).
Cohen, D. & Cuffin, B. Developing a more focal magnetic stimulator. Part I: some basic principles. J. Clin. Neurophysiol. 8, 102–111 (1991).
Ilmoniemi, R., Ruohonen, J. & Karhu, J. Transcranial magnetic stimulation — a new tool for functional imaging of the brain. Crit. Rev. Biomed. Eng. 27, 241–284 (1999).
Miranda, P., Hallett, M. & Basser, P. The electric field induced in the brain by magnetic stimulation: a 3D finite-element analysis of the effect of tissue heterogeneity and anisotropy. IEEE Trans. Biomed. Eng. 50, 1074–1085 (2003).
Silva, S., Basser, P. & Miranda, P. Elucidating the mechanisms and loci of neuronal excitation by transcranial magnetic stimulation using a finite element model of a cortical sulcus. Clin. Neurophysiol. 119, 2405–2413 (2008).
Salinas, F., Lancaster, J. & Fox, P. 3D modeling of the total electric field induced by transcranial magnetic stimulation using the boundary element method. Phys. Med. Biol. 54, 3631–3647 (2009).
Salvador, R., Silva, S., Basser, P. & Miranda, P. Determining which mechanisms lead to activation in the motor cortex: a modeling study of transcranial magnetic stimulation using realistic stimulus waveforms and sulcal geometry. Clin. Neurophysiol. 122, 748–758 (2011).
Opitz, A., Windhoff, M., Heidemann, R., Turner, R. & Thielscher, A. How the brain tissue shapes the electric field induced by transcranial magnetic stimulation. Neuroimage 58, 849–859 (2011).
Opitz, A., Zafar, N., Bockermann, V., Rohde, V. & Paulus, W. Validating computationally predicted TMS stimulation areas using direct electrical stimulation in patients with brain tumors near precentral regions. Neuroimage Clin. 4, 500–507 (2014).
Nummenmaa, A. et al. Comparison of spherical and anatomically realistic boundary element head models for transcranial magnetic stimulation navigation. Clin. Neurophysiol. 124, 1995–2007 (2013).
Nummenmaa, A. et al. Targeting of white matter tracts with transcranial magnetic stimulation. Brain Stimul. 7, 80–84 (2014).
Ziemann, U. TMS and drugs. Clin. Neurophysiol. 115, 1717–1729 (2004).
Paulus, W. et al. State of the art: pharmacologic effects on cortical excitability measures tested by transcranial magnetic stimulation. Brain Stimul. 1, 151–163 (2008).
Ziemann, U. et al. TMS and drugs revisited 2014. Clin. Neurophysiol. 126, 1847–1868 (2015).
Fox, P. et al. Imaging intra-cerebral connectivity by PET during TMS. Neuroreport 8, 2787–2791 (1997).
Di Lazzaro, V. et al. I-Wave origin and modulation. Brain Stimul. 5, 512–525 (2012).
Niehaus, L., Meyer, B. & Weyh, T. Influence of pulse configuration and direction of coil current on excitatory effects of magnetic motor cortex and nerve stimulation. Clin. Neurophysiol. 111, 75–80 (2000).
Peterchev, A., Goetz, S., Westin, G., Luber, B. & Lisanby, S. Pulse width dependence of motor threshold and input-output curve characterized with controllable pulse parameter transcranial magnetic stimulation. Clin. Neurophysiol. 124, 1364–1372 (2013).
Rushton, W. The effect upon the threshold for nervous excitation of the length of nerve exposed, and the angle between current and nerve. J. Physiol. 63, 357–377 (1927).
Amassian, V., Eberle, L., Maccabee, P. & Cracco, R. Modelling magnetic coil excitation of human cerebral cortex with a peripheral nerve immersed in a brain-shaped volume conductor: the significance of fiber bending in excitation. Electroencephalogr. Clin. Neurophysiol. 85, 291–301 (1992).
