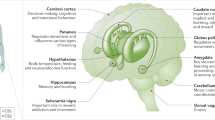
Key Points
-
Cannabis contains more than 100 unique ingredients that are known as 'cannabinoids', and the proportions of these vary widely across different strains of the plant. High-Δ9-tetrahydrocannabinol (Δ9-THC) varieties with negligible levels of cannabidiol (CBD) now dominate many Western markets and are more harmful than lower-Δ9-THC, higher-CBD varieties.
-
Like other recreational drugs, Δ9-THC increases release of dopamine and opioid peptides (in preclinical studies) and alters endocannabinoid processing in the mesocorticolimbic reward system. Long-term Δ9-THC exposure leads to a downregulation of brain cannabinoid receptor function that reverses following abstinence.
-
People who try cannabis are ninefold more likely to become addicted to it than to develop psychosis. Cannabis addiction is an increasing problem globally, and no effective pharmacological treatments currently exist — this remains a major unmet clinical need.
-
The association between cannabis use and psychosis can be influenced by several vulnerability factors, including genetics, environmental factors and the frequency and type of cannabis used. Evidence linking cannabis use with the development of depression and anxiety is less consistent, although these disorders are often comorbid with cannabis addiction.
-
The acute effects of cannabis on cognitive function are well documented, and the most robust, dose-related decrements are to working and episodic memory. Its long-term cognitive effects remain controversial, are influenced by many confounds and appear to subside a month after stopping use of the drug.
-
We should ensure that global legislative changes are informed by neuroscience and public health. They should mitigate against adolescent uptake and the availability of highly potent products, including synthetic agents such as 'spice', that act as full cannabinoid receptor agonists.
Abstract
In an increasing number of states and countries, cannabis now stands poised to join alcohol and tobacco as a legal drug. Quantifying the relative adverse and beneficial effects of cannabis and its constituent cannabinoids should therefore be prioritized. Whereas newspaper headlines have focused on links between cannabis and psychosis, less attention has been paid to the much more common problem of cannabis addiction. Certain cognitive changes have also been attributed to cannabis use, although their causality and longevity are fiercely debated. Identifying why some individuals are more vulnerable than others to the adverse effects of cannabis is now of paramount importance to public health. Here, we review the current state of knowledge about such vulnerability factors, the variations in types of cannabis, and the relationship between these and cognition and addiction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Whiting, P. F. et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 313, 2456–2473 (2015).
Curran, H. V. & Morgan, C. J. A. in Handbook of Cannabis Ch. 36 (ed. Pertwee, R. G.) (Oxford Univ. Press, 2014).
Volkow, N. D., Baler, R. D., Compton, W. M. & Weiss, S. R. Adverse health effects of marijuana use. N. Engl. J. Med. 370, 2219–2227 (2014).
Hall, W. What has research over the past two decades revealed about the adverse health effects of recreational cannabis use? Addiction 110, 19–35 (2015).
United Nations Office on Drugs and Crime. World drug report (UNODC, 2015).
D'Souza, D. C. et al. The psychotomimetic effects of intravenous Δ-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 29, 1558–1572 (2004).
Das, R. K. et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology 226, 781–792 (2013).
Leweke, F. et al. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2, e94 (2012).
Bergamaschi, M. M. et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naive social phobia patients. Neuropsychopharmacology 36, 1219–1226 (2011).
Morgan, C. J., Schafer, G., Freeman, T. P. & Curran, H. V. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br. J. Psychiatry 197, 285–290 (2010).
Englund, A. et al. Cannabidiol inhibits THC-elicited paranoid symptoms and hippocampal-dependent memory impairment. J. Psychopharmacol. 27, 19–27 (2013).
Pertwee, R. G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. Br. J. Pharmacol. 153, 199–215 (2008).
Muniyappa, R. et al. Metabolic effects of chronic cannabis smoking. Diabetes Care 36, 2415–2422 (2013).
Batalla, A. et al. Neuroimaging studies of acute effects of THC and CBD in humans and animals: a systematic review. Curr. Pharm. Des. 20, 2168–2185 (2014).
ElSohly, M. A. et al. Changes in cannabis potency over the last two decades (1995–2014): analysis of current data in the United States. Biol. Psychiatry http://dx.doi.org/10.1016/j.biopsych.2016.01.004, (2016).
Hardwick, S. & King, L. A. Home Office cannabis potency study 2008 (Home Office Scientific Development Branch United Kingdom, 2008).
Niesink, R. J., Rigter, S., Koeter, M. W. & Brunt, T. M. Potency trends of Δ9-tetrahydrocannabinol, cannabidiol and cannabinol in cannabis in the Netherlands: 2005–15. Addiction 110, 1941–1950 (2015).
Swift, W., Wong, A., Li, K. M., Arnold, J. C. & McGregor, I. S. Analysis of cannabis seizures in NSW, Australia: cannabis potency and cannabinoid profile. PLoS ONE 8, e70052 (2013).
Di Marzo, V. et al. Enhancement of anandamide formation in the limbic forebrain and reduction of endocannabinoid contents in the striatum of Δ9-tetrahydrocannabinol-tolerant rats. J. Neurochem. 74, 1627–1635 (2000).
Gonzalez, S. et al. Behavioral and molecular changes elicited by acute administration of SR141716 to Δ9-tetrahydrocannabinol-tolerant rats: an experimental model of cannabinoid abstinence. Drug Alcohol Depend. 74, 159–170 (2004).
Hillard, C. J. Chapter one — the endocannabinoid signaling system in the CNS: a primer. Int. Rev. Neurobiol. 125, 1–47 (2015).
Mechoulam, R., Hanuš, L. O., Pertwee, R. & Howlett, A. C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat. Rev. Neurosci. 15, 757–764 (2014).
Zhu, P. J. Endocannabinoid signaling and synaptic plasticity in the brain. Crit. Rev. Neurobiol. 18, 113–124 (2006).
