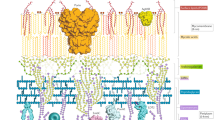
Key Points
-
Although tuberculosis (TB) kills about two million people each year, no new drug for its treatment has been introduced in the past 30 years. Knowledge of the mechanisms by which mycobacteria are able to circumvent host defence responses is essential to the quest for the development of new drug candidates for the treatment of TB.
-
Pathogenic mycobacteria use several mechanisms that allow them to survive in the hostile environment of the host cell. These include preventing phagosomal maturation, modifying the host apoptotic response and blocking host-signalling pathways that would otherwise trigger an antibacterial response.
-
Mycobacteria block phagosomal maturation through lipid molecules, such as Man-LAM, that inhibit signalling through Ca2+/calmodulin and phosphatidylinositol-3-kinase pathways. However, the details of the mechanisms that mycobacteria use to suppress these pathways are not well understood.
-
The suppression of host-cell apoptosis by pathogenic bacteria early in infection facilitates their proliferation and survival. This inhibition of apoptosis takes place through several mechanisms, including preventing increases in the Ca2+ concentration that trigger cytochrome c release from mitochondria, blocking the function of the pro-apoptotic protein Bad and inhibiting TNF-α production.
-
Pathogenic mycobacteria suppress the MAPK and JAK/STAT signalling pathways that are crucial for many innate and adaptive immune responses to infection. They suppress the sustained activation of the MAPKs p38 and ERK1/2 and are able to inhibit JAK/STAT signalling by inhibiting phosphorylation of JAKs and translocation of STAT to the nucleus, and by inducing the expression of the SOCS protein, which binds and inactivates JAKs.
-
The prevention of dendritic cell maturation and the activation of T cells by pathogenic bacteria is another method by which they minimize the host immune response. Binding of Man-LAM to DC-SIGN receptors on dendritic cells prevents their maturation and leads to the secretion of IL-10, an immunosuppressive chemokine.
-
The release of the complete genome sequence of Mycobacterium tuberculosis has identified eukaryotic-like protein kinases and phosphatases. In several bacterial species, protein kinases and phosphatases are important virulence mediators that disrupt host-signalling networks. Some of the mycobacterial kinases and phosphatases might modulate the eukaryotic signalling machinery for intracellular survival.
-
Mycobacterial kinases and phosphatases show potential as targets for TB therapeutics; kinase inhibitors suppress mycobacterial infection in vitro; inhibitors of both kinases and phosphatases have been developed for the treatment of other diseases. As our understanding of the mechanisms involved in mycobacterial survival increases, the range of other potential targets for anti-TB drugs will expand.
Abstract
Pathogenesis by mycobacteria requires the exploitation of host-cell signalling pathways to enhance the intracellular survival and persistence of the pathogen. The disruption of these pathways by mycobacteria causes impaired maturation of phagosomes into phagolysosomes, modulates host-cell apoptotic pathways and suppresses the host immune response. This review highlights the strategies employed by mycobacteria to subvert host-cell signalling and identifies key molecules involved in these processes that might serve as potential targets for new antimycobacterial therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Butler, D. New fronts in an old war. Nature 406, 670–672 (2000).
Rosenberger, C. M. & Finlay, B. B. Phagocyte sabotage: disruption of macrophage signalling by bacterial pathogens. Nature Rev. Mol. Cell Biol. 4, 385–396 (2003).
Beatty, W. L. et al. Trafficking and release of mycobacterial lipids from infected macrophages. Traffic 1, 235–247 (2000).
Brennan, P. J. & Nikaido, H. The envelope of mycobacteria. Annu. Rev. Biochem. 64, 29–63 (1995).
Ernst, J. D. Macrophage receptors for Mycobacterium tuberculosis. Infect. Immun. 66, 1277–1281 (1998).
