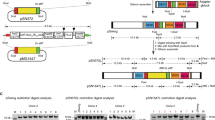
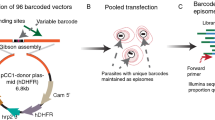
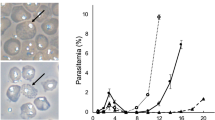
Key Points
-
The ability to genetically manipulate the malaria parasite to discover the functions of its genes has provided a powerful technique to gain detailed insight into Plasmodium spp. biology and to identify and characterize potential targets for antimalaria intervention.
-
The human malaria species Plasmodium falciparum and the rodent malaria species Plasmodium berghei have been the species predominantly used for gene manipulation studies. Although the genetic systems for these species vary in their efficiency and their approach and whether they are used in vitro or in vivo, their results can often be very complementary.
-
As a result of the haploid nature of malaria parasites, genes that have an important role in the blood stages of Plasmodium spp. cannot be genetically targeted for disruption using conventional knockout approaches, as this would lead to parasite death or severe growth defects. This has forced the development of conditional systems to rapidly regulate gene expression.
-
An array of conditional systems that are capable of regulating gene expression at the genome, transcript or protein level are now available for Plasmodium spp.. Each system has advantages and drawbacks, which should be considered carefully before choosing a system to regulate a gene of interest.
-
A variety of methods can be used to edit the Plasmodium genome. These include the use of piggyBac transposons that integrate randomly into the genome, or more precise editing tools such as the CRISPR–Cas9 system, zinc-finger nucleases and integrases.
-
Improvements to transfection efficiency and investment in the development of more robust genetic tools and phenotypic assays should lead to large-scale forward-genetic screens being possible in Plasmodium spp..
Abstract
Robust tools for analysing gene function in Plasmodium parasites, which are the causative agents of malaria, are being developed at an accelerating rate. Two decades after genetic technologies for use in Plasmodium spp. were first described, a range of genetic tools are now available. These include conditional systems that can regulate gene expression at the genome, transcriptional or protein level, as well as more sophisticated tools for gene editing that use piggyBac transposases, integrases, zinc-finger nucleases or the CRISPR–Cas9 system. In this Review, we discuss the molecular genetic systems that are currently available for use in Plasmodium falciparum and Plasmodium berghei, and evaluate the advantages and limitations of these tools. We examine the insights that have been gained into the function of genes that are important during the blood stages of the parasites, which may help to guide the development and improvement of drug therapies and vaccines.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
World Health Organisation. World malaria report (WHO, 2014).
Dondorp, A. M. et al. Artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 361, 455–467 (2009).
Olotu, A. et al. Four-year efficacy of RTS,S/AS01E and its interaction with malaria exposure. N. Engl. J. Med. 368, 1111–1120 (2013).
Duraisingh, M. T., Maier, A. G., Triglia, T. & Cowman, A. F. Erythrocyte-binding antigen 175 mediates invasion in Plasmodium falciparum utilizing sialic acid-dependent and -independent pathways. Proc. Natl Acad. Sci. USA 100, 4796–4801 (2003).
Baum, J., Maier, A. G., Good, R. T., Simpson, K. M. & Cowman, A. F. Invasion by P. falciparum merozoites suggests a hierarchy of molecular interactions. PLoS Pathog. 1, e37 (2005).
Stubbs, J. et al. Molecular mechanism for switching of P. falciparum invasion pathways into human erythrocytes. Science 309, 1384–1387 (2005).
Triglia, T., Duraisingh, M. T., Good, R. T. & Cowman, A. F. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol. Microbiol. 55, 162–174 (2005).
Lopaticki, S. et al. Reticulocyte and erythrocyte binding-like proteins function cooperatively in invasion of human erythrocytes by malaria parasites. Infect. Immun. 79, 1107–1117 (2011).
Reiling, L. et al. The Plasmodium falciparum erythrocyte invasion ligand PfRH4 as a target of functional and protective human antibodies against malaria. PLoS ONE 7, e45253 (2012).
Persson, K. E. et al. Erythrocyte-binding antigens of Plasmodium falciparum are targets of human inhibitory antibodies and function to evade naturally acquired immunity. J. Immunol. 191, 785–794 (2013).
Baum, J. et al. Reticulocyte-binding protein homologue 5 — an essential adhesin involved in invasion of human erythrocytes by Plasmodium falciparum. Int. J. Parasitol. 39, 371–380 (2009).
Crosnier, C. et al. Basigin is a receptor essential for erythrocyte invasion by Plasmodium falciparum. Nature 480, 534–537 (2011).