Curran, H. V., Brignell, C., Fletcher, S., Middleton, P. & Henry, J. Cognitive and subjective dose–response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 164, 61–70 (2002).
Crane, N. A., Schuster, R. M., Fusar-Poli, P. & Gonzalez, R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol. Rev. 23, 117–137 (2013).
Bossong, M. G. et al. Effects of Δ9-tetrahydrocannabinol on human working memory function. Biol. Psychiatry 71, 693–699 (2012).
Crean, R. D., Crane, N. A. & Mason, B. J. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J. Addict. Med. 5, 1–8 (2011).
D'Souza, D. C. et al. Blunted psychotomimetic and amnestic effects of Δ-9-tetrahydrocannabinol in frequent users of cannabis. Neuropsychopharmacology 33, 2505–2516 (2008).
Ramaekers, J. G. et al. Tolerance and cross-tolerance to neurocognitive effects of THC and alcohol in heavy cannabis users. Psychopharmacology 214, 391–401 (2011).
Hirvonen, J. et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol. Psychiatry 17, 642–649 (2012).This was the first study to report downregulation of CB1Rs in frequent cannabis users: the effects were reversed after 4 weeks of monitored abstinence, consistent with findings in rodents.
D'Souza, D. C. et al. Rapid changes in CB1 receptor availability in cannabis dependent males after abstinence from cannabis. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 1, 60–67 (2016).This paper reported a reversal of the downregulation of CB1Rs in frequent cannabis users after only 2 days.
Bhattacharyya, S. et al. Opposite effects of Δ-9-tetrahydrocannabinol and cannabidiol on human brain function and psychopathology. Neuropsychopharmacology 35, 764–774 (2010).
Yücel, M. et al. Hippocampal harms, protection and recovery following regular cannabis use. Transl. Psychiatry 6, e710 (2016).This cross-sectional study used hair analysis to examine the relationship between cannabinoids and hippocampal integrity, and found that chronic Δ9-THC exposure is associated with reduced hippocampal volume and N -acetylaspartate concentrations; however, these effects are not found in those individuals with CBD as well as Δ9-THC in hair or after extended abstinence from the drug.
Han, E., Chung, H. & Song, J. M. Segmental hair analysis for 11-nor-Δ9-tetrahydrocannabinol-9-carboxylic acid and the patterns of cannabis use. J. Anal. Toxicol. 36, 195–200 (2012).
Gonzalez, R. et al. Performance of young adult cannabis users on neurocognitive measures of impulsive behavior and their relationship to symptoms of cannabis use disorders. J. Clin. Exp. Neuropsychol. 34, 962–976 (2012).
Batalla, A. et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PLoS ONE 8, e55821 (2013).
Irimia, C., Polis, I. Y., Stouffer, D. & Parsons, L. H. Persistent effects of chronic Δ9-THC exposure on motor impulsivity in rats. Psychopharmacology 232, 3033–3043 (2015).
Kucewicz, M. T., Tricklebank, M. D., Bogacz, R. & Jones, M. W. Dysfunctional prefrontal cortical network activity and interactions following cannabinoid receptor activation. J. Neurosci. 31, 15560–15568 (2011).
Goldstein, R. Z. & Volkow, N. D. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 12, 652–669 (2011).
Lubman, D. I., Cheetham, A. & Yücel, M. Cannabis and adolescent brain development. Pharmacol. Ther. 148, 1–16 (2015).
Schneider, M. & Koch, M. The effect of chronic peripubertal cannabinoid treatment on deficient object recognition memory in rats after neonatal mPFC lesion. Eur. Neuropsychopharmacol. 17, 180–186 (2007).
Quinn, H. R. et al. Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 33, 1113–1126 (2008).
Cha, Y. M., White, A. M., Kuhn, C. M., Wilson, W. A. & Swartzwelder, H. Differential effects of Δ9-THC on learning in adolescent and adult rats. Pharmacol. Biochem. Behav. 83, 448–455 (2006).
Berghuis, P. et al. Endocannabinoids regulate interneuron migration and morphogenesis by transactivating the TrkB receptor. Proc. Natl Acad. Sci. USA 102, 19115–19120 (2005).
Kim, D. & Thayer, S. A. Cannabinoids inhibit the formation of new synapses between hippocampal neurons in culture. J. Neurosci. 21, RC146 (2001).
Mulder, J. et al. Endocannabinoid signaling controls pyramidal cell specification and long-range axon patterning. Proc. Natl Acad. Sci. USA 105, 8760–8765 (2008).
Verrico, C. D., Gu, H., Peterson, M. L., Sampson, A. R. & Lewis, D. A. Repeated Δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am. J. Psychiatry 171, 416–425 (2014).This study provided proof-of-concept evidence in non-human primates that chronic exposure to exogenous cannabinoids during adolescence selectively disrupts cognitive processes that were actively developing during the time window of exposure.
Jacobus, J. & Tapert, S. F. Effects of cannabis on the adolescent brain. Curr. Pharm. Des. 20, 2186–2193 (2014).
Cheetham, A. et al. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: a 4-year longitudinal and prospective study. Biol. Psychiatry 71, 684–692 (2012).
Lorenzetti, V., Solowij, N., Fornito, A., Lubman, D. I. & Yucel, M. The association between regular cannabis exposure and alterations of human brain morphology: an updated review of the literature. Curr. Pharm. Des. 20, 2138–2167 (2014).
Jager, G., Block, R. I., Luijten, M. & Ramsey, N. F. Cannabis use and memory brain function in adolescent boys: a cross-sectional multicenter functional magnetic resonance imaging study. J. Am. Acad. Child Adolesc. Psychiatry 49, 561–572 (2010).
Ehrenreich, H. et al. Specific attentional dysfunction in adults following early start of cannabis use. Psychopharmacology 142, 295–301 (1999).
Gruber, S. A., Sagar, K. A., Dahlgren, M. K., Racine, M. & Lukas, S. E. Age of onset of marijuana use and executive function. Psychol. Addict. Behav. 26, 496–506 (2012).