Schlesinger, L. S., Bellinger-Kawahara, C. G., Payne, N. R. & Horwitz, M. A. Phagocytosis of Mycobacterium tuberculosis is mediated by human monocyte complement receptors and complement component C3. J. Immunol. 144, 2771–2780 (1990).
Caron, E. & Hall, A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721 (1998).
Hellwig, S. M. et al. Targeting to Fcγ receptors, but not CR3 (CD11b/CD18), increases clearance of Bordetella pertussis. J. Infect. Dis. 183, 871–879 (2001).
Melo, M. D., Catchpole, I. R., Haggar, G. & Stokes, R. W. Utilization of CD11b knockout mice to characterize the role of complement receptor 3 (CR3, CD11b/CD18) in the growth of Mycobacterium tuberculosis in macrophages. Cell Immunol. 205, 13–23 (2000).
Vieira, O. V., Botelho, R. J. & Grinstein, S. Phagosome maturation: aging gracefully. Biochem. J. 366, 689–704 (2002).
Clemens, D. L. & Horwitz, M. A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagosomal maturation is inhibited. J. Exp. Med. 181, 257–270 (1995).
Sturgill-Koszycki, S. et al. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science 263, 678–681 (1994). This paper showed for the first time that mycobacterial phagosomes do not recruit the ATP-dependent H+ pumps that bring about acidification to their membranes.
Armstrong, J. A. & Hart, P. D. Phagosome–lysosome interactions in cultured macrophages infected with virulent tubercle bacilli. Reversal of the usual nonfusion pattern and observations on bacterial survival. J. Exp. Med. 142, 1–16 (1975). This is the first report of the failure of phagosomes in cells infected with pathogenic mycobacteria to fuse with lysosomes.
Russell, D. G. Mycobacterium tuberculosis: here today, and here tomorrow. Nature Rev. Mol. Cell Biol. 2, 569–577 (2001).
Ferrari, G., Langen, H., Naito, M. & Pieters, J. A coat protein on phagosomes involved in the intracellular survival of mycobacteria. Cell 97, 435–447 (1999).
Deretic, V. & Fratti, R. A. Mycobacterium tuberculosis phagosome. Mol. Microbiol. 31, 1603–1609 (1999).
Schuller, S., Neefjes, J., Ottenhoff, T., Thole, J. & Young, D. Coronin is involved in uptake of Mycobacterium bovis BCG in human macrophages but not in phagosome maintenance. Cell. Microbiol. 3, 785–793 (2001).
Via, L. E. et al. Arrest of mycobacterial phagosome maturation is caused by a block in vesicle fusion between stages controlled by Rab5 and Rab7. J. Biol. Chem. 272, 13326–13331 (1997). Together with reference 15, this paper describes the stable association of TACO and Rab5 with phagosomes containing mycobacteria.
Fratti, R. A., Backer, J. M., Gruenberg, J., Corvera, S. & Deretic, V. Role of phosphatidylinositol 3-kinase and Rab5 effectors in phagosomal biogenesis and mycobacterial phagosome maturation arrest. J. Cell Biol. 154, 631–644 (2001).
Christoforidis, S. et al. Phosphatidylinositol-3-OH kinases are Rab5 effectors. Nature Cell Biol. 1, 249–252 (1999).
Jaconi, M. E. et al. Cytosolic free calcium elevation mediates the phagosome–lysosome fusion during phagocytosis in human neutrophils. J. Cell Biol. 110, 1555–1564 (1990).
Wurmser, A. E., Gary, J. D. & Emr, S. D. Phosphoinositide-3-kinases and their FYVE domain-containing effectors as regulators of vacuolar/lysosomal membrane trafficking pathways. J. Biol. Chem. 274, 9129–9132 (1999).
Carafoli, E. Calcium signaling: a tale for all seasons. Proc. Natl Acad. Sci. USA 99, 1115–1122 (2002).