Roth, B. Mechanisms for electrical stimulation of excitable tissue. Crit. Rev. Biomed. Eng. 22, 253–305 (1994).
Maccabee, P., Amassian, V., Eberle, L. & Cracco, R. Magnetic coil stimulation of straight and bent amphibian and mammalian peripheral nerve in vitro: locus of excitation. J. Physiol. 460, 201–219 (1993).
Nagarajan, S., Durand, D. & Warman, E. Effects of induced electric fields on finite neuronal structures: a simulation study. IEEE Trans. Biomed. Eng. 40, 1175–1188 (1993).
D'Ostilio, K. et al. Effect of coil orientation on strength-duration time constant and I-wave activation with controllable pulse parameter transcranial magnetic stimulation. Clin. Neurophysiol. 127, 675–683 (2016).
Patton, H. & Amassian, V. Single and multiple-unit analysis of cortical stage of pyramidal tract activation. J. Neurophysiol. 17, 345–363 (1954).
Valls-Sole, J., Pascual-Leone, A., Wassermann, E. & Hallett, M. Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr. Clin. Neurophysiol. 85, 355–364 (1992).
McDonnell, M., Orekhov, Y. & Ziemann, U. The role of GABA(B) receptors in intracortical inhibition in the human motor cortex. Exp. Brain Res. 173, 86–93 (2006).
Romero, J., Anschel, D., Sparing, R., Gangitano, M. & Pascual-Leone, A. Subthreshold low frequency repetitive transcranial magnetic stimulation selectively decreases facilitation in the motor cortex. Clin. Neurophysiol. 113, 101–107 (2002).
Maeda, F., Keenan, J., Tormos, J., Topka, H. & Pascual-Leone, A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin. Neurophysiol. 111, 800–805 (2000).
Ridding, M. & Ziemann, U. Determinants of the induction of cortical plasticity by non-invasive brain stimulation in healthy subjects. J. Physiol. 588, 2291–2304 (2010).
Vernet, M. et al. Reproducibility of the effects of theta burst stimulation on motor cortical plasticity in healthy participants. Clin. Neurophysiol. 125, 320–326 (2014).
Hamada, M., Murase, N., Hasan, A., Balaratnam, M. & Rothwell, J. The role of interneuron networks in driving human motor cortical plasticity. Cereb. Cortex 23, 1593–1605 (2013).
Strafella, A., Paus, T., Fraraccio, M. & Dagher, A. Striatal dopamine release induced by repetitive transcranial magnetic stimulation of the human motor cortex. Brain 126, 2609–2615 (2003).
Cho, S. & Strafella, A. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS ONE 4, e6725 (2009).
Baeken, C. et al. The impact of HF-rTMS treatment on serotonin(2A) receptors in unipolar melancholic depression. Brain Stimul. 4, 104–111 (2011).
Baeken, C. & De Raedt, R. Neurobiological mechanisms of repetitive transcranial magnetic stimulation on the underlying neurocircuitry in unipolar depression. Dialogues Clin. Neurosci. 13, 139–145 (2011).
Bashir, S., Edwards, D. & Pascual-Leone, A. Neuronavigation increases the physiologic and behavioral effects of low-frequency rTMS of primary motor cortex in healthy subjects. Brain Topogr. 24, 54–64 (2011).
Plewnia, C., Lotze, M. & Gerloff, C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport 14, 609–612 (2003).
Schambra, H., Sawaki, L. & Cohen, L. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin. Neurophysiol. 114, 130–133 (2003).
Suppa, A. et al. Theta burst stimulation induces after-effects on contralateral primary motor cortex excitability in humans. J. Physiol. 586, 4489–4500 (2008).
Di Lazzaro, V. et al. The physiological basis of the effects of intermittent theta burst stimulation of the human motor cortex. J. Physiol. 586, 3871–3879 (2008).