Meier, M. H. et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc. Natl Acad. Sci. USA 109, E2657–E2664 (2012).
Blakemore, S. J., Burnett, S. & Dahl, R. E. The role of puberty in the developing adolescent brain. Hum. Brain Mapp. 31, 926–933 (2010).
Crane, N. A., Schuster, R. M., Mermelstein, R. J. & Gonzalez, R. Neuropsychological sex differences associated with age of initiated use among young adult cannabis users. J. Clin. Exp. Neuropsychol. 37, 389–401 (2015).
Lenroot, R. K. & Giedd, J. N. Sex differences in the adolescent brain. Brain Cogn. 72, 46–55 (2010).
Pope, H. G., Gruber, A. J., Hudson, J. I., Huestis, M. A. & Yurgelun-Todd, D. Neuropsychological performance in long-term cannabis users. Arch. Gen. Psychiatry 58, 909–915 (2001).
Schreiner, A. M. & Dunn, M. E. Residual effects of cannabis use on neurocognitive performance after prolonged abstinence: a meta-analysis. Exp. Clin. Psychopharmacol. 20, 420–429 (2012).
Sim-Selley, L. J. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev. Neurobiol. 15, 91–119 (2003).
Fried, P., Watkinson, B. & Gray, R. Neurocognitive consequences of marihuana — a comparison with pre-drug performance. Neurotoxicol. Teratol. 27, 231–239 (2005).
Moore, T. H. et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 370, 319–328 (2007).
Kessler, R. C. et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States: results from the National Comorbidity Survey. Arch. Gen. Psychiatry 51, 8–19 (1994).
Lopez-Quintero, C. et al. Probability and predictors of transition from first use to dependence on nicotine, alcohol, cannabis, and cocaine: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 115, 120–130 (2011).
Koob, G. F. & Volkow, N. D. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010).
Nestler, E. J. Is there a common molecular pathway for addiction? Nat. Neurosci. 8, 1445–1449 (2005).
Everitt, B. J. & Robbins, T. W. Drug addiction: updating actions to habits to compulsions ten years on. Annu. Rev. Psychol. 67, 23–50 (2016).
Badiani, A., Belin, D., Epstein, D., Calu, D. & Shaham, Y. Opiate versus psychostimulant addiction: the differences do matter. Nat. Rev. Neurosci. 12, 685–700 (2011).
Fratta, W. & Fattore, L. Molecular mechanisms of cannabinoid addiction. Curr. Opin. Neurobiol. 23, 487–492 (2013).
European Monitoring Centre for Drugs and Drug Addiction. European drug report (EMCDDA, 2015).
Budney, A. J., Hughes, J. R., Moore, B. A. & Vandrey, R. Review of the validity and significance of cannabis withdrawal syndrome. Am. J. Psychiatry 161, 1967–1977 (2004).
Allsop, D. J., Norberg, M. M., Copeland, J., Fu, S. & Budney, A. J. The Cannabis Withdrawal Scale development: patterns and predictors of cannabis withdrawal and distress. Drug Alcohol Depend. 119, 123–129 (2011).
Budney, A. J., Vandrey, R. G., Hughes, J. R., Moore, B. A. & Bahrenburg, B. Oral Δ9-tetrahydrocannabinol suppresses cannabis withdrawal symptoms. Drug Alcohol Depend. 86, 22–29 (2007).
Rodriguez de Fonseca, F., Carrera, M. R., Navarro, M., Koob, G. F. & Weiss, F. Activation of corticotropin-releasing factor in the limbic system during cannabinoid withdrawal. Science 276, 2050–2054 (1997).
Caberlotto, L., Rimondini, R., Hansson, A., Eriksson, S. & Heilig, M. Corticotropin-releasing hormone (CRH) mRNA expression in rat central amygdala in cannabinoid tolerance and withdrawal: evidence for an allostatic shift? Neuropsychopharmacology 29, 15–22 (2004).References 74 and 75 were the first to demonstrate that Δ9-THC withdrawal is associated with increased levels of the stress neuropeptide CRF in the amygdala, which probably contributes to negative affective states and diminished brain reward function; this withdrawal-related effect is also common to many other classes of abused drugs, including nicotine, alcohol and opiates.
Zorrilla, E. P., Logrip, M. L. & Koob, G. F. Corticotropin releasing factor: a key role in the neurobiology of addiction. Front. Neuroendocrinol. 35, 234–244 (2014).
Vandrey, R., Budney, A., Hughes, J. & Liguori, A. A within-subject comparison of withdrawal symptoms during abstinence from cannabis, tobacco, and both substances. Drug Alcohol Depend. 92, 48–54 (2008).
Justinova, Z., Goldberg, S. R., Heishman, S. J. & Tanda, G. Self-administration of cannabinoids by experimental animals and human marijuana smokers. Pharmacol. Biochem. Behav. 81, 285–299 (2005).This comprehensive review considers the reinforcing effects and abuse liability of Δ9-THC and related cannabinoids as investigated in both human and animal studies.
Freeman, T. P. et al. Just say 'know': how do cannabinoid concentrations influence users' estimates of cannabis potency and the amount they roll in joints? Addiction 109, 1686–1694 (2014).
van der Pol, P. et al. Cross-sectional and prospective relation of cannabis potency, dosing and smoking behaviour with cannabis dependence: an ecological study. Addiction 109, 1101–1109 (2014).
Freeman, T. & Winstock, A. Examining the profile of high-potency cannabis and its association with severity of cannabis dependence. Psychol. Med. 45, 3181–3189 (2015).
Morgan, C. J., Freeman, T. P., Schafer, G. L. & Curran, H. V. Cannabidiol attenuates the appetitive effects of Δ9-tetrahydrocannabinol in humans smoking their chosen cannabis. Neuropsychopharmacology 35, 1879–1885 (2010).
Field, M., Marhe, R. & Franken, I. H. The clinical relevance of attentional bias in substance use disorders. CNS Spectr. 19, 225–230 (2014).