Malik, Z. A., Iyer, S. S. & Kusner, D. J. Mycobacterium tuberculosis phagosomes exhibit altered calmodulin-dependent signal transduction: contribution to inhibition of phagosome-lysosome fusion and intracellular survival in human macrophages. J. Immunol. 166, 3392–3401 (2001). This paper gives a clear demonstration of the role of Ca2+- and calmodulin-mediated signalling pathways in blocking the maturation of phagosomes bearing pathogenic mycobacteria.
Rich, R. C. & Schulman, H. Substrate-directed function of calmodulin in autophosphorylation of Ca2+/calmodulin-dependent protein kinase II. J. Biol. Chem. 273, 28424–28429 (1998).
Peters, C. & Mayer, A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 396, 575–580 (1998).
Malik, Z. A., Denning, G. M. & Kusner, D. J. Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome–lysosome fusion and increased survival within human macrophages. J. Exp. Med. 191, 287–302 (2000).
Rojas, M., Garcia, L. F., Nigou, J., Puzo, G. & Olivier, M. Mannosylated lipoarabinomannan antagonizes Mycobacterium tuberculosis-induced macrophage apoptosis by altering Ca2+-dependent cell signaling. J. Infect. Dis. 182, 240–251 (2000).
Malik, Z. A. et al. Cutting edge: Mycobacterium tuberculosis blocks Ca2+ signaling and phagosome maturation in human macrophages via specific inhibition of sphingosine kinase. J. Immunol. 170, 2811–2815 (2003).
Spiegel, S. & Milstien, S. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 277, 25851–25854 (2002).
Patki, V. et al. Identification of an early endosomal protein regulated by phosphatidylinositol 3-kinase. Proc. Natl Acad. Sci. USA 94, 7326–7330 (1997).
Vergne, I., Chua, J. & Deretic, V. Tuberculosis toxin blocking phagosome maturation inhibits a novel Ca2+/calmodulin-PI3K hVPS34 cascade. J. Exp. Med. 198, 653–659 (2003).
Fratti, R. A., Chua, J., Vergne, I. & Deretic, V. Mycobacterium tuberculosis glycosylated phosphatidylinositol causes phagosome maturation arrest. Proc. Natl Acad. Sci. USA 100, 5437–5442 (2003). References 32 and 33 describe the host cell signalling molecules that are involved in mycobacterial phagosome maturation and also define mycobacterial glycolipids, such as Man-LAM, as critical mycobacterial virulence factors that block phagolysosomal trafficking.
Anes, E. et al. Selected lipids activate phagosome actin assembly and maturation resulting in killing of pathogenic mycobacteria. Nature Cell Biol. 5, 793–802 (2003).
Taunton, J. Actin filament nucleation by endosomes, lysosomes and secretory vesicles. Curr. Opin. Cell Biol. 13, 85–91 (2001).
Spira, A. et al. Apoptosis genes in human alveolar macrophages infected with virulent or attenuated M. tuberculosis. Am. J. Respir. Cell Mol. Biol. 29 545–551 (2003).
Keane, J., Remold, H. G. & Kornfeld, H. Virulent Mycobacterium tuberculosis strains evade apoptosis of infected alveolar macrophages. J. Immunol. 164, 2016–2020 (2000).
Nigou, J. et al. Mycobacterial lipoarabinomannans: modulators of dendritic cell function and the apoptotic response. Microbes Infect. 4, 945–953 (2002).
Szalai, G., Krishnamurthy, R. & Hajnoczky, G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 18, 6349–6361 (1999).
Maiti, D., Bhattacharyya, A. & Basu, J. Lipoarabinomannan from Mycobacterium tuberculosis promotes macrophage survival by phosphorylating Bad through a phosphatidylinositol 3-kinase/Akt pathway. J. Biol. Chem. 276, 329–333 (2001).
Esdar, C., Milasta, S., Maelicke, A. & Herget, T. Differentiation-associated apoptosis of neural stem cells is effected by Bcl-2 overexpression: impact on cell lineage determination. Eur. J. Cell Biol. 80, 539–553 (2001).
