Key Points
-
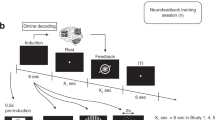
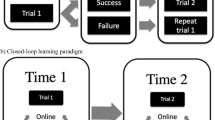
Neurofeedback is a type of biofeedback in which neural activity is measured and presented through one or more sensory channels to the participant in real time to facilitate self-regulation of the putative neural substrates that underlie a particular behaviour or pathology
-
Animal and human brain self-regulation has been demonstrated using various invasive and non-invasive recording methods and with different features of the brain signals, such as frequency spectra, functional connectivity or spatiotemporal patterns of brain activity
-
Neurofeedback provides the possibility of endogenously manipulating brain activity as an independent variable, making it a powerful neuroscientific tool
-
Neurofeedback training results in specific neural changes relevant to the trained brain circuit and the associated behavioural changes. These changes have been shown to last anywhere from hours to months after training and to correlate with changes in grey and white matter structure
-
The underlying neural circuitry relating to the process of brain self-regulation is becoming clearer. Accumulating evidence suggests the involvement of the thalamus and the dorsolateral prefrontal, posterior parietal and occipital cortices in neurofeedback control, and the dorsal and ventral striatum, anterior cingulate cortex and anterior insula in neurofeedback reward processing
-
Psychological factors, such as the differential influence of feedback, reward and experimental instructions, and other factors, such as sense of agency and locus of control, are now being investigated for their effects on neurofeedback
-
The demonstration of robust clinical effects remains a major hurdle in neurofeedback research. The results of randomized controlled trials in attention deficit and hyperactivity disorder and stroke rehabilitation have been mixed, and have been affected by differences in study design, difficulty of identifying responders and the scarcity of homogenous patient populations
-
Future neurofeedback research will probably clarify the psychological and neural mechanisms that may help to address issues in clinical translation
Abstract
Neurofeedback is a psychophysiological procedure in which online feedback of neural activation is provided to the participant for the purpose of self-regulation. Learning control over specific neural substrates has been shown to change specific behaviours. As a progenitor of brain–machine interfaces, neurofeedback has provided a novel way to investigate brain function and neuroplasticity. In this Review, we examine the mechanisms underlying neurofeedback, which have started to be uncovered. We also discuss how neurofeedback is being used in novel experimental and clinical paradigms from a multidisciplinary perspective, encompassing neuroscientific, neuroengineering and learning-science viewpoints.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kamiya, J. The first communications about operant conditioning of the EEG. J. Neurother. 15, 65–73 (2011).
Wolpaw, J. et al. Brain–computer interfaces: principles and practice (Oxford Univ. Press, 2012).
Shibata, K. et al. Perceptual learning incepted by decoded fMRI neurofeedback without stimulus presentation. Science 334, 1413–1415 (2011). This was the first study with real-time fMRI-based neurofeedback demonstrating that the adult primate early visual cortex is plastic enough for visual perceptual learning.
Sitaram, R. et al. Real-time support vector classification and feedback of multiple emotional brain states. Neuroimage 56, 753–765 (2011).
Niazi, A. M. et al. Online decoding of object-based attention using real-time fMRI. Eur. J. Neurosci. 39, 319–329 (2014).
deBettencourt, M. T. et al. Closed-loop training of attention with real-time brain imaging. Nat. Neurosci. 18, 470–475 (2015). This was the first study to use real-time fMRI neurofeedback to increase the cognitive potential of participants, which was indicated by fewer attention lapses.
Mayer, K. et al. Neurofeedback as a nonpharmacological treatment for adults with attention-deficit/hyperactivity disorder (ADHD): study protocol for a randomized controlled trial. Trials 16, 174 (2015).
Kim, D. Y. et al. The inclusion of functional connectivity information into fMRI-based neurofeedback improves its efficacy in the reduction of cigarette cravings. J. Cogn. Neurosci. 27, 1552–1572 (2015). This study showed the importance of how inclusion of functional connectivity information in the feedback signal improves self-regulation learning and behavioural outcome.
Lansbergen, M. M. et al. The increase in theta/beta ratio on resting-state EEG in boys with attention-deficit/hyperactivity disorder is mediated by slow alpha peak frequency. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 47–52 (2011).
Sulzer, J. et al. Real-time fMRI neurofeedback: progress and challenges. Neuroimage 76, 386–399 (2013).
Schafer, R. J. et al. Selective attention from voluntary control of neurons in prefrontal cortex. Science 332, 1568–1571 (2011).
Clancy, K. B. et al. Volitional modulation of optically recorded calcium signals during neuroprosthetic learning. Nat. Neurosci. 17, 807–809 (2014). This study provided a new insight into how neural ensemble dynamics change during learning.
Collinger, J. L. et al. High-performance neuroprosthetic control by an individual with tetraplegia. Lancet 381, 557–564 (2013).
Hochberg, L. R. et al. Neuronal ensemble control of prosthetic devices by a human with tetraplegia. Nature 442, 164–171 (2006).
Bouton, C. E. et al. Restoring cortical control of functional movement in a human with quadriplegia. Nature 533, 247–250 (2016).
Musall, S. et al. Effects of neural synchrony on surface EEG. Cereb. Cortex 24, 1045–1053 (2014).
Beatty, J. et al. Operant control of occipital theta thythm affects performance in a radar monitoring task. Science 183, 871–873 (1973).
Ros, T. et al. Mind over chatter: plastic up-regulation of the fMRI salience network directly after EEG neurofeedback. Neuroimage 65, 324–335 (2013).
Hanslmayr, S. et al. Increasing individual upper alpha power by neurofeedback improves cognitive performance in human subjects. Appl. Psychophysiol. Biofeedback 30, 1–10 (2005).
