Key Points
-
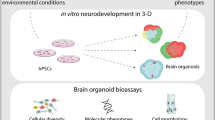
Organoid models provide an opportunity to model the unique features of human brain development in a complex tissue-like environment.
-
The intrinsic patterning of whole brain organoids in intrinsic organoid protocols promotes the generation of diverse brain regions.
-
Signalling molecules can be used to mimic in vivo patterning and increase the efficiency of directed differentiation in comparison to the intrinsic patterning of regional brain organoids.
-
There are a number of advantages of an in vitro system for experimentation purposes, including the relative ease of genomic manipulation and the possibility of using patient-derived cell lines.
-
Human brain organoids can model human development and shed light on human-specific diseases.
-
Protocols will benefit from systematic analysis of cell types generated and a robust comparison to in vivo counterparts.
-
Pre-patterned, region-specific organoids can be fused to model complex brain structures.
-
Current organoid protocols still face a number of limitations, including low reproducibility, incomplete cell type diversity and slow maturation.
Abstract
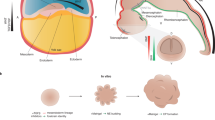
Understanding the development and dysfunction of the human brain is a major goal of neurobiology. Much of our current understanding of human brain development has been derived from the examination of post-mortem and pathological specimens, bolstered by observations of developing non-human primates and experimental studies focused largely on mouse models. However, these tissue specimens and model systems cannot fully capture the unique and dynamic features of human brain development. Recent advances in stem cell technologies that enable the generation of human brain organoids from pluripotent stem cells (PSCs) promise to profoundly change our understanding of the development of the human brain and enable a detailed study of the pathogenesis of inherited and acquired brain diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Tiscornia, G., Vivas, E. L. & Belmonte, J. C. I. Diseases in a dish: modeling human genetic disorders using induced pluripotent cells. Nat. Med. 17, 1570–1576 (2011).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013). This was the first paper to introduce a method for generating cerebral organoids. The organoids were used to model microcephaly.
Kadoshima, T. et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc. Natl Acad. Sci. USA 110, 20284–20289 (2013). This study provided a detailed exploration of brain organoid tissue including the temporal and spatial organization of cell type diversity and patterning.
Jo, J. et al. Midbrain-like organoids from human pluripotent stem cells contain functional dopaminergic and neuromelanin-producing neurons. Cell Stem Cell 19, 248–257 (2016).
Qian, X. et al. Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165, 1238–1254 (2016). This study used mini bioreactors to produce forebrain organoids with a well-defined OSVZ and demonstrated the presence of oRGs with defined molecular markers. Diverse neuronal cell types expressing molecular markers of all six cortical layers were observed.
Birey, F. et al. Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59 (2017). This study used pre-patterned pallial and subpallial spheroids to form fused forebrain spheroids, demonstrating interneuron diversity, migration and integration. This organoid model was used to model Timothy syndrome.
Sakaguchi, H. et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun. 6, 8896 (2015).
Monzel, A. S. et al. Derivation of human midbrain-specific organoids from neuroepithelial stem cells. Stem Cell Rep. 8, 1144–1154 (2017).
Muguruma, K., Nishiyama, A., Kawakami, H., Hashimoto, K. & Sasai, Y. Self-organization of polarized cerebellar tissue in 3D culture of human pluripotent stem cells. Cell Rep. 10, 537–550 (2015).
Suga, H. et al. Self-formation of functional adenohypophysis in three-dimensional culture. Nature 480, 57–62 (2011).
Eiraku, M. et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 3, 519–532 (2008).
Weitzer, G. in Handbook of Experimental Pharmacology (eds Barrett, J.E. et al.) 21–51 (Springer, 2004).
Zhang, S.-C., Wernig, M., Duncan, I. D., Brüstle, O. & Thomson, J. A. In vitro differentiation of transplantable neural precursors from human embryonic stem cells. Nat. Biotechnol. 19, 1129–1133 (2001).
Edri, R. et al. Analysing human neural stem cell ontogeny by consecutive isolation of Notch active neural progenitors. Nat. Commun. 6, 6500 (2015).
Shi, Y., Kirwan, P., Smith, J., Robinson, H. P. C. & Livesey, F. J. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat. Neurosci. 15, 477–486 (2012).
Ying, Q.-L., Stavridis, M., Griffiths, D., Li, M. & Smith, A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183–186 (2003).
Gaspard, N. et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 455, 351–357 (2008).
Chambers, S. M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Watanabe, K. et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat. Neurosci. 8, 288–296 (2005).
Eiraku, M. et al. Self-organizing optic-cup morphogenesis in three-dimensional culture. Nature 472, 51–56 (2011).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Fietz, S. A. et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 13, 690–699 (2010).