Vlachos, A. et al. Repetitive magnetic stimulation induces functional and structural plasticity of excitatory postsynapses in mouse organotypic hippocampal slice cultures. J. Neurosci. 32, 17514–17523 (2012).
Lenz, M. et al. Repetitive magnetic stimulation induces plasticity of excitatory postsynapses on proximal dendrites of cultured mouse CA1 pyramidal neurons. Brain Struct. Funct. 220, 3323–3337 (2015).
Müller-Dahlhaus, F., Ziemann, U. & Classen, J. Plasticity resembling spike-timing dependent synaptic plasticity: the evidence in human cortex. Front. Synapt. Neurosci. 2, 34 (2010).
Daskalakis, Z. et al. The effects of repetitive transcranial magnetic stimulation on cortical inhibition in healthy human subjects. Exp. Brain Res. 174, 403–412 (2006).
Barr, M., Farzan, F., Davis, K., Fitzgerald, P. & Daskalakis, Z. Measuring GABAergic inhibitory activity with TMS-EEG and its potential clinical application for chronic pain. J. Neuroimmune Pharmacol. 8, 535–546 (2013).
Lenz, M. et al. Repetitive magnetic stimulation induces plasticity of inhibitory synapses. Nat. Commun. 7, 10020 (2016).
Normann, C. et al. Associative long-term depression in the hippocampus is dependent on postsynaptic N-type Ca2+ channels. J. Neurosci. 20, 8290–8297 (2000).
Raymond, C. Different requirements for action potentials in the induction of different forms of long-term potentiation. J. Physiol. 586, 1859–1865 (2008).
Ziemann, U. et al. Consensus: motor cortex plasticity protocols. Brain Stimul. 1, 164–182 (2008).
Hoogendam, J., Ramakers, G. & Di Lazzaro, V. Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3, 95–118 (2010).
Tang, A., Thickbroom, G. & Rodger, J. Repetitive transcranial magnetic stimulation of the brain: mechanisms from animal and experimental models. Neuroscientist 23, 82–94 (2015).
Fitzgerald, P., Fountain, S. & Daskalakis, Z. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin. Neurophysiol. 117, 2584–2596 (2006).
Müller-Dahlhaus, F. & Vlachos, A. Unraveling the cellular and molecular mechanisms of repetitive magnetic stimulation. Front. Mol. Neurosci. 6, 50 (2013).
Pell, G., Roth, Y. & Zangen, A. Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: influence of timing and geometrical parameters and underlying mechanisms. Prog. Neurobiol. 93, 59–98 (2010).
Funke, K. & Benali, A. Modulation of cortical inhibition by rTMS — findings obtained from animal models. J. Physiol. 589, 4423–4435 (2011).
Banerjee, J., Sorrell, M., Celnik, P. & Pelled, G. Immediate effects of repetitive magnetic stimulation on single cortical pyramidal neurons. PLoS ONE 12, e0170528 (2017).
Yang, S., Tang, Y. & Zucker, R. Selective induction of LTP and LTD by postsynaptic [Ca2+]i elevation. J. Neurophysiol. 81, 781–787 (1999).
Murphy, S., Palmer, L., Nyffeler, T., Müri, R. & Larkum, M. Transcranial magnetic stimulation (TMS) inhibits cortical dendrites. Elife 5, e13598 (2016).
Tigaret, C., Olivo, V., Sadowski, J., Ashby, M. & Mellor, J. Coordinated activation of distinct Ca2+ sources and metabotropic glutamate receptors encodes Hebbian synaptic plasticity. Nat. Commun. 7, 10289 (2016).
Zanardini, R. et al. Effect of repetitive transcranial magnetic stimulation on serum brain derived neurotrophic factor in drug resistant depressed patients. J. Affect. Disord. 91, 83–86 (2006).
Cheeran, B. et al. A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol. 586, 5717–5725 (2008).
Chen, J. et al. Heterosynaptic long-term depression mediated by ATP released from astrocytes. Glia 61, 178–191 (2013).