Egner, T. et al. Ecological validity of neurofeedback: modulation of slow wave EEG enhances musical performance. Neuroreport 14, 1221–1224 (2003).
Bray, S. et al. Direct instrumental conditioning of neural activity using functional magnetic resonance imaging-derived reward feedback. J. Neurosci. 27, 7498–7507 (2007).
Blefari, M. L. et al. Improvement in precision grip force control with self-modulation of primary motor cortex during motor imagery. Front. Behav. Neurosci. 9, 18 (2015).
Sherwood, M. S. et al. Enhanced control of dorsolateral prefrontal cortex neurophysiology with real-time functional magnetic resonance imaging (rt-fMRI) neurofeedback training and working memory practice. Neuroimage 124, 214–223 (2016).
Caria, A. et al. Regulation of anterior insular cortex activity using real-time fMRI. Neuroimage 35, 1238–1246 (2007).
Li, X. et al. Volitional reduction of anterior cingulate cortex activity produces decreased cue craving in smoking cessation: a preliminary real-time fMRI study. Addict. Biol. 18, 739–748 (2013).
Keynan, J. N. et al. Limbic activity modulation guided by fMRI-inspired EEG improves implicit emotion regulation. Biol. Psychiatry 80, 490–496 (2016).
Zotev, V. et al. Self-regulation of human brain activity using simultaneous real-time fMRI and EEG neurofeedback. Neuroimage 85 (Pt.3), 985–995 (2014). This was the first study to implement a real-time neurofeedback system that allows participants to simultaneously self-regulate haemodynamic (fMRI) and electrophysiological (EEG) brain activity.
Fazli, S. et al. Enhanced performance by a hybrid NIRS–EEG brain computer interface. Neuroimage 59, 519–529 (2012).
Haynes, J. D. A. Primer on pattern-based approaches to fMRI: principles, pitfalls, and perspectives. Neuron 87, 257–270 (2015).
Haynes, J. D. et al. Decoding mental states from brain activity in humans. Nat. Rev. Neurosci. 7, 523–534 (2006).
Norman, K. A. et al. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn. Sci. 10, 424–430 (2006).
LaConte, S. M. et al. Real-time fMRI using brain-state classification. Hum. Brain Mapp. 28, 1033–1044 (2007).
Megumi, F. et al. Functional MRI neurofeedback training on connectivity between two regions induces long-lasting changes in intrinsic functional network. Front. Hum. Neurosci. 9, 160 (2015).
Ruiz, S. et al. Acquired self-control of insula cortex modulates emotion recognition and brain network connectivity in schizophrenia. Hum. Brain Mapp. 34, 200–212 (2013).
Rota, G. et al. Reorganization of functional and effective connectivity during real-time fMRI–BCI modulation of prosody processing. Brain Lang. 117, 123–132 (2011).
Kajal, D. S. et al. P113. Learning volitional control of functional connectivity: effects on behaviour. Clin. Neurophysiol. 126, e104 (2015).
Sacchet, M. D. et al. Volitional control of neuromagnetic coherence. Front. Neurosci. 6, 189 (2012). This was the first study to demonstrate the feasibility of performing neurofeedback training based on coherence between two circumscribed brain areas using magnetoencephalography.
Koush, Y. et al. Learning control over emotion networks through connectivity-based neurofeedback. Cereb. Cortex http://dx.doi.org/10.1093/cercor/bhv311 (2015). This study introduced a novel approach for top-down modulation of emotion using effective connectivity feedback with real-time fMRI.
Lewis-Peacock, J. A. et al. Competition between items in working memory leads to forgetting. Nat. Commun. 5, 5768 (2014).
Kamitani, Y. et al. Decoding the visual and subjective contents of the human brain. Nat. Neurosci. 8, 679–685 (2005).
Wiestler, T. et al. Skill learning strengthens cortical representations of motor sequences. eLife 2, e00801 (2013).
Ray, A. M. et al. A subject-independent pattern-based brain–computer interface. Front. Behav. Neurosci. 9, 269 (2015). This paper introduced a new method to perform real-time pattern classification of EEG signals from a group support vector model for neurofeedback training of individuals, eliminating the need for calibrating the classifier on subject-specific data as it wasd one in traditional approaches of pattern classification.
Lee, S. et al. Detection of cerebral reorganization induced by real-time fMRI feedback training of insula activation a multivariate investigation. Neurorehabil. Neural Repair 25, 259–267 (2011). This study showed the change in connectivity pattern of the brain due to learned volitional control of a circumscribed brain area.
Fetz, E. E. Operant conditioning of cortical unit activity. Science 163, 955–958 (1969). This was the first study to demonstrate the feasibility of realizing volitional control of a single neuron by operant training.
Koralek, A. C. et al. Corticostriatal plasticity is necessary for learning intentional neuroprosthetic skills. Nature 483, 331–335 (2012).
Koralek, A. C. et al. Temporally precise cell-specific coherence develops in corticostriatal networks during learning. Neuron 79, 865–872 (2013).
Jackson, A. et al. The Neurochip BCI: towards a neural prosthesis for upper limb function. IEEE Trans. Neural Syst. Rehabil. Eng. 14, 187–190 (2006).
Jackson, A. et al. Long-term motor cortex plasticity induced by an electronic neural implant. Nature 444, 56–60 (2006).
Gruzelier, J. H. EEG-neurofeedback for optimising performance. III: a review of methodological and theoretical considerations. Neurosci. Biobehav. Rev. 44, 159–182 (2014).
Chein, J. M. et al. Neuroimaging studies of practice-related change: fMRI and meta-analytic evidence of a domain-general control network for learning. Brain Res. Cognitive Brain Res. 25, 607–623 (2005).
Scharnowski, F. et al. Manipulating motor performance and memory through real-time fMRI neurofeedback. Biol. Psychol. 108, 85–97 (2015).