Hansen, D. V., Lui, J. H., Parker, P. R. L. & Kriegstein, A. R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561 (2010).
Shi, Y., Kirwan, P. & Livesey, F. J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat. Protoc. 7, 1836–1846 (2012).
Paşca, A. M. et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678 (2015).
Bagley, J. A., Reumann, D., Bian, S., Lévi-Strauss, J. & Knoblich, J. A. Fused cerebral organoids model interactions between brain regions. Nat. Methods 14, 743–751 (2017).
Lindborg, B. A. et al. Rapid induction of cerebral organoids from human induced pluripotent stem cells using a chemically defined hydrogel and defined cell culture medium. Stem Cells Transl Med. 5, 970–979 (2016).
Quadrato, G. et al. Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53 (2017). This study analysed gene expression in over 80,000 individual cells and found that organoids can generate a broad diversity of cells that are related to endogenous classes, including cells from the cerebral cortex and the retina. Neuronal activity within organoids was also controlled using light stimulation of photosensitive cells.
Camp, J. G. et al. Human cerebral organoids recapitulate gene expression programs of fetal neocortex development. Proc. Natl Acad. Sci. USA 112, 15672–15677 (2015).
Suzuki, I. K. & Vanderhaeghen, P. Is this a brain which I see before me? Modeling human neural development with pluripotent stem cells. Development 142, 3138–3150 (2015).
Harrison-Uy, S. J. & Pleasure, S. J. Wnt signaling and forebrain development. Cold Spring Harb. Perspect. Biol. 4, a008094 (2012).
Hikasa, H. & Sokol, S. Y. Wnt signaling in vertebrate axis specification. Cold Spring Harb. Perspect. Biol. 5, a007955 (2012).
Backman, M. et al. Effects of canonical Wnt signaling on dorso-ventral specification of the mouse telencephalon. Dev. Biol. 279, 155–168 (2005).
ten Berge, D. et al. Wnt signaling mediates self-organization and axis formation in embryoid bodies. Cell Stem Cell 3, 508–518 (2008).
Kirkeby, A. et al. Generation of regionally specified neural progenitors and functional neurons from human embryonic stem cells under defined conditions. Cell Rep. 1, 703–714 (2012).
Nasu, M. et al. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS ONE 7, e53024 (2012).
Chenn, A. & Walsh, C. A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297, 365–369 (2002).
Machon, O. et al. A dynamic gradient of Wnt signaling controls initiation of neurogenesis in the mammalian cortex and cellular specification in the hippocampus. Dev. Biol. 311, 223–237 (2007).
Otani, T., Marchetto, M. C., Gage, F. H., Simons, B. D. & Livesey, F. J. 2D and 3D stem cell models of primate cortical development identify species-specific differences in progenitor behavior contributing to brain size. Cell Stem Cell 18, 467–480 (2016).
Smart, I. H. M., Dehay, C., Giroud, P., Berland, M. & Kennedy, H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 12, 37–53 (2002).
Lukaszewicz, A. et al. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron 47, 353–364 (2005).
Zecevic, N., Chen, Y. & Filipovic, R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J. Comp. Neurol. 491, 109–122 (2005).
Dehay, C. & Kennedy, H. Cell-cycle control and cortical development. Nat. Rev. Neurosci. 8, 438–450 (2007).
Martínez- Cerdeño, V. et al. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS ONE 7, e30178 (2012).
Lui, J. H., Hansen, D. V. & Kriegstein, A. R. Development and evolution of the human neocortex. Cell 146, 18–36 (2011).
Fish, J. L., Dehay, C., Kennedy, H. & Huttner, W. B. Making bigger brains-the evolution of neural-progenitor-cell division. J. Cell Sci. 121, 2783–2793 (2008).
Wang, X., Tsai, J.-W., LaMonica, B. & Kriegstein, A. R. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat. Neurosci. 14, 555–561 (2011).
Pollen, A. A. et al. Molecular identity of human outer radial glia during cortical development. Cell 163, 55–67 (2015).
Ostrem, B., Di Lullo, E. & Kriegstein, A. oRGs and mitotic somal translocation - a role in development and disease. Curr. Opin. Neurobiol. 42, 61–67 (2017).
Bershteyn, M. et al. Human iPSC-derived cerebral organoids model cellular features of lissencephaly and reveal prolonged mitosis of outer radial glia. Cell Stem Cell 20, 435–449.e4 (2017). This study used brain organoids to model lissencephaly. The authors identified a mitotic defect in oRGs and used single-cell RNA sequencing to identify cells presenting an oRG transcriptional signature in human brain organoids.