Letellier, M. et al. Astrocytes regulate heterogeneity of presynaptic strengths in hippocampal networks. Proc. Natl Acad. Sci. USA 113, E2685–E2694 (2016).
Etiévant, A. et al. Repetitive transcranial magnetic stimulation induces long-lasting changes in protein expression and histone acetylation. Sci. Rep. 5, 16873 (2015).
Ceccanti, M. et al. Deep TMS on alcoholics: effects on cortisolemia and dopamine pathway modulation. A pilot study. Can. J. Physiol. Pharmacol. 93, 283–290 (2015).
Löffler, S. et al. The effect of repetitive transcranial magnetic stimulation on monoamine outflow in the nucleus accumbens shell in freely moving rats. Neuropharmacology 63, 898–904 (2012).
Keck, M. et al. Repetitive transcranial magnetic stimulation increases the release of dopamine in the mesolimbic and mesostriatal system. Neuropharmacology 43, 101–109 (2002).
Zangen, A. & Hyodo, K. Transcranial magnetic stimulation induces increases in extracellular levels of dopamine and glutamate in the nucleus accumbens. Neuroreport 13, 2401–2405 (2002).
Ikemoto, S. & Bonci, A. Neurocircuitry of drug reward. Neuropharmacology 76, 329–341 (2014).
Baik, J. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 7, 152 (2013).
Volman, S. et al. New insights into the specificity and plasticity of reward and aversion encoding in the mesolimbic system. J. Neurosci. 33, 17569–17576 (2013).
Pignatelli, M. & Bonci, A. Role of dopamine neurons in reward and aversion: a synaptic plasticity perspective. Neuron 86, 1145–1157 (2015).
Diana, M. The dopamine hypothesis of drug addiction and its potential therapeutic value. Front. Psychiatry 2, 64 (2011).
Lüscher, C. The emergence of a circuit model for addiction. Annu. Rev. Neurosci. 39, 257–276 (2016).
Argilli, E., Sibley, D., Malenka, R., England, P. & Bonci, A. Mechanism and time course of cocaine-induced long-term potentiation in the ventral tegmental area. J. Neurosci. 28, 9092–9100 (2008).
Bellone, C. & Lüscher, C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat. Neurosci. 9, 636–641 (2006).
Chen, B. et al. Cocaine but not natural reward self-administration nor passive cocaine infusion produces persistent LTP in the VTA. Neuron 59, 288–297 (2008).
Good, C. & Lupica, C. Afferent-specific AMPA receptor subunit composition and regulation of synaptic plasticity in midbrain dopamine neurons by abused drugs. J. Neurosci. 30, 7900–7909 (2010).
Mameli, M., Balland, B., Luján, R. & Lüscher, C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science 317, 530–533 (2007).
Saal, D., Dong, Y., Bonci, A. & Malenka, R. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37, 577–582 (2003).
Ungless, M., Whistler, J., Malenka, R. & Bonci, A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587 (2001).
Bock, R. et al. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat. Neurosci. 16, 632–638 (2013).
Hsiang, H. et al. Manipulating a “cocaine engram” in mice. J. Neurosci. 34, 14115–14127 (2014).
Kasanetz, F. et al. Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328, 1709–1712 (2010).
Kasanetz, F. et al. Prefrontal synaptic markers of cocaine addiction-like behavior in rats. Mol. Psychiatry 18, 729–737 (2013).
Volkow, N., Wang, G. & Fowler, J. Imaging studies of cocaine in the human brain and studies of the cocaine addict. Ann. N. Y. Acad. Sci. 820, 41–54 (1997).
Volkow, N., Koob, G. & McLellan, A. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 374, 363–371 (2016).
Wiers, C., Cabrera, E., Skarda, E., Volkow, N. & Wang, G. PET imaging for addiction medicine: from neural mechanisms to clinical considerations. Prog. Brain Res. 224, 175–201 (2016).