Sulzer, J. et al. Neurofeedback-mediated self-regulation of the dopaminergic midbrain. Neuroimage 75C, 176–184 (2013).
Caria, A. et al. Volitional control of anterior insula activity modulates the response to aversive stimuli. A real-time functional magnetic resonance imaging study. Biol. Psychiatry 68, 425–432 (2010).
Lawrence, E. J. et al. Self-regulation of the anterior insula: reinforcement learning using real-time fMRI neurofeedback. Neuroimage 88, 113–124 (2013).
Ros, T. et al. Endogenous control of waking brain rhythms induces neuroplasticity in humans. Eur. J. Neurosci. 31, 770–778 (2010).
Sitaram, R. et al. Acquired control of ventral premotor cortex activity by feedback training: an exploratory real-time FMRI and TMS study. Neurorehabil. Neural Repair 26, 256–265 (2012).
Scholz, J. et al. Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 (2009).
Ghaziri, J. et al. Neurofeedback training induces changes in white and gray matter. Clin. EEG Neurosci. 44, 265–272 (2013).
Marder, E. et al. Variability, compensation and homeostasis in neuron and network function. Nat. Rev. Neurosci. 7, 563–574 (2006).
Maffei, A. et al. Network homeostasis: a matter of coordination. Curr. Opin. Neurobiol. 19, 168–173 (2009).
Ros, T. et al. Tuning pathological brain oscillations with neurofeedback: a systems neuroscience framework. Front. Hum. Neurosci. 8, 1008 (2014).
Ros, T. et al. Neurofeedback tunes scale-free dynamics in spontaneous brain activity. Cereb. Cortex. http://dx.doi.org/10.1093/cercor/bhw285 (2016).
Harmelech, T. et al. The day-after effect: long term, Hebbian-like restructuring of resting-state fMRI patterns induced by a single epoch of cortical activation. J. Neurosci. 33, 9488–9497 (2013).
Gevensleben, H. et al. Neurofeedback training in children with ADHD: 6-month follow-up of a randomised controlled trial. Eur. Child Adolesc. Psychiatry 19, 715–724 (2010). This study explored the long-term effect of neurofeedback training in children with ADHD, showing that the behavioural effect of neurofeedback training persists even after 6 months.
Engelbregt, H. J. et al. Short and long-term effects of sham-controlled prefrontal EEG-neurofeedback training in healthy subjects. Clin. Neurophysiol. 127, 1931–1937 (2016).
Chapin, J. K. et al. Real-time control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat. Neurosci. 2, 664–670 (1999).
Fetz, E. E. Volitional control of neural activity: implications for brain–computer interfaces. J. Physiol. 579, 571–579 (2007).
Allison, B. Z. et al. in Human–Computer Interaction Series (eds Tan, D. & Vanderdonckt, J.) 35–54 (Springer, 2010).
Hammer, E. M. et al. Psychological predictors of SMR–BCI performance. Biol. Psychol. 89, 80–86 (2012).
Sepúlveda, P. et al. How feedback, motor imagery, and reward influence brain self-regulation using real-time fMRI. Hum. Brain Mapp. 37, 3153–3171 (2016). This study investigated several factors (for example, reward, instruction and feedback) that influence the learning process during neurofeedback training, showing that participants who are trained with visual feedback without explicit instruction for using mental imagery show an increase in BOLD self-regulation compared with other participants who do not receive explicit instructions.
Kober, S. E. et al. Learning to modulate one's own brain activity: the effect of spontaneous mental strategies. Front. Hum. Neurosci. 7, 695 (2013).
Ramot, M. et al. Covert neurofeedback without awareness shapes cortical network spontaneous connectivity. Proc. Natl Acad. Sci. USA 113, E2413–E2420 (2016). This study demonstrated that volitional control of brain activations could be learned when participants were unaware that they were undergoing neurofeedback training and did not have any explicit awareness of the feedback signal.
Rockstroh, B. et al. Slow Cortical Potentials and Behavior 2nd edn (Urban & Schwarzenberg, 1989).
Lacroix, J. M. et al. A comparison of the mechanisms and some properties of instructed sudomotor and cardiac control. Biofeedback Self Regul. 3, 105–132 (1978).
Utz, S. W. The effect of instructions on cognitive strategies and performance in biofeedback. J. Behav. Med. 17, 291–308 (1994).
Dunn, T. G. et al. The learning process in biofeedback: Is it feed-forward or feedback? Biofeedback Self Regul. 11, 143–156 (1986).
Siniatchkin, M. et al. Neurofeedback — the significance of reinforcement and the search for an appropriate strategy for the success of self-regulation. Appl. Psychophysiol. Biofeedback 25, 167–175 (2000).
Greer, S. M. et al. Control of nucleus accumbens activity with neurofeedback. Neuroimage 96, 237–244 (2014).
MacInnes, J. J. et al. Cognitive neurostimulation: learning to volitionally sustain ventral tegmental area activation. Neuron 89, 1331–1342 (2016).
Auer, T. et al. Training efficiency and transfer success in an extended real-time functional MRI neurofeedback training of the somatomotor cortex of healthy subjects. Front. Hum. Neurosci. 9, 547 (2015).
Johnson, K. A. et al. Intermittent “real-time” fMRI feedback is superior to continuous presentation for a motor imagery task: a pilot study. J. Neuroimaging 22, 58–66 (2012).
Beier, G. Kontrollüberzeugungen im Umgang mit Technik: ein Persönlichkeitsmerkmal mit Relevanz für die Gestaltung technischer Systeme (in German) (Humboldt Univ. Berlin, 2004).
Witte, M. et al. Control beliefs can predict the ability to up-regulate sensorimotor rhythm during neurofeedback training. Front. Hum. Neurosci. 7, 478 (2013).
Evans, N. et al. Visual feedback dominates the sense of agency for brain–machine actions. PLoS ONE 10, e0130019 (2015).