Douglas, A. D. et al. The blood-stage malaria antigen PfRH5 is susceptible to vaccine-inducible cross-strain neutralizing antibody. Nature Commun. 2, 601 (2011).
Triglia, T., Wang, P., Sims, P. F., Hyde, J. E. & Cowman, A. F. Allelic exchange at the endogenous genomic locus in Plasmodium falciparum proves the role of dihydropteroate synthase in sulfadoxine-resistant malaria. EMBO J. 17, 3807–3815 (1998).
Fidock, D. A. et al. Mutations in the P. falciparum digestive vacuole transmembrane protein PfCRT and evidence for their role in chloroquine resistance. Mol. Cell 6, 861–871 (2000).
Reed, M. B., Saliba, K. J., Caruana, S. R., Kirk, K. & Cowman, A. F. Pgh1 modulates sensitivity and resistance to multiple antimalarials in Plasmodium falciparum. Nature 403, 906–909 (2000). References 15 and 16 are landmark papers in the field of antimalarial drug resistance and describe research that makes extensive use of allelic replacement technology.
Sidhu, A. B., Verdier-Pinard, D. & Fidock, D. A. Chloroquine resistance in Plasmodium falciparum malaria parasites conferred by pfcrt mutations. Science 298, 210–213 (2002).
Sidhu, A. B., Valderramos, S. G. & Fidock, D. A. pfmdr1 mutations contribute to quinine resistance and enhance mefloquine and artemisinin sensitivity in Plasmodium falciparum. Mol. Microbiol. 57, 913–926 (2005).
Franke-Fayard, B. et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc. Natl Acad. Sci. USA 102, 11468–11473 (2005).
Ekland, E. H. & Fidock, D. A. Advances in understanding the genetic basis of antimalarial drug resistance. Curr. Opin. Microbiol. 10, 363–370 (2007).
Guttery, D. S., Holder, A. A. & Tewari, R. Sexual development in Plasmodium: lessons from functional analyses. PLoS Pathog. 8, e1002404 (2012).
Prudencio, M., Mota, M. M. & Mendes, A. M. A toolbox to study liver stage malaria. Trends Parasitol. 27, 565–574 (2011).
van Dijk, M. R. et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104, 153–164 (2001). This is an important early study that shows the power of gene-knockout technology in both rodent and human malaria parasites.
Tomas, A. M. et al. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 20, 3975–3983 (2001).
van Schaijk, B. C. et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 149, 216–222 (2006).
van Dijk, M. R. et al. Genetically attenuated, 36p-deficient malarial sporozoites induce protective immunity and apoptosis of infected liver cells. Proc. Natl Acad. Sci. USA 102, 12194–12199 (2005).
Mueller, A. K. et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc. Natl Acad. Sci. USA 102, 3022–3027 (2005).
Mueller, A. K., Labaied, M., Kappe, S. H. & Matuschewski, K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature 433, 164–167 (2005).
Tarun, A. S. et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J. Infect. Dis. 196, 608–616 (2007).
van Schaijk, B. C. et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS ONE 3, e3549 (2008).
Ishino, T., Chinzei, Y. & Yuda, M. Two proteins with 6-cys motifs are required for malarial parasites to commit to infection of the hepatocyte. Mol. Microbiol. 58, 1264–1275 (2005).
Van Buskirk, K. M. et al. Preerythrocytic, live-attenuated Plasmodium falciparum vaccine candidates by design. Proc. Natl Acad. Sci. USA 106, 13004–13009 (2009).
Mikolajczak, S. A. et al. A next-generation genetically attenuated Plasmodium falciparum parasite created by triple gene deletion. Mol. Ther. 22, 1707–1715 (2014).
van Schaijk, B. C. et al. A genetically attenuated malaria vaccine candidate based on P. falciparum b9/slarp gene-deficient sporozoites. eLife http://dx.doi.org/10.7554/eLife.03582 (2014).
Fidock, D. A. & Wellems, T. E. Transformation with human dihydrofolate reductase renders malaria parasites insensitive to WR99210 but does not affect the intrinsic activity of proguanil. Proc. Natl Acad. Sci. USA 94, 10931–10936 (1997). This crucial study provided the P. falciparum research field with its most powerful selectable marker approach.
Deitsch, K., Driskill, C. & Wellems, T. Transformation of malaria parasites by the spontaneous uptake and expression of DNA from human erythrocytes. Nucleic Acids Res. 29, 850–853 (2001).
van Dijk, M. R., Waters, A. P. & Janse, C. J. Stable transfection of malaria parasite blood stages. Science 268, 1358–1362 (1995). This seminal paper was the first to describe transfection in rodent malaria.