Hatzigiakoumis, D., Martinotti, G., Giannantonio, M. & Janiri, L. Anhedonia and substance dependence: clinical correlates and treatment options. Front. Psychiatry 2, 10 (2011).
Stein, D. & Manyedi, E. Psychoactive substances: position statement on harm reduction. S. Afr. Med. J. 106, 11223 (2016).
George, O., Koob, G. & Vendruscolo, L. Negative reinforcement via motivational withdrawal is the driving force behind the transition to addiction. Psychopharmacology (Berl.) 231, 3911–3917 (2014).
Koob, G. & Le Moal, M. Plasticity of reward neurocircuitry and the 'dark side' of drug addiction. Nat. Neurosci. 8, 1442–1444 (2005).
Yagishita, S. et al. A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science 345, 1616–1620 (2014).
Dan, Y. & Poo, M. Spike timing-dependent plasticity: from synapse to perception. Physiol. Rev. 86, 1033–1048 (2006).
Spiga, S. et al. Hampered long-term depression and thin spine loss in the nucleus accumbens of ethanol-dependent rats. Proc. Natl Acad. Sci. USA 111, E3745–3754 (2014).
Kasai, H., Matsuzaki, M., Noguchi, J., Yasumatsu, N. & Nakahara, H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 26, 360–368 (2003).
Freund, T., Powell, J. & Smith, A. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience 13, 1189–1215 (1984).
Terraneo, A. et al. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur. Neuropsychopharmacol. 26, 37–44 (2016).
Politi, E., Fauci, E., Santoro, A. & Smeraldi, E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am. J. Addict. 17, 345–346 (2008).
Rapinesi, C. et al. Add-on high frequency deep transcranial magnetic stimulation (dTMS) to bilateral prefrontal cortex reduces cocaine craving in patients with cocaine use disorder. Neurosci. Lett. 629, 43–47 (2016).
Bolloni, C. et al. Bilateral transcranial magnetic stimulation of the prefrontal cortex reduces cocaine intake: a pilot study. Front. Psychiatry 7, 133 (2016).
Keck, M. et al. Acute transcranial magnetic stimulation of frontal brain regions selectively modulates the release of vasopressin, biogenic amines and amino acids in the rat brain. Eur. J. Neurosci. 12, 3713–3720 (2000).
Deng, Z., Lisanby, S. & Peterchev, A. Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul. 6, 1–13 (2013).
Addolorato, G. et al. Deep transcranial magnetic stimulation of the dorsolateral prefrontal cortex in alcohol use disorder patients: effects on dopamine transporter availability and alcohol intake. Eur. Neuropsychopharmacol. 27, 450–461 (2017).
Dinur-Klein, L. et al. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol. Psychiatry 76, 742–749 (2014).
Alger, B. Endocannabinoid signaling in neural plasticity. Curr. Top. Behav. Neurosci. 1, 141–172 (2009).
Castillo, P., Younts, T., Chávez, A. & Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 76, 70–81 (2012).
Iremonger, K., Wamsteeker Cusulin, J. & Bains, J. Changing the tune: plasticity and adaptation of retrograde signals. Trends Neurosci. 36, 471–479 (2013).
Abraham, W. Metaplasticity: tuning synapses and networks for plasticity. Nat. Rev. Neurosci. 9, 387 (2008).
Camprodon, J., Martínez-Raga, J., Alonso-Alonso, M., Shih, M. & Pascual-Leone, A. One session of high frequency repetitive transcranial magnetic stimulation (rTMS) to the right prefrontal cortex transiently reduces cocaine craving. Drug Alcohol Depend. 86, 91–94 (2007).
Mishra, B., Praharaj, S., Katshu, M., Sarkar, S. & Nizamie, S. Comparison of anticraving efficacy of right and left repetitive transcranial magnetic stimulation in alcohol dependence: a randomized double-blind study. J. Neuropsychiatry Clin. Neurosci. 27, e54–e59 (2015).