Hindocha, C. et al. Acute effects of Δ9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur. Neuropsychopharmacol. 25, 325–334 (2015).
Haney, M. et al. Oral cannabidiol does not alter the subjective, reinforcing or cardiovascular effects of smoked cannabis. Neuropsychopharmacology http://dx.doi.org/10.1038/npp.2015.367, (2016).
Braida, D., Iosue, S., Pegorini, S. & Sala, M. Δ9-tetrahydrocannabinol-induced conditioned place preference and intracerebroventricular self-administration in rats. Eur. J. Pharmacol. 506, 63–69 (2004).
Zangen, A., Solinas, M., Ikemoto, S., Goldberg, S. R. & Wise, R. A. Two brain sites for cannabinoid reward. J. Neurosci. 26, 4901–4907 (2006).
Gardner, E. L. et al. Facilitation of brain stimulation reward by Δ9-tetrahydrocannabinol. Psychopharmacology 96, 142–144 (1988).
Vlachou, S., Nomikos, G. G. & Panagis, G. CB1 cannabinoid receptor agonists increase intracranial self-stimulation thresholds in the rat. Psychopharmacology 179, 498–508 (2005).
Vlachou, S., Nomikos, G. G., Stephens, D. N. & Panagis, G. Lack of evidence for appetitive effects of Δ9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav. Pharmacol. 18, 311–319 (2007).
Sanudo-Pena, M. C. et al. Endogenous cannabinoids as an aversive or counter-rewarding system in the rat. Neurosci. Lett. 223, 125–128 (1997).
Cheer, J. F., Kendall, D. A. & Marsden, C. A. Cannabinoid receptors and reward in the rat: a conditioned place preference study. Psychopharmacology 151, 25–30 (2000).
Winstock, A. R. & Barratt, M. J. Synthetic cannabis: a comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend. 131, 106–111 (2013).
Justinova, Z., Tanda, G., Redhi, G. H. & Goldberg, S. R. Self-administration of Δ9-tetrahydrocannabinol (THC) by drug naive squirrel monkeys. Psychopharmacology 169, 135–140 (2003).
Fattore, L., Cossu, G., Martellotta, C. M. & Fratta, W. Intravenous self-administration of the cannabinoid CB1 receptor agonist WIN 55,212-2 in rats. Psychopharmacology 156, 410–416 (2001).
Lecca, D., Cacciapaglia, F., Valentini, V. & Di Chiara, G. Monitoring extracellular dopamine in the rat nucleus accumbens shell and core during acquisition and maintenance of intravenous WIN 55,212-2 self-administration. Psychopharmacology 188, 63–74 (2006).
Parsons, L. H. & Hurd, Y. L. Endocannabinoid signaling in reward and addiction. Nat. Rev. Neurosci. 16, 579–594 (2015).
Serrano, A. & Parsons, L. H. Endocannabinoid influence in drug reinforcement, dependence and addiction-related behaviors. Pharmacol. Ther. 132, 215–241 (2011).
Ceccarini, J. et al. [18F]MK-9470 PET measurement of cannabinoid CB1 receptor availability in chronic cannabis users. Addict. Biol. 20, 357–367 (2014).
Breivogel, C. S. et al. Chronic Δ9-tetrahydrocannabinol treatment produces a time-dependent loss of cannabinoid receptors and cannabinoid receptor-activated G proteins in rat brain. J. Neurochem. 73, 2447–2459 (1999).
Dudok, B. et al. Cell-specific STORM super-resolution imaging reveals nanoscale organization of cannabinoid signaling. Nat. Neurosci. 18, 75–86 (2015).This study used nanoscale imaging and electrophysiological techniques to demonstrate greater CB1R expression and influence on perisomatically projecting versus dendritically projecting GABAergic interneurons in the mouse hippocampus, and that persistent deficits in hippocampal LTP following chronic Δ9-THC exposure result from near-complete loss of CB1R at somatic synapses.
Sim-Selley, L. J. et al. Prolonged recovery rate of CB1 receptor adaptation after cessation of long-term cannabinoid administration. Mol. Pharmacol. 70, 986–996 (2006).
Hoffman, A. F., Oz, M., Caulder, T. & Lupica, C. R. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J. Neurosci. 23, 4815–4820 (2003).
Mato, S. et al. A single in vivo exposure to Δ9-THC blocks endocannabinoid-mediated synaptic plasticity. Nat. Neurosci. 7, 585–586 (2004).References 103 and 104 were among the first to demonstrate that Δ9-THC exposure disrupts synaptic plasticity of nucleus accumbens neurons in rodents.
Schlosburg, J. E. et al. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 11, 342–352 (2009).
Castelli, M. P. et al. Dysregulation of the endogenous cannabinoid system in adult rats prenatally treated with the cannabinoid agonist WIN 55,212-2. Eur. J. Pharmacol. 573, 11–19 (2007).
Morgan, C. J. et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br. J. Psychiatry 202, 381–382 (2013).
Muhl, D. et al. Increased CB2 mRNA and anandamide in human blood after cessation of cannabis abuse. Naunyn Schmiedebergs Arch. Pharmacol. 387, 691–695 (2014).
Bossong, M. G. et al. Further human evidence for striatal dopamine release induced by administration of Δ9-tetrahydrocannabinol (THC): selectivity to limbic striatum. Psychopharmacology 232, 2723–2729 (2015).
Nutt, D. J., Lingford-Hughes, A., Erritzoe, D. & Stokes, P. A. The dopamine theory of addiction: 40 years of highs and lows. Nat. Rev. Neurosci. 16, 305–312 (2015).
Cheer, J. F., Wassum, K. M., Heien, M. L., Phillips, P. E. & Wightman, R. M. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J. Neurosci. 24, 4393–4400 (2004).
Chen, J., Marmur, R., Pulles, A., Paredes, W. & Gardner, E. L. Ventral tegmental microinjection of Δ9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana's psychoactive ingredient. Brain Res. 621, 65–70 (1993).