Brazil, D. P., Park, J. & Hemmings, B. A. PKB binding proteins. Getting in on the Akt. Cell 111, 293–303 (2002).
Balcewicz-Sablinska, M. K., Keane, J., Kornfeld, H. & Remold, H. G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNF-α. J. Immunol. 161, 2636–2641 (1998).
Bhattacharyya, A. et al. Execution of macrophage apoptosis by Mycobacterium avium through apoptosis signal-regulating kinase 1/p38 mitogen-activated protein kinase signaling and caspase 8 activation. J. Biol. Chem. 278, 26517–26525 (2003).
Kobayashi, S. D. et al. Bacterial pathogens modulate an apoptosis differentiation program in human neutrophils. Proc. Natl Acad. Sci. USA 100, 10948–10953 (2003).
Johnson, G. L. & Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298, 1911–1912 (2002).
Roach, S. K. & Schorey, J. S. Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria. Infect. Immun. 70, 3040–3052 (2002).
Blumenthal, A., Ehlers, S., Ernst, M., Flad, H. D. & Reiling, N. Control of mycobacterial replication in human macrophages: roles of extracellular signal-regulated kinases 1 and 2 and p38 mitogen-activated protein kinase pathways. Infect. Immun. 70, 4961–4967 (2002).
Reiling, N., Blumenthal, A., Flad, H. D., Ernst, M. & Ehlers, S. Mycobacteria-induced TNF-α and IL-10 formation by human macrophages is differentially regulated at the level of mitogen-activated protein kinase activity. J. Immunol. 167, 3339–3345 (2001).
Schorey, J. S. & Cooper, A. M. Macrophage signalling upon mycobacterial infection: the MAP kinases lead the way. Cell. Microbiol. 5, 133–142 (2003).
Orth, K. Function of the Yersinia effector YopJ. Curr. Opin. Microbiol. 5, 38–43 (2002).
Orth, K. et al. Inhibition of the mitogen-activated protein kinase kinase superfamily by a Yersinia effector. Science 285, 1920–1923 (1999).
Lin, S. L., Le, T. X. & Cowen, D. S. SptP, a Salmonella typhimurium type III-secreted protein, inhibits the mitogen-activated protein kinase pathway by inhibiting Raf activation. Cell. Microbiol. 5, 267–275 (2003).
Tse, H. M., Josephy, S. I., Chan, E. D., Fouts, D. & Cooper, A. M. Activation of the mitogen-activated protein kinase signaling pathway is instrumental in determining the ability of Mycobacterium avium to grow in murine macrophages. J. Immunol. 168, 825–833 (2002).
van den, B. B. et al. p38 mitogen-activated protein kinase inhibition increases cytokine release by macrophages in vitro and during infection in vivo. J. Immunol. 166, 582–587 (2001).
Godl, K. et al. An efficient proteomics method to identify the cellular targets of protein kinase inhibitors. Proc. Natl Acad. Sci. USA 100, 15434–15439 (2003).
Riedel, D. D. & Kaufmann, S. H. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65, 4620–4623 (1997).
Perskvist, N., Zheng, L. & Stendahl, O. Activation of human neutrophils by Mycobacterium tuberculosis H37Ra involves phospholipase Cγ2, Shc adapter protein, and p38 mitogen-activated protein kinase. J. Immunol. 164, 959–965 (2000).
Mitsuyama, T., Takeshige, K. & Minakami, S. Tyrosine phosphorylation is involved in the respiratory burst of electropermeabilized human neutrophils at a step before diacylglycerol formation by phospholipase C. FEBS Lett. 322, 280–284 (1993).
Knutson, K. L., Hmama, Z., Herrera-Velit, P., Rochford, R. & Reiner, N. E. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. Role of the Src homology 2 containing tyrosine phosphatase 1. J. Biol. Chem. 273, 645–652 (1998).