Hinterberger, T. et al. Neuronal mechanisms underlying control of a brain–computer interface. Eur. J. Neurosci. 21, 3169–3181 (2005).
Ninaus, M. et al. Neural substrates of cognitive control under the belief of getting neurofeedback training. Front. Hum. Neurosci. 7, 914 (2013). This study investigated the cognitive mechanism underpinning the perception of control during neurofeedback training and showed that a broad frontoparietal and cingulo-opercular network was engaged by participants who were attempting to control the feedback signal, although only sham feedback was provided to the participants.
Emmert, K. et al. Meta-analysis of real-time fMRI neurofeedback studies using individual participant data: How is brain regulation mediated? Neuroimage 124, 806–812 (2015). This meta-analysis of several past neurofeedback studies investigated neural correlates of self-regulation, showing that the anterior insula and basal ganglia are key components of the regulation network.
Craig, A. D. How do you feel — now? The anterior insula and human awareness. Nat. Rev. Neurosci. 10, 59–70 (2009).
Harmelech, T. et al. Differential magnetic resonance neurofeedback modulations across extrinsic (visual) and intrinsic (default-mode) nodes of the human cortex. J. Neurosci. 35, 2588–2595 (2015).
Hetu, S. et al. The neural network of motor imagery: an ALE meta-analysis. Neurosci. Biobehav. Rev. 37, 930–949 (2013).
Amiez, C. et al. Modulation of feedback related activity in the rostral anterior cingulate cortex during trial and error exploration. Neuroimage 63, 1078–1090 (2012).
Gevensleben, H. et al. Neurofeedback of slow cortical potentials: neural mechanisms and feasibility of a placebo-controlled design in healthy adults. Front. Hum. Neurosci. 8, 990 (2014).
Lubar, J. O. et al. Electroencephalographic biofeedback of SMR and beta for treatment of attention deficit disorders in a clinical setting. Biofeedback Self Regul. 9, 1–23 (1984).
Chabot, R. J. et al. The clinical role of computerized EEG in the evaluation and treatment of learning and attention disorders in children and adolescents. J. Neuropsychiatry Clin. Neurosci. 13, 171–186 (2001).
Dustman, R. E. et al. Life-span changes in EEG spectral amplitude, amplitude variability and mean frequency. Clin. Neurophysiol. 110, 1399–1409 (1999).
Poil, S. S. et al. Age dependent electroencephalographic changes in attention-deficit/hyperactivity disorder (ADHD). Clin. Neurophysiol. 125, 1626–1638 (2014).
Ogrim, G. et al. Predicting the clinical outcome of stimulant medication in pediatric attention-deficit/hyperactivity disorder: data from quantitative electroencephalography, event-related potentials, and a go/no-go test. Neuropsychiatr. Dis. Treat. 10, 231–242 (2014).
Janssen, T. W. et al. A randomized controlled trial into the effects of neurofeedback, methylphenidate, and physical activity on EEG power spectra in children with ADHD. J. Child Psychol. Psychiatry 57, 633–644 (2016).
Lubar, J. F. et al. Evaluation of the effectiveness of EEG neurofeedback training for ADHD in a clinical setting as measured by changes in T.O.V.A. scores, behavioral ratings, and WISC-R performance. Biofeedback Self Regul. 20, 83–99 (1995).
Gevensleben, H. et al. Distinct EEG effects related to neurofeedback training in children with ADHD: a randomized controlled trial. Int. J. Psychophysiol. 74, 149–157 (2009).
Steiner, N. J. et al. Neurofeedback and cognitive attention training for children with attention-deficit hyperactivity disorder in schools. J. Dev. Behav. Pediatr. 35, 18–27 (2014).
Gevensleben, H. et al. Is neurofeedback an efficacious treatment for ADHD? A randomised controlled clinical trial. J. Child Psychol. Psychiatry 50, 780–789 (2009).
Duric, N. S. et al. Neurofeedback for the treatment of children and adolescents with ADHD: a randomized and controlled clinical trial using parental reports. BMC Psychiatry 12, 107 (2012).
Meisel, V. et al. Neurofeedback and standard pharmacological intervention in ADHD: a randomized controlled trial with six-month follow-up. Biol. Psychol. 94, 12–21 (2013).
Arns, M. et al. Efficacy of neurofeedback treatment in ADHD: the effects on inattention, impulsivity and hyperactivity: a meta-analysis. Clin. EEG Neurosci. 40, 180–189 (2009).
Sonuga-Barke, E. et al. Computer-based cognitive training for ADHD: a review of current evidence. Child Adolesc. Psychiatr. Clin. N. Am. 23, 807–824 (2014).
Micoulaud-Franchi, J. A. et al. EEG neurofeedback treatments in children with ADHD: an updated meta-analysis of randomized controlled trials. Front. Hum. Neurosci. 8, 906 (2014).
van Dongen-Boomsma, M. et al. A randomized placebo-controlled trial of electroencephalographic (EEG) neurofeedback in children with attention-deficit/hyperactivity disorder. J. Clin. Psychiatry 74, 821–827 (2013).
Arnold, L. E. et al. EEG neurofeedback for ADHD: double-blind sham-controlled randomized pilot feasibility trial. J. Atten. Disord. 17, 410–419 (2013).
Arns, M. et al. Evaluation of neurofeedback in ADHD: the long and winding road. Biol. Psychol. 95, 108–115 (2014).
Zuberer, A. et al. Are treatment effects of neurofeedback training in children with ADHD related to the successful regulation of brain activity? A review on the learning of regulation of brain activity and a contribution to the discussion on specificity. Front. Hum. Neurosci. 9, 135 (2015). This comprehensive review described the efficacy and specificity of neurofeedback training in children with ADHD.