Tanda, G., Pontieri, F. E. & Di Chiara, G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science 276, 2048–2050 (1997).
Diana, M., Melis, M., Muntoni, A. L. & Gessa, G. L. Mesolimbic dopaminergic decline after cannabinoid withdrawal. Proc. Natl Acad. Sci. USA 95, 10269–10273 (1998).
Tanda, G., Loddo, P. & Di Chiara, G. Dependence of mesolimbic dopamine transmission on Δ9-tetrahydrocannabinol. Eur. J. Pharmacol. 376, 23–26 (1999).
Bloomfield, M. A. et al. Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol. Psychiatry 75, 470–478 (2014).
Ghazzaoui, R. & Abi-Dargham, A. Imaging dopamine transmission parameters in cannabis dependence. Prog. Neuropsychopharmacol. Biol. Psychiatry 52, 28–32 (2014).
Manzanares, J. et al. Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Brain Res. Mol. Brain Res. 55, 126–132 (1998).
Valverde, O. et al. Δ9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur. J. Neurosci. 13, 1816–1824 (2001).
Braida, D., Pozzi, M., Parolaro, D. & Sala, M. Intracerebral self-administration of the cannabinoid receptor agonist CP 55,940 in the rat: interaction with the opioid system. Eur. J. Pharmacol. 413, 227–234 (2001).
Justinova, Z., Tanda, G., Munzar, P. & Goldberg, S. R. The opioid antagonist naltrexone reduces the reinforcing effects of Δ9-tetrahydrocannabinol (THC) in squirrel monkeys. Psychopharmacology 173, 186–194 (2004).
Haney, M. et al. Naltrexone maintenance decreases cannabis self-administration and subjective effects in daily cannabis smokers. Neuropsychopharmacology 40, 2489–2498 (2015).
Delfs, J. M., Zhu, Y., Druhan, J. P. & Aston-Jones, G. Noradrenaline in the ventral forebrain is critical for opiate withdrawal-induced aversion. Nature 403, 430–434 (2000).
Moranta, D., Esteban, S. & Garcia-Sevilla, J. A. Chronic treatment and withdrawal of the cannabinoid agonist WIN 55,212-2 modulate the sensitivity of presynaptic receptors involved in the regulation of monoamine syntheses in rat brain. Naunyn Schmiedebergs Arch. Pharmacol. 379, 61–72 (2009).
Page, M. E., Oropeza, V. C. & Van Bockstaele, E. J. Local administration of a cannabinoid agonist alters norepinephrine efflux in the rat frontal cortex. Neurosci. Lett. 431, 1–5 (2008).
Jentsch, J. D., Andrusiak, E., Tran, A., Bowers, M. B. & Roth, R. H. Δ9-tetrahydrocannabinol increases prefrontal cortical catecholaminergic utilization and impairs spatial working memory in the rat: blockade of dopaminergic effects with HA966. Neuropsychopharmacology 16, 426–432 (1997).
Carvalho, A. F. & Van Bockstaele, E. J. Cannabinoid modulation of noradrenergic circuits: implications for psychiatric disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 38, 59–67 (2012).
Coffey, C., Carlin, J. B., Lynskey, M., Li, N. & Patton, G. C. Adolescent precursors of cannabis dependence: findings from the Victorian Adolescent Health Cohort Study. Br. J. Psychiatry 182, 330–336 (2003).
Hines, L. A. et al. Onset of opportunity to use cannabis and progression from opportunity to dependence: are influences consistent across transitions? Drug Alcohol Depend. 160, 57–64 (2016).
Hindocha, C. et al. Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend. 148, 165–171 (2015).
Chen, C.-Y., O'Brien, M. S. & Anthony, J. C. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug Alcohol Depend. 79, 11–22 (2005).
Verweij, K. J. et al. Genetic and environmental influences on cannabis use initiation and problematic use: a meta-analysis of twin studies. Addiction 105, 417–430 (2010).
Agrawal, A. & Lynskey, M. T. Candidate genes for cannabis use disorders: findings, challenges and directions. Addiction 104, 518–532 (2009).
Uhl, G. R. et al. “Higher order” addiction molecular genetics: convergent data from genome-wide association in humans and mice. Biochem. Pharmacol. 75, 98–111 (2008).
Cooper, K., Chatters, R., Kaltenthaler, E. & Wong, R. Psychological and psychosocial interventions for cannabis cessation in adults: a systematic review short report. Health Technol. Assess. 19, 1–130 (2015).
Marshall, K., Gowing, L., Ali, R. & Le Foll, B. Pharmacotherapies for cannabis dependence. Cochrane Database Syst. Rev. 12, CD008940 (2014).
Mason, B. J. et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology 37, 1689–1698 (2012).
Gray, K. M. et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am. J. Psychiatry 169, 805–812 (2012).
Levin, F. R. et al. Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 116, 142–150 (2011).
Allsop, D. J. et al. Nabiximols as an agonist replacement therapy during cannabis withdrawal: a randomized clinical trial. JAMA Psychiatry 71, 281–291 (2014).
Lutz, B., Marsicano, G., Maldonado, R. & Hillard, C. J. The endocannabinoid system in guarding against fear, anxiety and stress. Nat. Rev. Neurosci. 16, 705–718 (2015).
Murillo-Rodriguez, E. et al. The emerging role of the endocannabinoid system in the sleep–wake cycle modulation. Cent. Nerv. Syst. Agents Med. Chem. 11, 189–196 (2011).
Weinstein, A. M. & Gorelick, D. A. Pharmacological treatment of cannabis dependence. Curr. Pharm. Des. 17, 1351–1358 (2011).
Clapper, J. R., Mangieri, R. A. & Piomelli, D. The endocannabinoid system as a target for the treatment of cannabis dependence. Neuropharmacology 56 (Suppl. 1), 235–243 (2009).
van der Pol, P. et al. Mental health differences between frequent cannabis users with and without dependence and the general population. Addiction 108, 1459–1469 (2013).