Decker, T., Stockinger, S., Karaghiosoff, M., Muller, M. & Kovarik, P. IFNs and STATs in innate immunity to microorganisms. J. Clin. Invest 109, 1271–1277 (2002).
Shtrichman, R. & Samuel, C. E. The role of γ-interferon in antimicrobial immunity. Curr. Opin. Microbiol. 4, 251–259 (2001).
MacMicking, J. D., Taylor, G. A. & McKinney, J. D. Immune control of tuberculosis by IFN-γ-inducible LRG-47. Science 302, 654–659 (2003).
Hussain, S., Zwilling, B. S. & Lafuse, W. P. Mycobacterium avium infection of mouse macrophages inhibits IFN-γ Janus kinase–STAT signaling and gene induction by down-regulation of the IFN-γ receptor. J. Immunol. 163, 2041–2048 (1999).
Ting, L. M., Kim, A. C., Cattamanchi, A. & Ernst, J. D. Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J. Immunol. 163, 3898–3906 (1999).
Dupuis, S. et al. Impairment of mycobacterial but not viral immunity by a germline human STAT1 mutation. Science 293, 300–303 (2001).
Imai, K., Kurita-Ochiai, T. & Ochiai, K. Mycobacterium bovis bacillus Calmette–Guerin infection promotes SOCS induction and inhibits IFN-γ-stimulated JAK/STAT signaling in J774 macrophages. FEMS Immunol. Med. Microbiol. 39, 173–180 (2003).
Van Kooyk, Y. & Geijtenbeek, T. B. DC-SIGN: escape mechanism for pathogens. Nature Rev. Immunol. 3, 697–709 (2003).
Tsuji, S. et al. Maturation of human dendritic cells by cell wall skeleton of Mycobacterium bovis bacillus Calmette–Guerin: involvement of toll-like receptors. Infect. Immun. 68, 6883–6890 (2000).
Underhill, D. M., Ozinsky, A., Smith, K. D. & Aderem, A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc. Natl Acad. Sci. USA 96, 14459–14463 (1999).
Lopez, M. et al. The 19-kDa Mycobacterium tuberculosis protein induces macrophage apoptosis through Toll-like receptor-2. J. Immunol. 170, 2409–2416 (2003).
Aliprantis, A. O. et al. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science 285, 736–739 (1999).
Geijtenbeek, T. B. et al. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197, 7–17 (2003).
Tailleux, L. et al. DC-SIGN is the major Mycobacterium tuberculosis receptor on human dendritic cells. J. Exp. Med. 197, 121–127 (2003). Together with reference 73, these authors describe how mycobacteria enter dendritic cells and that certain receptor pathways allow the pathogens to escape bactericidal killing by modulating TLR signalling pathways.
Nigou, J., Zelle-Rieser, C., Gilleron, M., Thurnher, M. & Puzo, G. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166, 7477–7485 (2001).
Redpath, S., Ghazal, P. & Gascoigne, N. R. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 9, 86–92 (2001).
Engering, A., Geijtenbeek, T. B. & van Kooyk, Y. Immune escape through C-type lectins on dendritic cells. Trends Immunol. 23, 480–485 (2002).
Hamilton, V. T., Stone, D. M., Pritchard, S. M. & Cantor, G. H. Bovine leukemia virus gp30 transmembrane (TM) protein is not tyrosine phosphorylated: examining potential interactions with host tyrosine-mediated signaling. Virus Res. 90, 155–169 (2002).
Pancholi, P., Mirza, A., Bhardwaj, N. & Steinman, R. M. Sequestration from immune CD4+ T cells of mycobacteria growing in human macrophages. Science 260, 984–986 (1993).
Wojciechowski, W., DeSanctis, J., Skamene, E. & Radzioch, D. Attenuation of MHC class II expression in macrophages infected with Mycobacterium bovis bacillus Calmette–Guerin involves class II transactivator and depends on the Nramp1 gene. J. Immunol. 163, 2688–2696 (1999).