Toga, A. & Thompson, P. Mapping brain asymmetry. Nat. Rev. Neurosci. 4, 37–48 (2003).
Trouche, S. et al. Recoding a cocaine-place memory engram to a neutral engram in the hippocampus. Nat. Neurosci. 19, 564–567 (2016).
Corbit, L., Nie, H. & Janak, P. Habitual responding for alcohol depends upon both AMPA and D2 receptor signaling in the dorsolateral striatum. Front. Behav. Neurosci. 8, 301 (2014).
Kling, J., Yarita, M., Yamamoto, T. & Matsumiya, Y. Memory for conditioned taste aversions is diminished by transcranial magnetic stimulation. Physiol. Behav. 48, 713–717 (1990).
Valero-Cabré, A., Pascual-Leone, A. & Rushmore, R. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur. J. Neurosci. 27, 765–774 (2008).
Cash, R., Murakami, T., Chen, R., Thickbroom, G. & Ziemann, U. Augmenting plasticity induction in human motor cortex by disinhibition stimulation. Cereb. Cortex 26, 58–69 (2016).
Sewerin, S. et al. Enhancing the effect of repetitive I-wave paired-pulse TMS (iTMS) by adjusting for the individual I-wave periodicity. BMC Neurosci. 12, 45 (2011).
Zrenner, C., Belardinelli, P., Müller-Dahlhaus, F. & Ziemann, U. Closed-loop neuroscience and non-invasive brain stimulation: a tale of two loops. Front. Cell Neurosci. 10, 92 (2016).
Fox, M., Liu, H. & Pascual-Leone, A. Identification of reproducible individualized targets for treatment of depression with TMS based on intrinsic connectivity. Neuroimage 66, 151–160 (2013).
Hanlon, C., Dowdle, L. & Jones, J. Biomarkers for success: using neuroimaging to predict relapse and develop brain stimulation treatments for cocaine-dependent individuals. Int. Rev. Neurobiol. 129, 125–156 (2016).
Luber, B. et al. Using neuroimaging to individualize TMS treatment for depression: toward a new paradigm for imaging-guided intervention. Neuroimage 148, 1–7 (2017).
Ruohonen, J. & Karhu, J. Navigated transcranial magnetic stimulation. Neurophysiol. Clin. 40, 7–17 (2010).
Ahveninen, J. et al. Evidence for distinct human auditory cortex regions for sound location versus identity processing. Nat. Comm. 4, 2585 (2013).
Fox, M., Buckner, R., White, M., Greicius, M. & Pascual-Leone, A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol. Psychiatry 72, 595–603 (2012).
Fattore, L. & Diana, M. Drug addiction: an affective-cognitive disorder in need of a cure. Neurosci. Biobehav. Rev. 65, 341–361 (2016).
Davey, K., Epstein, C., George, M. & Bohning, D. Modeling the effects of electrical conductivity of the head on the induced electric field in the brain during magnetic stimulation. Clin. Neurophysiol. 114, 2204–2209 (2003).
Wagner, T., Zahn, M., Grodzinsky, A. & Pascual-Leone, A. Three-dimensional head model simulation of transcranial magnetic stimulation. IEEE Trans. Biomed. Eng. 51, 1586–1598 (2004).
Chen, M. & Mogul, D. Using increased structural detail of the cortex to improve the accuracy of modeling the effects of transcranial magnetic stimulation on neocortical activation. IEEE Trans. Biomed. Eng. 57, 1216–1226 (2010).
Opitz, A. et al. Physiological observations validate finite element models for estimating subject-specific electric field distributions induced by transcranial magnetic stimulation of the human motor cortex. Neuroimage 81, 253–264 (2013).
Deng, Z., Lisanby, S. & Peterchev, A. Coil design considerations for deep transcranial magnetic stimulation. Clin. Neurophysiol. 125, 1202–1212 (2014).