Degenhardt, L., Hall, W. & Lynskey, M. Testing hypotheses about the relationship between cannabis use and psychosis. Drug Alcohol Depend. 71, 37–48 (2003).
Flórez-Salamanca, L. et al. Probability and predictors of cannabis use disorders relapse: results of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Drug Alcohol Depend. 132, 127–133 (2013).
Christensen, R., Kristensen, P. K., Bartels, E. M., Bliddal, H. & Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet 370, 1706–1713 (2007).
Nissen, S. E. et al. Effect of rimonabant on progression of atherosclerosis in patients with abdominal obesity and coronary artery disease: the STRADIVARIUS randomized controlled trial. JAMA 299, 1547–1560 (2008).
Valverde, O. & Torrens, M. CB1 receptor-deficient mice as a model for depression. Neuroscience 204, 193–206 (2012).
Zanelati, T. V., Biojone, C., Moreira, F. A., Guimaraes, F. S. & Joca, S. R. Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT1A receptors. Br. J. Pharmacol. 159, 122–128 (2010).
Moreira, F. A. & Wotjak, C. T. in Behavioral Neurobiology of Anxiety and Its Treatment (eds Stein, M. B. & Steckler, T.) 429–450 (Springer, 2010).
Sidhpura, N. & Parsons, L. H. Endocannabinoid-mediated synaptic plasticity and addiction-related behavior. Neuropharmacology 61, 1070–1087 (2011).
Morgan, C. et al. Sub-chronic impact of cannabinoids in street cannabis on cognition, psychotic-like symptoms and psychological well-being. Psychol. Med. 42, 391–400 (2012).
Buckner, J. D. & Carroll, K. M. Effect of anxiety on treatment presentation and outcome: results from the Marijuana Treatment Project. Psychiatry Res. 178, 493–500 (2010).
Van Dam, N. T., Bedi, G. & Earleywine, M. Characteristics of clinically anxious versus non-anxious regular, heavy marijuana users. Addict. Behav. 37, 1217–1223 (2012).
Schafer, G. et al. Investigating the interaction between schizotypy, divergent thinking and cannabis use. Conscious. Cogn. 21, 292–298 (2012).
Weiland, B. J. et al. Daily marijuana use is not associated with brain morphometric measures in adolescents or adults. J. Neurosci. 35, 1505–1512 (2015).
Yücel, M. et al. The impact of cannabis use on cognitive functioning in patients with schizophrenia: a meta-analysis of existing findings and new data in a first-episode sample. Schizophr. Bull. 38, 316–330 (2012).
Hindocha, C., Freeman, T. P., Winstock, A. R. & Lynskey, M. T. Vaping cannabis (marijuana) has the potential to reduce tobacco smoking in cannabis users. Addiction 111, 375 (2015).
Hasin, D. S. et al. Prevalence of marijuana use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 72, 1235–1242 (2015).
Nutt, D. J., King, L. A. & Nichols, D. E. Effects of Schedule I drug laws on neuroscience research and treatment innovation. Nat. Rev. Neurosci. 14, 577–585 (2013).
Mokrysz, C. et al. Are IQ and educational outcomes in teenagers related to their cannabis use? A prospective cohort study. J. Psychopharmacol. 30, 159–168 (2016).
Jackson, N. J. et al. Impact of adolescent marijuana use on intelligence: results from two longitudinal twin studies. Proc. Natl Acad. Sci. USA 113, E500–E508 (2016).References 163 and 164 both examined associations between adolescent cannabis use and IQ by using data from large longitudinal cohorts in the United Kingdom and United States; both papers suggest that there are non-causal explanations for the observed associations between adolescent cannabis use and lower IQ.
Temple, E. C., Brown, R. F. & Hine, D. W. The 'grass ceiling': limitations in the literature hinder our understanding of cannabis use and its consequences. Addiction 106, 238–244 (2011).An insightful review covering methodological inconsistencies in cannabis research, offering useful guidance for future research.
Fergusson, D. M., Horwood, L. J. & Beautrais, A. L. Cannabis and educational achievement. Addiction 98, 1681–1692 (2003).
Silins, E. et al. Young adult sequelae of adolescent cannabis use: an integrative analysis. Lancet Psychiatry 1, 286–293 (2014).
Lynskey, M. T. & Hall, W. The effects of adolescent cannabis use on educational attainment: a review. Addiction 95, 1621–1630 (2000).
Townsend, L., Flisher, A. J. & King, G. A systematic review of the relationship between high school dropout and substance use. Clin. Child Fam. Psychol. Rev. 10, 295–317 (2007).
Verweij, K. J., Huizink, A. C., Agrawal, A., Martin, N. G. & Lynskey, M. T. Is the relationship between early-onset cannabis use and educational attainment causal or due to common liability? Drug Alcohol Depend. 133, 580–586 (2013).
Bloomfield, M. A., Morgan, C. J., Kapur, S., Curran, H. V. & Howes, O. D. The link between dopamine function and apathy in cannabis users: an [18F]-DOPA PET imaging study. Psychopharmacology 231, 2251–2259 (2014).
McCaffrey, D. F., Liccardo Pacula, R., Han, B. & Ellickson, P. Marijuana use and high school dropout: the influence of unobservables. Health Econ. 19, 1281–1299 (2010).
Hooper, S. R., Woolley, D. & De Bellis, M. D. Intellectual, neurocognitive, and academic achievement in abstinent adolescents with cannabis use disorder. Psychopharmacology 231, 1467–1477 (2014).
Stiby, A. I. et al. Adolescent cannabis and tobacco use and educational outcomes at age 16: birth cohort study. Addiction 110, 658–668 (2015).
Grant, J. D. et al. Associations of alcohol, nicotine, cannabis, and drug use/dependence with educational attainment: evidence from cotwin-control analyses. Alcohol. Clin. Exp. Res. 36, 1412–1420 (2012).
Large, M., Sharma, S., Compton, M. T., Slade, T. & Nielssen, O. Cannabis use and earlier onset of psychosis: a systematic meta-analysis. Arch. Gen. Psychiatry 68, 555–561 (2011).