Mariotti, S. et al. Mycobacterium tuberculosis subverts the differentiation of human monocytes into dendritic cells. Eur. J. Immunol. 32, 3050–3058 (2002).
Stenger, S., Niazi, K. R. & Modlin, R. L. Down-regulation of CD1 on antigen-presenting cells by infection with Mycobacterium tuberculosis. J. Immunol. 161, 3582–3588 (1998).
Noss, E. H. et al. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167, 910–918 (2001).
Pai, R. K., Convery, M., Hamilton, T. A., Boom, W. H. & Harding, C. V. Inhibition of IFN-γ-induced class II transactivator expression by a 19-kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171, 175–184 (2003).
Hakansson, S., Galyov, E. E., Rosqvist, R. & Wolf-Watz, H. The Yersinia YpkA Ser/Thr kinase is translocated and subsequently targeted to the inner surface of the HeLa cell plasma membrane. Mol. Microbiol. 20, 593–603 (1996).
Galyov, E. E., Hakansson, S., Forsberg, A. & Wolf-Watz, H. A secreted protein kinase of Yersinia pseudotuberculosis is an indispensable virulence determinant. Nature 361, 730–732 (1993).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 (1998). A landmark paper in mycobacteriology, as it led to the identification of several potential mycobacterial drug targets, including several serine/threonine protein kinases and tyrosine phosphatases.
Chaba, R., Raje, M. & Chakraborti, P. K. Evidence that a eukaryotic-type serine/threonine protein kinase from Mycobacterium tuberculosis regulates morphological changes associated with cell division. Eur. J. Biochem. 269, 1078–1085 (2002).
Av-Gay, Y., Jamil, S. & Drews, S. J. Expression and characterization of the Mycobacterium tuberculosis serine/threonine protein kinase PknB. Infect. Immun. 67, 5676–5682 (1999).
Peirs, P., de Wit, L., Braibant, M., Huygen, K. & Content, J. A serine/threonine protein kinase from Mycobacterium tuberculosis. Eur. J. Biochem. 244, 604–612 (1997).
Koul, A. et al. Serine/threonine protein kinases PknF and PknG of Mycobacterium tuberculosis: characterization and localization. Microbiology 147, 2307–2314 (2001).
Eiglmeier, K. et al. The decaying genome of Mycobacterium leprae. Lepr. Rev. 72, 387–398 (2001).
Sassetti, C. M., Boyd, D. H. & Rubin, E. J. Genes required for mycobacterial growth defined by high density mutagenesis. Mol. Microbiol. 48, 77–84 (2003). These authors characterized the genes essential for mycobacterial growth using a technique involving random mutagenesis of the mycobacterial genome with transposons. They identified several STPKs as essential for mycobacterial growth in vitro.
Av-Gay, Y., Drews, S. J. & Cowley, S. Tuberculosis drug targets. PCT WO-03/074728–A2 (2003).
Kennelly, P. J. & Potts, M. Fancy meeting you here! A fresh look at 'prokaryotic' protein phosphorylation. J. Bacteriol. 178, 4759–4764 (1996).
Persson, C., Carballeira, N., Wolf-Watz, H. & Fallman, M. The PTPase YopH inhibits uptake of Yersinia, tyrosine phosphorylation of p130cas and FAK, and the associated accumulation of these proteins in peripheral focal adhesions. EMBO J. 16, 2307–2318 (1997).
Black, D. S. & Bliska, J. B. Identification of p130cas as a substrate of Yersinia YopH (Yop51), a bacterial protein tyrosine phosphatase that translocates into mammalian cells and targets focal adhesions. EMBO J. 16, 2730–2744 (1997).
Fu, Y. & Galan, J. E. A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401, 293–297 (1999).
Koul, A. et al. Cloning and characterization of secretory tyrosine phosphatases of Mycobacterium tuberculosis. J. Bacteriol. 182, 5425–5432 (2000).