Goonewardene, R. et al. Transfection of the malaria parasite and expression of firefly luciferase. Proc. Natl Acad. Sci. USA 90, 5234–5236 (1993).
Wu, Y., Sifri, C. D., Lei, H.-H., Su, X.-Z. & Wellems, T. E. Transfection of Plasmodium falciparum within human red blood cells. Proc. Natl Acad. Sci. USA 92, 973–977 (1995). This seminal paper was the first to describe stable transfection of P. falciparum.
Wu, Y., Kirkman, L. A. & Wellems, T. E. Transformation of Plasmodium falciparum malaria parasites by homologous integration of plasmids that confer resistance to pyrimethamine. Proc. Natl Acad. Sci. USA 93, 1130–1134 (1996).
Crabb, B. S. & Cowman, A. F. Characterization of promoters and stable transfection by homologous and nonhomologous recombination in Plasmodium falciparum. Proc. Natl Acad. Sci. USA 93, 7289–7294 (1996).
van Dijk, M. R., Janse, C. J. & Waters, A. P. Expression of a Plasmodium gene introduced into subtelomeric regions of Plasmodium berghei chromosomes. Science 271, 662–665 (1996).
de Koning-Ward, T. F., Janse, C. J. & Waters, A. P. The development of genetic tools for dissecting the biology of malaria parasites. Annu. Rev. Microbiol. 54, 157–185 (2000).
Mota, M. M., Thathy, V., Nussenzweig, R. S. & Nussenzweig, V. Gene targeting in the rodent malaria parasite Plasmodium yoelii. Mol. Biochem. Parasitol. 113, 271–278 (2001).
Jongco, A. M., Ting, L. M., Thathy, V., Mota, M. M. & Kim, K. Improved transfection and new selectable markers for the rodent malaria parasite Plasmodium yoelii. Mol. Biochem. Parasitol. 146, 242–250 (2006).
Spence, P. J. et al. Transformation of the rodent malaria parasite Plasmodium chabaudi. Nature Protoc. 6, 553–561 (2011).
van der Wel, A. M. et al. Transfection of the primate malaria parasite Plasmodium knowlesi using entirely heterologous constructs. J. Exp. Med. 185, 1499–1503 (1997).
Kocken, C. H., van der Wel, A. & Thomas, A. W. Plasmodium cynomolgi: transfection of blood-stage parasites using heterologous DNA constructs. Exp. Parasitol. 93, 58–60 (1999).
Kocken, C. H. et al. Plasmodium knowlesi provides a rapid in vitro and in vivo transfection system that enables double-crossover gene knockout studies. Infect. Immun. 70, 655–660 (2002).
Moon, R. W. et al. Adaptation of the genetically tractable malaria pathogen Plasmodium knowlesi to continuous culture in human erythrocytes. Proc. Natl Acad. Sci. USA 110, 531–536 (2013).
Sanchez, C. P., Pfahler, J., Del Portillo, H. A. & Lanzer, M. Transient transfection of Plasmodium vivax blood-stage parasites. Methods Mol. Biol. 923, 151–159 (2013).
Moraes Barros, R. R. et al. Editing the Plasmodium vivax genome, using zinc-finger nucleases. J. Infect. Dis. 211, 125–129 (2015).
Crabb, B. S. et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 89, 287–296 (1997). This paper describes the first gene knockout in P. falciparum and the power of the technology to assess virulence mechanisms.
O'Donnell, R. A. et al. A genetic screen for improved plasmid segregation reveals a role for Rep20 in the interaction of Plasmodium falciparum chromosomes. EMBO J. 21, 1231–1239 (2002).
Crabb, B. S. et al. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol. Biol. 270, 263–276 (2004).
Braks, J. A., Franke-Fayard, B., Kroeze, H., Janse, C. J. & Waters, A. P. Development and application of a positive-negative selectable marker system for use in reverse genetics in Plasmodium. Nucleic Acids Res. 34, e39 (2006).
Maier, A., Braks, J., Waters, A. & Cowman, A. Negative selection using yeast cytosine deaminase/uracil phosphoribosyl transferase in Plasmodium falciparum for targeted gene deletion by double crossover recombination. Mol. Biochem. Parasitol. 150, 118–121 (2006).
Janse, C. J., Ramesar, J. & Waters, A. P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nature Protoc. 1, 346–356 (2006).