Morgan, C. J. A., Freeman, T. P., Powell, J. & Curran, H. V. AKT1 genotype moderates the acute psychotomimetic effects of naturalistically smoked cannabis in young cannabis smokers. Transl. Psychiatry 6, e738 (2016).This is the largest study to date conducted on the acute response to cannabis and shows that psychotic-like symptoms in young healthy cannabis users are predicted by their AKT1 genotype, replicating the same effect observed in schizophrenia (see references 178 and 179).
Di Forti, M. et al. Confirmation that the AKT1 (rs2494732) genotype influences the risk of psychosis in cannabis users. Biol. Psychiatry 72, 811–816 (2012).
van Winkel, R., van Beveren, N. J., Simons, C. & Genetic Risk and Outcome of Psychosis (GROUP) Investigators. AKT1 moderation of cannabis-induced cognitive alterations in psychotic disorder. Neuropsychopharmacology 36, 2529–2537 (2011).
Di Forti, M. et al. Proportion of patients in south London with first-episode psychosis attributable to use of high potency cannabis: a case-control study. Lancet Psychiatry 2, 233–238 (2015).The risk of individuals having a psychotic disorder was roughly threefold higher in those saying they used skunk (which contains high concentrations of Δ9-THC and negligible CBD) and fivefold higher in those reporting daily skunk use, compared with non-users; hash (which has a lower Δ9-THC content and more CBD) did not affect risk of a psychotic disorder.
Morgan, C. J. & Curran, H. V. Effects of cannabidiol on schizophrenia-like symptoms in people who use cannabis. Br. J. Psychiatry 192, 306–307 (2008).The first study to show that psychosis-like symptoms are greater in those whose hair contains only Δ9-THC than in those whose hair contains both Δ9-THC and CBD or no cannabinoids — suggesting a protective effect of CBD.
Leweke, F. M., Giuffrida, A., Wurster, U., Emrich, H. M. & Piomelli, D. Elevated endogenous cannabinoids in schizophrenia. Neuroreport 10, 1665–1669 (1999).
Koethe, D. et al. Anandamide elevation in cerebrospinal fluid in initial prodromal states of psychosis. Br. J. Psychiatry 194, 371–372 (2009).
Di Marzo, V. Targeting the endocannabinoid system: to enhance or reduce? Nat. Rev. Drug Discov. 7, 438–455 (2008).
Volk, D. W. & Lewis, D. A. The role of endocannabinoid signaling in cortical inhibitory neuron dysfunction in schizophrenia. Biol. Psychiatry http://dx.doi.org/10.1016/j.biopsych.2015.06.015, (2015).
American Psychiatric Association. (eds) Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Publishing, 2013).
American Psychiatric Association. (eds) Diagnostic and Statistical Manual of Mental Disorders 4th edn text revision (American Psychiatric Publishing, 2000).
Kandel, D. B. et al. Stages and Pathways of Drug Involvement: Examining the Gateway Hypothesis. (Cambridge Univ. Press, 2002).
Fergusson, D. M., Boden, J. M. & Horwood, L. J. Cannabis use and other illicit drug use: testing the cannabis gateway hypothesis. Addiction 101, 556–569 (2006).
Lynskey, M. T. et al. Escalation of drug use in early-onset cannabis users versus co-twin controls. JAMA 289, 427–433 (2003).
Cadoni, C., Simola, N., Espa, E., Fenu, S. & Di Chiara, G. Strain dependence of adolescent cannabis influence on heroin reward and mesolimbic dopamine transmission in adult Lewis and Fischer 344 rats. Addict. Biol. 20, 132–142 (2015).
Tomasiewicz, H. C. et al. Proenkephalin mediates the enduring effects of adolescent cannabis exposure associated with adult opiate vulnerability. Biol. Psychiatry 72, 803–810 (2012).
Higuera-Matas, A. et al. Augmented acquisition of cocaine self-administration and altered brain glucose metabolism in adult female but not male rats exposed to a cannabinoid agonist during adolescence. Neuropsychopharmacology 33, 806–813 (2008).
Szutorisz, H. et al. Parental THC exposure leads to compulsive heroin-seeking and altered striatal synaptic plasticity in the subsequent generation. Neuropsychopharmacology 39, 1315–1323 (2014).
MacCoun, R. & Reuter, P. Evaluating alternative cannabis regimes. Br. J. Psychiatry 178, 123–128 (2001).
Panlilio, L. V., Zanettini, C., Barnes, C., Solinas, M. & Goldberg, S. R. Prior exposure to THC increases the addictive effects of nicotine in rats. Neuropsychopharmacology 38, 1198–1208 (2013).
Solinas, M., Panlilio, L. & Goldberg, S. Exposure to Δ9-tetrahydrocannabinol (THC) increases subsequent heroin taking but not heroin's reinforcing efficacy: a self-administration study in rats. Neuropsychopharmacology 29, 1301–1311 (2004).
Panlilio, L. V., Solinas, M., Matthews, S. A. & Goldberg, S. R. Previous exposure to THC alters the reinforcing efficacy and anxiety-related effects of cocaine in rats. Neuropsychopharmacology 32, 646–657 (2007).
Patton, G. C., Coffey, C., Carlin, J. B., Sawyer, S. M. & Lynskey, M. Reverse gateways? Frequent cannabis use as a predictor of tobacco initiation and nicotine dependence. Addiction 100, 1518–1525 (2005).
Rubino, T. et al. Chronic Δ9-tetrahydrocannabinol during adolescence provokes sex-dependent changes in the emotional profile in adult rats: behavioral and biochemical correlates. Neuropsychopharmacology 33, 2760–2771 (2008).
Carlezon, W. A. & Thomas, M. J. Biological substrates of reward and aversion: a nucleus accumbens activity hypothesis. Neuropharmacology 56 (Suppl. 1), 122–132 (2009).