Singh, R. et al. Disruption of mptpB impairs the ability of Mycobacterium tuberculosis to survive in guinea pigs. Mol. Microbiol. 50, 751–762 (2003). This paper describes, for the first time, the role of protein tyrosine phosphatases in the pathogenesis of M. tuberculosis and possible implications for interaction with IFN-γ signalling pathways in host cells.
Stewart, G., Robertson, B. D. & Young, D. B. Tuberculosis: A problem with persistence. Nature Rev. Microbiol. 1, 97–105 (2003)
Shawver, L. K., Slamon, D. & Ullrich, A. Smart drugs: tyrosine kinase inhibitors in cancer therapy. Cancer Cell 1, 117–123 (2002).
Prabhakaran, K., Harris, E. B. & Randhawa, B. Regulation by protein kinase of phagocytosis of Mycobacterium leprae by macrophages. J. Med. Microbiol. 49, 339–342 (2000).
Drews, S. J., Hung, F. & Av-Gay, Y. A protein kinase inhibitor as an antimycobacterial agent. FEMS Microbiol. Lett. 205, 369–374 (2001). This is an excellent demonstration of the use of kinase inhibitors as antimycobacterial agents.
Young, T. A., Delagoutte, B., Endrizzi, J. A., Falick, A. M. & Alber, T. Structure of Mycobacterium tuberculosis PknB supports a universal activation mechanism for Ser/Thr protein kinases. Nature Struct. Biol. (2003).
Muller, G. Medicinal chemistry of target family-directed masterkeys. Drug Discov. Today 8, 681–691 (2003).
Stock, A. M., Robinson, V. L. & Goudreau, P. N. Two-component signal transduction. Annu. Rev. Biochem. 69, 183–215 (2000).
Zahrt, T. C. & Deretic, V. Mycobacterium tuberculosis signal transduction system required for persistent infections. Proc. Natl Acad. Sci. USA 98, 12706–12711 (2001).
Parish, T., Smith, D. A., Roberts, G., Betts, J. & Stoker, N. G. The senX3-regX3 two-component regulatory system of Mycobacterium tuberculosis is required for virulence. Microbiology 149, 1423–1435 (2003).
Johnson, T. O., Ermolieff, J. & Jirousek, M. R. Protein tyrosine phosphatase 1B inhibitors for diabetes. Nature Rev. Drug Discov. 1, 696–709 (2002).
Chen, Y. T. & Seto, C. T. Divalent and trivalent α-ketocarboxylic acids as inhibitors of protein tyrosine phosphatases. J. Med. Chem. 45, 3946–3952 (2002).
Smith, C. V., Sharma, V. & Sacchettini, J. C. TB drug discovery: addressing issues of persistence and resistance. Tuberculosis 84, 45–55 (2004).
Khasnobis, S., Escuyer, V. E. & Chatterjee, D. Emerging therapeutic targets in tuberculosis: post-genomic era. Expert. Opin. Ther. Targets. 6, 21–40 (2002).
Barry, C. E. Preclinical candidates and targets for tuberculosis therapy. Curr. Opin. Investig. Drugs 2, 198–201 (2001).
Darwin, K. H., Ehrt, S., Gutierrez-Ramos, J. C., Weich, N. & Nathan, C. F. The proteasome of Mycobacterium tuberculosis is required for resistance to nitric oxide. Science 302, 1963–1966 (2003).
Schnappinger, D. et al. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198, 693–704 (2003).
Weinrauch, Y. & Zychlinsky, A. The induction of apoptosis by bacterial pathogens. Annu. Rev. Microbiol. 53, 155–187 (1999).
Yrlid, U. & Wick, M. J. Salmonella-induced apoptosis of infected macrophages results in presentation of a bacteria-encoded antigen after uptake by bystander dendritic cells. J. Exp. Med. 191, 613–624 (2000).