Yunokuchi, K. & Cohen, D. Developing a more focal magnetic stimulator. Part II: Fabricating coils and measuring induced current distributions. J. Clin. Neurophysiol. 8, 112–120 (1991).
Brasil-Neto, J. et al. Optimal focal transcranial magnetic activation of the human motor cortex: effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol. 9, 132–136 (1992).
Pascual-Leone, A., Cohen, L., Brasil-Neto, J. & Hallett, M. Non-invasive differentiation of motor cortical representation of hand muscles by mapping of optimal current directions. Electroencephalogr. Clin. Neurophysiol. 93, 42–48 (1994).
Werhahn, K. et al. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseous muscle. Electroencephalogr. Clin. Neurophysiol. 93, 138–146 (1994).
Day, B. et al. Electric and magnetic stimulation of human motor cortex: surface EMG and single motor unit responses. J. Physiol. 412, 449–473 (1989).
Amassian, V., Quirk, G. J. & Stewart, M. A comparison of corticospinal activation by magnetic coil and electrical stimulation of monkey motor cortex. Electro-encephalogr. Clin. Neurophysiol. 77, 390–401 (1990).
Wilson, S., Day, B., Thickbroom, G. & Mastaglia, F. Spatial differences in the sites of direct and indirect activation of corticospinal neurones by magnetic stimulation. Electroencephalogr. Clin. Neurophysiol. 101, 255–261 (1996).
Nakamura, H., Kitagawa, H., Kawaguchi, Y. & Tsuji, H. Direct and indirect activation of human corticospinal neurons by transcranial magnetic and electrical stimulation. Neurosci. Lett. 210, 45–48 (1996).
Di Lazzaro, V. et al. Comparison of descending volleys evoked by transcranial magnetic and electric stimulation in conscious humans. Electroencephalogr. Clin. Neurophysiol. 109, 397–401 (1998).
Di Lazzaro, V. & Ziemann, U. The contribution of transcranial magnetic stimulation in the functional evaluation of microcircuits in human motor cortex. Front. Neural Circuits 7, 18 (2013).
Mountcastle, V. Perceptual Neuroscience: The Cerebral Cortex (Harvard Univ. Press, 1998).
Goldstein, R. & Volkow, N. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669 (2011).
Ferenczi, E. & Deisseroth, K. Illuminating next-generation brain therapies. Nat. Neurosci. 19, 414–416 (2016).
Kujirai, T. et al. Corticocortical inhibition in human motor cortex. J. Physiol. 471, 501–519 (1993).
Ziemann, U., Rothwell, J. & Ridding, M. Interaction between intracortical inhibition and facilitation in human motor cortex. J. Physiol. 496, 873–881 (1996).
Di Lazzaro, V. et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin. Neurophysiol. 111, 794–799 (2000).
Tokimura, H., Ridding, M., Tokimura, Y., Amassian, V. & Rothwell, J. Short latency facilitation between pairs of threshold magnetic stimuli applied to human motor cortex. Electroencephalogr. Clin. Neurophysiol. 101, 263–272 (1996).
Ilic, T. et al. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J. Physiol. 545, 153–167 (2002).
Ziemann, U. et al. Demonstration of facilitatory I wave interaction in the human motor cortex by paired transcranial magnetic stimulation. J. Physiol. 511, 181–190 (1998).
Hanajima, R. et al. Mechanisms of intracortical I-wave facilitation elicited with paired-pulse magnetic stimulation in humans. J. Physiol. 538, 253–261 (2002).
Claus, D., Weis, M., Jahnke, U., Plewe, A. & Brunhölzl, C. Corticospinal conduction studied with magnetic double stimulation in the intact human. J. Neurol. Sci. 111, 180–188 (1992).
Hamada, M. et al. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J. Physiol. 586, 3927–3947 (2008).
Khedr, E., Gilio, F. & Rothwell, J. Effects of low frequency and low intensity repetitive paired pulse stimulation of the primary motor cortex. Clin. Neurophysiol. 115, 1259–1263 (2004).