Liechti, M. D. et al. First clinical trial of tomographic neurofeedback in attention-deficit/hyperactivity disorder: evaluation of voluntary cortical control. Clin. Neurophysiol. 123, 1989–2005 (2012).
Lansbergen, M. M. et al. ADHD and EEG-neurofeedback: a double-blind randomized placebo-controlled feasibility study. J. Neural Transm. (Vienna) 118, 275–284 (2011).
Gelade, K. et al. An RCT into the effects of neurofeedback on neurocognitive functioning compared to stimulant medication and physical activity in children with ADHD. Eur. Child Adolesc. Psychiatry http://dx.doi.org/10.1007/s00787-016-0902-x (2016). This report of an RCT compared the effect of neurofeedback training, pharmacological treatment and physical therapy in children with ADHD.
Mazaheri, A. et al. Differential oscillatory electroencephalogram between attention-deficit/hyperactivity disorder subtypes and typically developing adolescents. Biol. Psychiatry 76, 422–429 (2014).
Arns, M. et al. EEG phenotypes predict treatment outcome to stimulants in children with ADHD. J. Integr. Neurosci. 7, 421–438 (2008).
Kanazawa, O. Reappraisal of abnormal EEG findings in children with ADHD: on the relationship between ADHD and epileptiform discharges. Epilepsy Behav. 41, 251–256 (2014).
Buyck, I. et al. Task-related electroencephalographic deviances in adults with attention deficit hyperactivity disorder. Neuropsychology 29, 433–444 (2015).
Missonnier, P. et al. EEG anomalies in adult ADHD subjects performing a working memory task. Neuroscience 241, 135–146 (2013).
Heinrich, H. et al. Training of slow cortical potentials in attention-deficit/hyperactivity disorder: evidence for positive behavioral and neurophysiological effects. Biol. Psychiatry 55, 772–775 (2004).
Mayer, K. et al. One size fits all? Slow cortical potentials neurofeedback: a review. J. Atten. Disord. 17, 393–409 (2013).
Doehnert, M. et al. Slow cortical potential neurofeedback in attention deficit hyperactivity disorder: is there neurophysiological evidence for specific effects? J. Neural Transm. (Vienna) 115, 1445–1456 (2008).
Gani, C. et al. Long term effects after feedback of slow cortical potentials and of theta-beta amplitudes in children with attention-deficit/hyperactivity disorder (ADHD). Int. J. Bioelectromagn. 10, 209–232 (2008).
Thibault, R. T. et al. The self-regulating brain and neurofeedback: experimental science and clinical promise. Cortex 74, 247–261 (2016).
Borkovec, T. D. et al. Problems with the use of placebo conditions in psychotherapy research, suggested alternatives, and some strategies for the pursuit of the placebo phenomenon. J. Clin. Psychol. 61, 805–818 (2005).
Kessler, R. C. et al. The effects of temporally secondary co-morbid mental disorders on the associations of DSM-IV ADHD with adverse outcomes in the US National Comorbidity Survey Replication Adolescent Supplement (NCS-A). Psychol. Med. 44, 1779–1792 (2014).
Querne, L. et al. Effects of methylphenidate on default-mode network/task-positive network synchronization in children with ADHD. J. Atten. Disord. http://dx.doi.org/10.1177/1087054713517542 (2014).
Chabernaud, C. et al. Dimensional brain-behavior relationships in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry 71, 434–442 (2012).
Wen, X. et al. Top-down regulation of default mode activity in spatial visual attention. J. Neurosci. 33, 6444–6453 (2013).
Kelly, A. M. et al. Competition between functional brain networks mediates behavioral variability. Neuroimage 39, 527–537 (2008).
Hlinka, J. et al. Slow EEG pattern predicts reduced intrinsic functional connectivity in the default mode network: an inter-subject analysis. Neuroimage 53, 239–246 (2010).
Jann, K. et al. BOLD correlates of EEG alpha phase-locking and the fMRI default mode network. Neuroimage 45, 903–916 (2009).
Mantini, D. et al. Electrophysiological signatures of resting state networks in the human brain. Proc. Natl Acad. Sci. USA 104, 13170–13175 (2007).
Heinrich, H. et al. EEG spectral analysis of attention in ADHD: implications for neurofeedback training? Front. Hum. Neurosci. 8, 611 (2014).
Calautti, C. et al. Functional neuroimaging studies of motor recovery after stroke in adults: a review. Stroke 34, 1553–1566 (2003).
Sung, W. H. et al. Efficacy of coupling inhibitory and facilitatory repetitive transcranial magnetic stimulation to enhance motor recovery in hemiplegic stroke patients. Stroke 44, 1375–1382 (2013).
Taub, E. The behavior-analytic origins of constraint-induced movement therapy: an example of behavioral neurorehabilitation. Behav. Anal. 35, 155–178 (2012).
Wolbrecht, E. T. et al. Optimizing compliant, model-based robotic assistance to promote neurorehabilitation. IEEE Trans. Neural Syst. Rehabil. Eng. 16, 286–297 (2008).
Buch, E. et al. Think to move: a neuromagnetic brain–computer interface (BCI) system for chronic stroke. Stroke 39, 910–917 (2008).
Ang, K. K. et al. Facilitating effects of transcranial direct current stimulation on motor imagery brain–computer interface with robotic feedback for stroke rehabilitation. Arch. Phys. Med. Rehabil. 96, S79–S87 (2015).
Ramos-Murguialday, A. et al. Brain–machine interface in chronic stroke rehabilitation: a controlled study. Ann. Neurol. 74, 100–108 (2013). This study provided haptic feedback in a BCI training paradigm, demonstrating improvement in motor function in patients with chronic stroke.
Small, S. L. et al. Brain repair after stroke — a novel neurological model. Nat. Rev. Neurol. 9, 698–707 (2013). This study introduced a new model of neural repair after stroke that is based on the notion that specific brain networks are reorganized in response to physical and behavioural intervention.