Schaible, U. E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nature Med. 9, 1039–1046 (2003).
Acknowledgements
We are grateful to K. Drilica (Public Health Research Institute, New York, USA) and to G. Bacher, H. Daub and G. Müller (Axxima AG) for critical reading of the manuscript, and to Y. Av-Gay (University of British Columbia, Vancouver, Canada) and J. Pieters (University of Basel, Switzerland) for helpful conversations. Our thanks go to I. Bhattacharya (MPI-Martinsried, Germany) for help with the references and to our colleagues at Axxima for useful insights into mycobacterial drug development.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez
Infectious Disease Information
LocusLink
SwissProt
FURTHER INFORMATION
Glossary
- MACROPHAGES
-
Cells that belong to the mononuclear phagocyte system and are responsible for phagocytosis of foreign material.
- PHAGOSOME
-
A vesicle that is formed by invagination of the plasma membrane during endocytosis and fuses with primary lysosomes to degrade engulfed material.
- LYSOSOME
-
Membrane-limited cellular organelles with a low internal pH that contain acid hydrolases for the degradation of polymers such as proteins, RNA, DNA, polysaccharides and lipids.
- INTERFERON
-
A cytokine that activates the innate immune response, thereby preventing replication of pathogens.
- PHOSPHATIDYLINOSITOL-3-KINASE
-
(PI3K). PI3Ks are a conserved family of lipid kinases that phosphorylate the 3′-OH group of the inositol ring of membrane-bound phosphatidylinositides.
- G-PROTEIN COUPLED RECEPTORS
-
(GPCRs). These cell surface receptors, which are characterized by seven transmembrane domains, are coupled to small G-proteins. Activation of GPCRs induces binding of GTP to the G-proteins, which leads to stimulation, or repression, of downstream signalling events.
- NEUTROPHILS
-
Polynuclear leucocytes belonging to the myeloid lineage that migrate to sites of infection or wounds and mediate the inflammatory response.
- CYTOKINES
-
Low-molecular-weight proteins that are important for immunity, inflammation and development, and which contribute to the pathophysiology of acute and chronic infections.
- INNATE IMMUNE RESPONSE
-
A cellular defence reaction to counteract invading pathogens such as bacteria and viruses. It uses interferon-dependent signalling and leads to the activation of genes that are responsible for bactericidal or antiviral responses.
- ISOGENIC
-
Having identical genotypes.
- MORPHOTYPE
-
A member of one form of a polymorphic species.
- CREB
-
cAMP response element (CRE)-binding protein. It stimulates the basal transcription of CRE-containing genes and mediates induction of transcription following phosphorylation by protein kinases.
- ADAPTIVE IMMUNE RESPONSE
-
This involves specificity and immunological memory. It is mediated by T and B cells through activation of cytotoxic CD8+ T cells for pathogen killing, or by interaction with CD4+ T cells for antibody production.
- GLYOXYLATE SHUNT
-
A biochemical pathway that is used by plants and microorganisms to metabolize acetate or long-chain fatty acids as a source of energy.
Rights and permissions
About this article
Cite this article
Koul, A., Herget, T., Klebl, B. et al. Interplay between mycobacteria and host signalling pathways. Nat Rev Microbiol 2, 189–202 (2004). https://doi.org/10.1038/nrmicro840
Issue Date:
DOI: https://doi.org/10.1038/nrmicro840
This article is cited by
-
Microbiota in a long survival discourse with the human host
Archives of Microbiology (2023)
-
Host-directed therapy against tuberculosis: Concept and recent developments
Journal of Biosciences (2023)
-
Bovine milk somatic cell transcriptomic response to Staphylococcus aureus is dependent on strain genotype
BMC Genomics (2021)
-
The mycobacterial cell envelope — a moving target
Nature Reviews Microbiology (2020)
-
Revealing eukaryotic histone-modifying mechanisms through bacterial infection
Seminars in Immunopathology (2020)