Glass, M., Dragunow, M. & Faull, R. L. Cannabinoid receptors in the human brain: a detailed anatomical and quantitative autoradiographic study in the fetal, neonatal and adult human brain. Neuroscience 77, 299–318 (1997).
Wang, X., Dow-Edwards, D., Keller, E. & Hurd, Y. L. Preferential limbic expression of the cannabinoid receptor mRNA in the human fetal brain. Neuroscience 118, 681–694 (2003).
Herkenham, M. et al. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 11, 563–583 (1991).
Egertova, M. & Elphick, M. R. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB1 . J. Comp. Neurol. 422, 159–171 (2000).
Tsou, K., Brown, S., Sanudo-Pena, M. C., Mackie, K. & Walker, J. M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 83, 393–411 (1998).
Hoffman, A. F. & Lupica, C. R. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J. Neurophysiol. 85, 72–83 (2001).
Pistis, M., Muntoni, A. L., Pillolla, G. & Gessa, G. L. Cannabinoids inhibit excitatory inputs to neurons in the shell of the nucleus accumbens: an in vivo electrophysiological study. Eur. J. Neurosci. 15, 1795–1802 (2002).
Tzavara, E. T., Wade, M. & Nomikos, G. G. Biphasic effects of cannabinoids on acetylcholine release in the hippocampus: site and mechanism of action. J. Neurosci. 23, 9374–9384 (2003).
Pisanu, A., Acquas, E., Fenu, S. & Di Chiara, G. Modulation of Δ9-THC-induced increase of cortical and hippocampal acetylcholine release by μ opioid and D1 dopamine receptors. Neuropharmacology 50, 661–670 (2006).
Pistis, M. et al. Δ9-tetrahydrocannabinol decreases extracellular GABA and increases extracellular glutamate and dopamine levels in the rat prefrontal cortex: an in vivo microdialysis study. Brain Res. 948, 155–158 (2002).
Higuera-Matas, A. et al. Periadolescent exposure to cannabinoids alters the striatal and hippocampal dopaminergic system in the adult rat brain. Eur. Neuropsychopharmacol. 20, 895–906 (2010).
Ellgren, M., Spano, S. M. & Hurd, Y. L. Adolescent cannabis exposure alters opiate intake and opioid limbic neuronal populations in adult rats. Neuropsychopharmacology 32, 607–615 (2007).
Morel, L. J., Giros, B. & Dauge, V. Adolescent exposure to chronic Δ9-tetrahydrocannabinol blocks opiate dependence in maternally deprived rats. Neuropsychopharmacology 34, 2469–2476 (2009).
van der Pol, P. et al. Predicting the transition from frequent cannabis use to cannabis dependence: a three-year prospective study. Drug Alcohol Depend. 133, 352–359 (2013).
Acknowledgements
This work was supported by grants from the US National Institutes of Health to L.H.P. (AA020404, AA006420, AA022249 and AA017447) and by grants from the UK Medical Research Council to H.V.C. and C.J.A.M. (G0800268; MR/K015524/1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
C.J.A.M. has been a consultant for Janssen and GlaxoSmithKline. D.A.L. currently receives investigator-initiated research support from Pfizer and in 2012–2014 served as a consultant in the areas of target identification and validation and new compound development for Autifony, Bristol-Myers Squibb, Concert Pharmaceuticals and Sunovion. H.V.C., T.P.F., C.M. and L.H.P. declare no competing interests.
PowerPoint slides
Glossary
- Psychosis
-
A mental disturbance characterized by aberrant perceptions (hallucinations) and thoughts (delusions) that causes an individual to lose touch with external reality.
- Long-term potentiation
-
(LTP). A lasting increase in the strength of neurotransmission at a synapse that is implicated in learning and memory.
- Long-term depression
-
(LTD). An enduring decrease in the strength of neurotransmission at a synapse that is implicated learning and memory.
- Episodic memory
-
Personal, contextualized autobiographical memory of past experiences.
- Working memory
-
The capacity to hold information 'online' (maintenance) and manipulate it.
- Cannabis abuse
-
Cannabis use that is problematic for various aspects of an individual's life (for example, causing occupational, educational or social problems) or that is carried out in dangerous contexts.
- Cannabis dependence
-
A group of severe consequences of repeated cannabis use, including tolerance to effects, withdrawal symptoms upon cessation, dysregulation of use, increased involvement with cannabis at the expense of other activities, and continued use despite the problems it causes.
- Reinforcement
-
A learning process through which particular stimuli or events (such as familiar drug-taking environments, or pleasant drug effects) influence the likelihood or strength of behaviour, such as drug seeking.
- Intracranial self-stimulation
-
(ICSS). An operant paradigm in which animals perform a behavioural response to receive brief electrical pulses into specific regions in the brain reward pathways.
- Conditioned place preference
-
A Pavlovian conditioning procedure used to index the motivational properties of drug experience. Typically, the time spent in an environment associated with drug intoxication is compared with that spent in a neutral context.
Rights and permissions
About this article
Cite this article
Curran, H., Freeman, T., Mokrysz, C. et al. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci 17, 293–306 (2016). https://doi.org/10.1038/nrn.2016.28
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2016.28
This article is cited by
-
Attention-deficit/hyperactivity disorder (ADHD) symptoms and their relation to diagnosed ADHD, sociodemographic characteristics, and substance use among patients receiving opioid agonist therapy: a Norwegian cohort study
BMC Psychiatry (2023)
-
Differentiating people who use cannabis heavily through latent class analysis
Substance Abuse Treatment, Prevention, and Policy (2023)
-
Adolescents and youths’ opinions about the factors associated with cannabis use: a qualitative study based on the I-Change model
BMC Nursing (2023)
-
Effects of cannabidiol on anandamide levels in individuals with cannabis use disorder: findings from a randomised clinical trial for the treatment of cannabis use disorder
Translational Psychiatry (2023)
-
Impairments of Sociocognitive Functions in Individuals with Behavioral Addictions: A Review Article
Journal of Gambling Studies (2023)