Pichiorri, F. et al. Brain–computer interface boosts motor imagery practice during stroke recovery. Ann. Neurol. 77, 851–865 (2015).
Varkuti, B. et al. Resting state changes in functional connectivity correlate with movement recovery for BCI and robot-assisted upper-extremity training after stroke. Neurorehabil. Neural Repair 27, 53–62 (2013).
Liew, S. L. et al. Improving motor corticothalamic communication after stroke using real-time fMRI connectivity-based neurofeedback. Neurorehabil. Neural Repair. 30, 671–675 (2015).
Chiew, M. et al. Investigation of fMRI neurofeedback of differential primary motor cortex activity using kinesthetic motor imagery. Neuroimage 61, 21–31 (2012).
Stoeckel, L. E. et al. Optimizing real time fMRI neurofeedback for therapeutic discovery and development. Neuromage Clin. 5, 245–255 (2014). This comprehensive review investigated the current status of neurofeedback techniques as a therapeutic tool and described the future steps that are needed to optimize their development and application.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders 5th edn (American Psychiatric Publishing, 2013).
Blankertz, B. et al. Neurophysiological predictor of SMR-based BCI performance. Neuroimage 51, 1303–1309 (2010).
Halder, S. et al. Prediction of brain–computer interface aptitude from individual brain structure. Front. Hum. Neurosci. 7, 105 (2013).
Ninaus, M. et al. Brain volumetry and self-regulation of brain activity relevant for neurofeedback. Biol. Psychol. 110, 126–133 (2015).
Emmert, K. et al. Active pain coping is associated with the response in real-time fMRI neurofeedback during pain. Brain Imaging Behav. http://dx.doi.org/10.1007/s11682-016-9547-0 (2016).
Rao, R. P. et al. A direct brain-to-brain interface in humans. PLoS ONE 9, e111332 (2014).
Zhdanov, A. et al. An internet-based real-time audiovisual link for dual MEG recordings. PLoS ONE 10, e0128485 (2015).
Feinberg, D. A. et al. Multiplexed echo planar imaging for sub-second whole brain FMRI and fast diffusion imaging. PLoS ONE 5, e15710 (2010). This study introduced an innovative methodological development that enables acquisition of fMRI images at very high temporal resolution, potentially suitable for more-effective real-time fMRI feedback.
Chen, S. et al. Optogenetics based rat–robot control: optical stimulation encodes “stop” and “escape” commands. Ann. Biomed. Eng. 43, 1851–1864 (2015). This pioneering study introduced a unique idea to control biorobots using optics rather than traditional electric brain stimulation.
Kasashima-Shindo, Y. et al. Brain–computer interface training combined with transcranial direct current stimulation in patients with chronic severe hemiparesis: proof of concept study. J. Rehabil. Med. 47, 318–324 (2015).
Logothetis, N. K. et al. Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 (2001). This study pioneered the understanding of the physiological basis of BOLD signal and suggested that the BOLD reflects the input of intracortical processing neurons rather than their spiking output.
Buzsaki, G. et al. The origin of extracellular fields and currents — EEG, ECoG, LFP and spikes. Nat. Rev. Neurosci. 13, 407–420 (2012).
Hari, R. et al. Spatial resolution of neuromagnetic records: theoretical calculations in a spherical model. Electroencephalogr. Clin. Neurophysiol. 71, 64–72 (1988).
Nunez, P. L. et al. A theoretical and experimental study of high resolution EEG based on surface Laplacians and cortical imaging. Electroencephalogr. Clin. Neurophysiol. 90, 40–57 (1994).
Cunningham, J. P. et al. Methods for estimating neural firing rates, and their application to brain–machine interfaces. Neural Netw. 22, 1235–1246 (2009).
Hwang, E. J. et al. The utility of multichannel local field potentials for brain–machine interfaces. J. Neural Eng. 10, 046005 (2013).
Oswal, A. et al. Optimising beamformer regions of interest analysis. Neuroimage 102 (Pt. 2), 945–954 (2014). This paper introduced a new two-step approach to perform source reconstruction using the beam-forming method, by first taking into account prior specification of channels pertaining to a brain region of interest.
Congedo, M. et al. Low-resolution electromagnetic tomography neurofeedback. IEEE Trans. Neural Syst. Rehabil. Eng. 12, 387–397 (2004). This study developed a pioneering method to solve the inverse problem in EEG for neurofeedback.
Viswanathan, A. et al. Neurometabolic coupling in cerebral cortex reflects synaptic more than spiking activity. Nat. Neurosci. 10, 1308–1312 (2007).
Shmuel, A. et al. Spatio-temporal point-spread function of fMRI signal in human gray matter at 7 Tesla. Neuroimage 35, 539–552 (2007).
Villringer, A. et al. in Brain Mapping: The Methods (eds Toga, A. W. & Mazziotta, J. C.) (Academic Press, 2002).
de Pasquale, F. et al. Temporal dynamics of spontaneous MEG activity in brain networks. Proc. Natl Acad. Sci. USA 107, 6040–6045 (2010).
Brookes, M. J. et al. Measuring functional connectivity using MEG: methodology and comparison with fcMRI. Neuroimage 56, 1082–1104 (2011).
Scheeringa, R. et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron 69, 572–583 (2011).
Conner, C. R. et al. Variability of the relationship between electrophysiology and BOLD–fMRI across cortical regions in humans. J. Neurosci. 31, 12855–12865 (2011). This paper investigated the variation in BOLD response in different brain areas, which has implications for how we model the BOLD response function.
Bridwell, D. A. et al. The spatiospectral characterization of brain networks: fusing concurrent EEG spectra and fMRI maps. Neuroimage 69, 101–111 (2013).