Thickbroom, G., Byrnes, M., Edwards, D. & Mastaglia, F. Repetitive paired-pulse TMS at I-wave periodicity markedly increases corticospinal excitability: a new technique for modulating synaptic plasticity. Clin. Neurophysiol. 117, 61–66 (2006).
Hamada, M. et al. Origin of facilitation in repetitive, 1.5 ms interval, paired pulse transcranial magnetic stimulation (rPPS) of the human motor cortex. Clin. Neurophysiol. 118, 1596–1601 (2007).
Fitzgerald, P. et al. A comparative study of the effects of repetitive paired transcranial magnetic stimulation on motor cortical excitability. J. Neurosci. Methods 165, 265–269 (2007).
Acknowledgements
Supported by the US National Institute on Drug Abuse Intramural Research Program (A.B., L.L.), the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (L.L.), the University of Sassari Department of Antidrug Policies and the Ministero dell'Istruzione dell'Università e della Ricerca (MIUR) (M.D.), the Dr Ralph and Marian Falk Medical Research Trust and NIH grants R01MH106512 and S10OD020080 (T.R.) and NIH grants R00EB015445 and R01MH111829 (A.N.). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding organizations.
Author information
Authors and Affiliations
Contributions
A.B. made substantial contribution to discussion of content and reviewed or edited the manuscript before submission.
M D., T.R., M.M. A.N. and L.L. contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Conventional rTMS
-
A form of TMS sequences where the pulses are given at regular intervals (for example, 1 Hz or 20 Hz).
- Direct mechanism
-
(D-mechanism). A mechanism in TMS in which the pyramidal neurons are directly activated by the TMS-induced E-fields.
- Electric field
-
(E-field). The field induced by the TMS coil. When the E-field interacts with a conducting medium, this drives electric currents.
- Indirect mechanism
-
(I-mechanism). A mechanism in TMS in which the pyramidal neurons are activated trans-synaptically, that is, indirectly.
- Motor threshold
-
(MT). The minimum TMS intensity that must be applied to the motor cortex to induce a peripheral muscle contraction.
- Quadripulse stimulation
-
(QPS). A form of patterned TMS where the TMS pulses are arranged in more complex patterns than in conventional rTMS.
- Repetitive paired-pulse TMS
-
(rppTMS). A form of patterned TMS where the TMS pulses are arranged in more complex patterns than in conventional rTMS.
- Repetitive TMS
-
(rTMS). A form of TMS in which individual TMS pulses are presented at regular time intervals (for example, 1 Hz, 20 Hz). Also known as 'conventional rTMS'.
- Theta burst stimulation
-
(TBS). A form of patterned TMS where the TMS pulses are arranged in more complex patterns than in conventional rTMS.
- TMS navigator
-
A device that enables accurate tracking of the TMS coil position relative to the subject's head. Often integrated with MRI of the subject's head.
Rights and permissions
About this article
Cite this article
Diana, M., Raij, T., Melis, M. et al. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nat Rev Neurosci 18, 685–693 (2017). https://doi.org/10.1038/nrn.2017.113
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.113
This article is cited by
-
Active versus sham DLPFC-NAc rTMS for depressed adolescents with anhedonia using resting-state functional magnetic resonance imaging (fMRI): a study protocol for a randomized placebo-controlled trial
Trials (2024)
-
Assessment of rTMS treatment effects for methamphetamine addiction based on EEG functional connectivity
Cognitive Neurodynamics (2024)
-
Clinical application of repetitive transcranial magnetic stimulation in improving functional impairments post-stroke: review of the current evidence and potential challenges
Neurological Sciences (2024)
-
Driving innovation in addiction treatment: role of transcranial magnetic stimulation
Journal of Neural Transmission (2024)
-
Preventing incubation of drug craving to treat drug relapse: from bench to bedside
Molecular Psychiatry (2023)