Li, Y. et al. Induction of expansion and folding in human cerebral organoids. Cell Stem Cell 20, 385–396 (2017).
Luo, C. et al. Cerebral organoids recapitulate epigenomic signatures of the human fetal brain. Cell Rep. 17, 3369–3384 (2016).
Lodato, S. & Arlotta, P. Generating neuronal diversity in the mammalian cerebral cortex. Annu. Rev. Cell Dev. Biol. 31, 699–720 (2015).
Voronova, A. et al. Migrating interneurons secrete fractalkine to promote oligodendrocyte formation in the developing mammalian brain. Neuron 94, 500–516.e9 (2017).
Bertrand, N., Castro, D. S. & Guillemot, F. Proneural genes and the specification of neural cell types. Nat. Rev. Neurosci. 3, 517–530 (2002).
Renner, M. et al. Self-organized developmental patterning and differentiation in cerebral organoids. EMBO J. 36, 1316–1329 (2017).
Subramanian, L. & Tole, S. Mechanisms underlying the specification, positional regulation, and function of the cortical hem. Cereb. Cortex 19 (Suppl. 1), i90–i95 (2009).
Caronia-Brown, G., Yoshida, M., Gulden, F., Assimacopoulos, S. & Grove, E. A. The cortical hem regulates the size and patterning of neocortex. Development 141, 2855–2865 (2014).
Furuta, Y., Piston, D. W. & Hogan, B. L. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development 124, 2203–2212 (1997).
Grove, E. A., Tole, S., Limon, J., Yip, L. & Ragsdale, C. W. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125, 2315–2325 (1998).
Guo, J. & Anton, E. S. Decision making during interneuron migration in the developing cerebral cortex. Trends Cell Biol. 24, 342–351 (2014).
Javaherian, A. & Kriegstein, A. A stem cell niche for intermediate progenitor cells of the embryonic cortex. Cereb. Cortex 19, i70–i77 (2009).
Stubbs, D. et al. Neurovascular congruence during cerebral cortical development. Cereb. Cortex 19, i32–i41 (2009).
Cunningham, C. L., Martínez- Cerdeño, V. & Noctor, S. C. Microglia regulate the number of neural precursor cells in the developing cerebral cortex. J. Neurosci. 33, 4216–4233 (2013).
Hockemeyer, D. & Jaenisch, R. Induced pluripotent stem cells meet genome editing. Cell Stem Cell 18, 573–586 (2016).
Sun, Y. & Ding, Q. Genome engineering of stem cell organoids for disease modeling. Protein Cell 8, 315–327 (2017).
Iefremova, V. et al. An organoid-based model of cortical development identifies non-cell-autonomous defects in wnt signaling contributing to Miller–Dieker syndrome. Cell Rep. 19, 50–59 (2017).
Tang, H. et al. Zika virus infects human cortical neural progenitors and attenuates their growth. Cell Stem Cell 18, 587–590 (2016).
Garcez, P. P. et al. Zika virus impairs growth in human neurospheres and brain organoids. Science 352, 816–818 (2016).
Cugola, F. R. et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534, 267–271 (2016). This report used a brain organoid model to demonstrate that ZIKV infection results in a reduction of proliferative zones and in disrupted cortical layers.
Dang, J. et al. Zika virus depletes neural progenitors in human cerebral organoids through activation of the innate immune receptor TLR3. Cell Stem Cell 19, 258–265 (2016).
Retallack, H. et al. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl Acad. Sci. USA 113, 14408–14413 (2016).
Nowakowski, T. J. et al. Expression analysis highlights AXL as a candidate zika virus entry receptor in neural stem cells. Cell Stem Cell 18, 591–596 (2016).
Wells, M. F. et al. Genetic ablation of AXL does not protect human neural progenitor cells and cerebral organoids from zika virus infection. Cell Stem Cell 19, 703–708 (2016).
Meertens, L. et al. Axl mediates ZIKA virus entry in human glial cells and modulates innate immune responses. Cell Rep. 18, 324–333 (2017).
Wen, Z., Christian, K. M., Song, H. & Ming, G.-L. Modeling psychiatric disorders with patient-derived iPSCs. Curr. Opin. Neurobiol. 36, 118–127 (2016).
Flaherty, E. K. & Brennand, K. J. Using hiPSCs to model neuropsychiatric copy number variations (CNVs) has potential to reveal underlying disease mechanisms. Brain Res. 1655, 283–293 (2017).
Brennand, K. J. et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature 473, 221–225 (2011).
Madison, J. M. et al. Characterization of bipolar disorder patient-specific induced pluripotent stem cells from a family reveals neurodevelopmental and mRNA expression abnormalities. Mol. Psychiatry 20, 703–717 (2015).