Whittingstall, K. et al. Frequency-band coupling in surface EEG reflects spiking activity in monkey visual cortex. Neuron 64, 281–289 (2009).
Monto, S. et al. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J. Neurosci. 28, 8268–8272 (2008).
He, B. J. et al. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc. Natl Acad. Sci. USA 105, 16039–16044 (2008).
Casey, B. J. et al. A neurodevelopmental perspective on the research domain criteria (RDoC) framework. Biol. Psychiatry 76, 350–353 (2014).
Menon, V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15, 483–506 (2011).
Calhoun, V. D. et al. The chronnectome: time-varying connectivity networks as the next frontier in fMRI data discovery. Neuron 84, 262–274 (2014). This paper introduced a new method for computing time-varying properties of functional connectivity to better understand the neural mechanism of different brain functions.
Dance, A. Neuroscience: connectomes make the map. Nature 526, 147–149 (2015).
Boes, A. D. et al. Network localization of neurological symptoms from focal brain lesions. Brain 138, 3061–3075 (2015).
Sheline, Y. I. et al. Resting state functional connectivity in preclinical Alzheimer's disease. Biol. Psychiatry 74, 340–347 (2013).
Fedota, J. R. et al. Resting-state functional connectivity and nicotine addiction: prospects for biomarker development. Ann. NY Acad. Sci. 1349, 64–82 (2015).
Lerman, C. et al. Large-scale brain network coupling predicts acute nicotine abstinence effects on craving and cognitive function. JAMA Psychiatry 71, 523–530 (2014).
Hebb, D. O. The Organization of Behavior (Wiley & Sons, 1949). This pioneer work proposed the Hebb's rule for explaining neural changes during learning.
Cooke, S. F. et al. Stimulus-selective response plasticity in the visual cortex: an assay for the assessment of pathophysiology and treatment of cognitive impairment associated with psychiatric disorders. Biol. Psychiatry 71, 487–495 (2012).
Caporale, N. et al. Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31, 25–46 (2008).
Gallistel, C. R. et al. The neuroscience of learning: beyond the Hebbian synapse. Annu. Rev. Psychol. 64, 169–200 (2013).
Gruart, A. et al. Functional basis of associative learning and their relationships with long-term potentiation evoked in the involved neural circuits: lessons from studies in behaving mammals. Neurobiol. Learn. Mem. 124, 3–18 (2015).
Daniel, R. et al. Striatal activations signal prediction errors on confidence in the absence of external feedback. Neuroimage 59, 3457–3467 (2012).
Schultz, W. et al. Explicit neural signals reflecting reward uncertainty. Philos. Trans. R. Soc. Lond. B Biol. Sci. 363, 3801–3811 (2008).
Schultz, W. et al. A neural substrate of prediction and reward. Science 275, 1593–1599 (1997).
Montague, P. R. et al. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J. Neurosci. 16, 1936–1947 (1996). This paper discussed a theoretical framework to predict future reward and errors based on brain activity.
Ashby, F. G. et al. The role of the basal ganglia in category learning. Psychol. Learn. Motiv. 47, 1–36 (2006).
Schultz, W. Getting formal with dopamine and reward. Neuron 36, 241–263 (2002).
Skinner, B. F. The operational analysis of psychological terms. Psychol. Rev. 52, 270–277 (1945). This pioneering work proposed the theory of operant conditioning, which is considered to be a form of learning during neurofeedback training.
Mulholland, T. B. in Biofeedback and Behavior (eds Beatty, J. & Legewie, H.) 95–106 (Plenum Press, 1977).
Christoffersen, G. R. et al. Electrophysiological CNS-processes related to associative learning in humans. Behav. Brain Res. 296, 211–232 (2016).
Lang, P. J. et al. Learning to control heart rate: effects of varying incentive and criterion of success on task performance. Psychophysiology 13, 378–385 (1976).
Cano-de-la-Cuerda, R. et al. Theories and control models and motor learning: clinical applications in neuro-rehabilitation. Neurologia 30, 32–41 (2015).
Lacroix, J. M. in Consciousness and Self-Regulation (eds Davidson, R. J., Schwartz, G. E., & Shapiro, D.) 137–162 (Plenum Press, 1986).
Lacroix, J. M. et al. The acquisition of autonomic control through biofeedback: some tests of discrimination theory. Psychophysiology 18, 559–572 (1981).
Black, A. et al. in Biofeedback: Theory and Research (eds Schwartz, G. E. & Beatty, J.) 89–127 (Academic Press, 1977).
Pessiglione, M. et al. Subliminal instrumental conditioning demonstrated in the human brain. Neuron 59, 561–567 (2008).
Bijleveld, E. et al. Unconscious reward cues increase invested effort, but do not change speed-accuracy tradeoffs. Cognition 115, 330–335 (2010).
Shea, N. et al. Dual-process theories and consciousness: the case for 'Type Zero' cognition. Neurosci. Conscious. http://dx.doi.org/10.1093/nc/niw005 (2016). This paper discussed different approaches of conscious and unconscious information processing.
Birbaumer, N. et al. Learned regulation of brain metabolism. Trends Cogn. Sci. 17, 295–302 (2013).
VanLehn, K. Cognitive skill acquisition. Annu. Rev. Psychol. 47, 513–539 (1996).
Yin, H. H. et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat. Neurosci. 12, 333–341 (2009).
Young, B. M. et al. Dose-response relationships using brain–computer interface technology impact stroke rehabilitation. Front. Hum. Neurosci. 9, 361 (2015). This paper investigated dose–response in BCI therapy, showing the effect of dose and intensity on behavioural change.
Murray, S. O. et al. Attention increases neural selectivity in the human lateral occipital complex. Nat. Neurosci. 7, 70–74 (2004).
Alvarez, J. A. et al. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol. Rev. 16, 17–42 (2006).
Ball, G. et al. Executive functions and prefrontal cortex: a matter of persistence? Front. Syst. Neurosci. 5, 3 (2011).
Seeley, W. W. et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 27, 2349–2356 (2007).
Llinas, R. R. et al. Bursting of thalamic neurons and states of vigilance. J. Neurophysiol. 95, 3297–3308 (2006).
Acknowledgements
The authors thank S. Ruiz and D. Schnyer for their valuable help in reviewing the manuscript before submission. R.S. is supported by the Comisión Nacional de Investigación Científica neurofeedback Tecnológica de Chile (Conicyt) through Fondo Nacional de Desarrollo Científico neurofeedback Tecnológico, Fondecyt (project number 11121153); Vicerrectoría de Investigación de la Pontificia Universidad Católica de Chile (Proyecto de Investigación Interdisciplina number15/2013); Institute for Medical and Biological Engineering and Department of Psychiatry, Pontificia Universidad Católica de Chile; the Medical Faculty of the University of Tübingen through the Fortüne funding (project number 2114-1-0); the Singapore–Bäden–Württemberg Life Sciences grant; the ERA-Net (European Research Area)–New INDIGO project funded by the Bundesministerium für Bildung und Forschung (BMBF) (project number 01DQ13004). N.W. is supported by the BRAINTRAIN European research network (Collaborative Project, grant agreement number 602186); the European Research Council (ERC) (grant agreement number 616905); and a Centre Grant by the Wellcome Trust (0915/Z/10/Z). F.S. is supported by the Swiss National Science Foundation (BSSG10_155915). L.S. is supported by the US National Institutes of Health (NIH) (K23DA032612); the Norman E. Zinberg Fellowship in Addiction Psychiatry at Harvard Medical School; the Charles A. King Trust; the McGovern Institute Neurotechnology Program; and private funds to the Massachusetts General Hospital Department of Psychiatry. N.B. is supported by Deutsche Forschungsgemeinschaft (DFG); EU Project LUMINOUS; BMBF, MOTOR-BIC und EMOIO; Eva und Horst Köhler Stiftung; Baden–Württemberg-Stiftung; and EU Project BRAINTRAIN. J.S. is supported by NIH 5K12HD073945-02.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
N.W. is supported by the Wellcome Trust Centre for Neuroimaging and has an institutional research agreement with and receives support from Siemens Healthcare. The remaining authors have no competing interests.
Related links
FURTHER INFORMATION
PowerPoint slides
Glossary
- Biofeedback
-
It provides an explicit indicator of some physiological process, such as heartbeat or brain activation, so that an individual can attempt to regulate that activation or guide behaviour.
- Brain–machine interfaces
-
(BMIs). Brain–machine interfaces, sometimes called direct neural or brain–computer interfaces, are direct communication pathways between the brain and external devices.
- Synchronization
-
Simultaneous oscillations of membrane potentials in a network of neurons that are connected with electrical synapses.
- Biomarkers
-
Biological features (physical, physiological or behavioural) that act as robust predictors of one or more experimental or clinical outcomes.
- Coherence
-
A measure of how stable the frequency and/or phase relationship is between two neural sites; it reflects the amount of information that is shared between two sensors or channels.
- Multivariate pattern analyses
-
(MVPAs). These are statistical and mathematical approaches for finding regularities and patterns in the data.
- Adaptive neurofeedback
-
Previously, and perhaps imprecisely, referred to as 'closed-loop', adaptive neurofeedback changes an experimental task in real time on the basis of neural activity.
- Fractional anisotropy
-
A property of white matter pathways of the brain that relates to the diffusion of water molecules along axonal pathways and is measured by diffusion tensor imaging; it is represented by a value ranging from 0, indicating no specific directionality, to 1, indicating one prominent directionality.
- Homeostatic plasticity
-
The capacity of neurons to regulate their own excitability relative to network activity; it is observed in neurofeedback as an opposite and paradoxical change in brain activity after the training.
- Operant conditioning
-
A process by which an organism learns a new association between two paired stimuli: a neutral stimulus and one that already evoked a reflexive response.
- Locus of control
-
A psychological construct that determines the subjective feeling of being in control.
- Sense of agency
-
The feeling that the individual causes the change.
- Slow cortical potentials
-
(SCPs). These are slow event-related direct-current shifts that can be detected on the electroencephalogram. Slow cortical potential shifts in the electrical negative direction reflect the depolarization of large cortical cell assemblies, reducing their excitation threshold.
- Fugl-Meyer scores
-
Performance-based impairment index for assessing motor functioning, balance, sensation and joint functioning in patients with post-stroke hemiplegia.
Rights and permissions
About this article
Cite this article
Sitaram, R., Ros, T., Stoeckel, L. et al. Closed-loop brain training: the science of neurofeedback. Nat Rev Neurosci 18, 86–100 (2017). https://doi.org/10.1038/nrn.2016.164
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrn.2016.164
This article is cited by
-
Neurofeedback training of executive function in autism spectrum disorder: distinct effects on brain activity levels and compensatory connectivity changes
Journal of Neurodevelopmental Disorders (2024)
-
Multivariate patterns of brain functional connectome associated with COVID-19-related negative affect symptoms
Translational Psychiatry (2024)
-
A review about synergistic effects of transcranial direct current stimulation (tDCS) in combination with motor imagery (MI)-based brain computer interface (BCI) on post-stroke rehabilitation
Research on Biomedical Engineering (2024)
-
Decoding the Debate: A Comparative Study of Brain-Computer Interface and Neurofeedback
Applied Psychophysiology and Biofeedback (2024)
-
Exploring electroencephalographic infraslow neurofeedback treatment for chronic low back pain: a double-blinded safety and feasibility randomized placebo-controlled trial
Scientific Reports (2023)