Mertens, J. et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 527, 95–99 (2015).
Marchetto, M. C. N. et al. A model for neural development and treatment of Rett syndrome using human induced pluripotent stem cells. Cell 143, 527–539 (2010).
Quadrato, G., Brown, J. & Arlotta, P. The promises and challenges of human brain organoids as models of neuropsychiatric disease. Nat. Med. 22, 1220–1228 (2016).
Mariani, J. et al. FOXG1-Dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell 162, 375–390 (2015).
Serrano-Pozo, A., Frosch, M. P., Masliah, E. & Hyman, B. T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 1, a006189 (2011).
Choi, S. H. et al. A three-dimensional human neural cell culture model of Alzheimer's disease. Nature 515, 274–278 (2014).
Raja, W. K. et al. Self-organizing 3D human neural tissue derived from induced pluripotent stem cells recapitulate alzheimer's disease phenotypes. PLoS ONE 11, e0161969 (2016). This report used Alzheimer disease (AD) patient-derived iPSCs to recapitulate AD-like pathologies such as age-dependent amyloid aggregation, hyperphosphorylated tau protein, and endosome abnormalities in organoids and showed that treatment of patient-derived organoids with β-and γ-secretase inhibitors significantly reduces amyloid and tau pathology.
Salloway, S. et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer's disease. N. Engl. J. Med. 370, 322–333 (2014).
Doody, R. S. et al. A phase 3 trial of semagacestat for treatment of Alzheimer's disease. N. Engl. J. Med. 369, 341–350 (2013).
Doody, R. S. et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer's disease. N. Engl. J. Med. 370, 311–321 (2014).
Nowakowski, T. J., Pollen, A. A., Sandoval-Espinosa, C. & Kriegstein, A. R. Transformation of the radial glia scaffold demarcates two stages of human cerebral cortex development. Neuron 91, 1219–1227 (2016).
Acknowledgements
The authors thank A. Bhaduri, J. Spatazza, T. Nowakowski, M. Bershteyn, H. Retallack, M. A. Mostajo Radji and A. Pollen for their scientific discussions and thoughtful input. The authors are very grateful to T. Nowakowski for his help with the illustrations. This work was supported by funding from the US National Institutes of Health R35NS097305.
Author information
Authors and Affiliations
Contributions
E.D. researched the data for the article and provided a substantial contribution to discussions of the content. E.D. and A.R.K. contributed equally to writing the article and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Self-organization
-
The capacity to autonomously generate the architectural complexity of vertebrate organs.
- Inductive signals
-
Molecular signals that can influence the developmental fate of a cell.
- Outer radial glial cells
-
A subclass of radial glial cell residing primarily in the outer subventricular zone.
- Morphogens
-
Secreted factors that can induce different cell fates across a sheet of cells in a concentration-dependent manner by forming gradients.
- Bioreactors
-
A manufactured or engineered device that supports a biologically active environment.
- Intermediate progenitors
-
Transient-amplifying cells that can produce neurons or new intermediate progenitor cells.
- RNA sequencing
-
(RNA-seq). A high-throughput method to sequence whole-genome cDNA in order to obtain quantitative measures of all expressed RNAs in a tissue.
- Principal component analysis
-
A mathematical algorithm that reduces the dimensionality of data while retaining important variation.
- Single-cell transcriptional profiling
-
RNA sequencing of single cells.
- Regulatory elements
-
Sequences of a gene that are involved in regulation of genetic transcription.
- Epigenome
-
A multitude of biochemical modifications to DNA that have key roles in regulating genome structure and function, including the timing, strength, and memory of gene expression.
- Forebrain organizing centres
-
Groups of cells that send signals that induce distinct fates in neighbouring cells, resulting in spatial patterning in the forebrain.
- Viral tropism
-
The specificity of a virus for a particular host cell.
Rights and permissions
About this article
Cite this article
Di Lullo, E., Kriegstein, A. The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 18, 573–584 (2017). https://doi.org/10.1038/nrn.2017.107
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2017.107
This article is cited by
-
Characterizing neuroinflammation and identifying prenatal diagnostic markers for neural tube defects through integrated multi-omics analysis
Journal of Translational Medicine (2024)
-
The 37TrillionCells initiative for improving global healthcare via cell-based interception and precision medicine: focus on neurodegenerative diseases
Molecular Brain (2024)
-
Complex activity and short-term plasticity of human cerebral organoids reciprocally connected with axons
Nature Communications (2024)
-
CUL4B mutations impair human cortical neurogenesis through PP2A-dependent inhibition of AKT and ERK
Cell Death & Disease (2024)
-
Apically localized PANX1 impacts neuroepithelial expansion in human cerebral organoids
Cell Death Discovery (2024)