Janse, C. J. et al. A genotype and phenotype database of genetically modified malaria-parasites. Trends Parasitol. 27, 31–39 (2011).
de Koning-Ward, T. F. et al. The selectable marker human dihydrofolate reductase enables sequential genetic manipulation of the Plasmodium berghei genome. Mol. Biochem. Parasitol. 106, 199–212 (2000).
Lin, J. W. et al. A novel 'Gene Insertion/Marker Out' (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS ONE 6, e29289 (2011).
Janse, C. J., Franke-Fayard, B. & Waters, A. P. Selection by flow-sorting of genetically transformed, GFP-expressing blood stages of the rodent malaria parasite, Plasmodium berghei. Nature Protoc. 1, 614–623 (2006).
Meissner, M. et al. Tetracycline analogue-regulated transgene expression in Plasmodium falciparum blood-stages using Toxoplasma gondii transactivators. Proc. Natl Acad. Sci. USA 102, 2980–2985 (2005). This was the first paper to show regulatable gene expression in P. falciparum.
Hoess, R. H., Ziese, M. & Sternberg, N. P1 site-specific recombination: nucleotide sequence of the recombining sites. Proc. Natl Acad. Sci. USA 79, 3398–3402 (1982).
McLeod, M., Craft, S. & Broach, J. R. Identification of the crossover site during FLP-mediated recombination in the Saccharomyces cerevisiae plasmid 2 microns circle. Mol. Cell. Biol. 6, 3357–3367 (1986).
van Schaijk, B. C., Vos, M. W., Janse, C. J., Sauerwein, R. W. & Khan, S. M. Removal of heterologous sequences from Plasmodium falciparum mutants using FLPe-recombinase. PLoS ONE 5, e15121 (2010).
O'Neill, M. T., Phuong, T., Healer, J., Richard, D. & Cowman, A. F. Gene deletion from Plasmodium falciparum using FLP and Cre recombinases: implications for applied site-specific recombination. Int. J. Parasitol. 41, 117–123 (2011).
Jullien, N., Sampieri, F., Enjalbert, A. & Herman, J. P. Regulation of Cre recombinase by ligand-induced complementation of inactive fragments. Nucleic Acids Res. 31, e131 (2003).
Jullien, N. et al. Conditional transgenesis using dimerizable Cre (DiCre). PLoS ONE 2, e1355 (2007).
Andenmatten, N. et al. Conditional genome engineering in Toxoplasma gondii uncovers alternative invasion mechanisms. Nature Methods 10, 125–127 (2013).
Collins, C. R. et al. Robust inducible Cre recombinase activity in the human malaria parasite Plasmodium falciparum enables efficient gene deletion within a single asexual erythrocytic growth cycle. Mol. Microbiol. 88, 687–701 (2013).
Yap, A. et al. Conditional expression of apical membrane antigen 1 in Plasmodium falciparum shows it is required for erythrocyte invasion by merozoites. Cell. Microbiol. 16, 642–656 (2014).
Ecker, A., Lewis, R. E., Ekland, E. H., Jayabalasingham, B. & Fidock, D. A. Tricks in Plasmodium's molecular repertoire — escaping 3′UTR excision-based conditional silencing of the chloroquine resistance transporter gene. Int. J. Parasitol. 42, 969–974 (2012).
Combe, A. et al. Clonal conditional mutagenesis in malaria parasites. Cell Host Microbe 5, 386–396 (2009).
Lacroix, C. et al. FLP/FRT-mediated conditional mutagenesis in pre-erythrocytic stages of Plasmodium berghei. Nature Protoc. 6, 1412–1428 (2011). This is an important and innovative new approach for conditional mutagenesis in rodent malaria.
Falae, A. et al. Role of Plasmodium berghei cGMP-dependent protein kinase in late liver stage development. J. Biol. Chem. 285, 3282–3288 (2010).
Bargieri, D. Y. et al. Apical membrane antigen 1 mediates apicomplexan parasite attachment but is dispensable for host cell invasion. Nature Commun. 4, 2552 (2013).
Suarez, C., Volkmann, K., Gomes, A. R., Billker, O. & Blackman, M. J. The malarial serine protease SUB1 plays an essential role in parasite liver stage development. PLoS Pathog. 9, e1003811 (2013).
Tawk, L. et al. A key role for Plasmodium subtilisin-like SUB1 protease in egress of malaria parasites from host hepatocytes. J. Biol. Chem. 288, 33336–33346 (2013).
Baum, J. et al. Molecular genetics and comparative genomics reveal RNAi is not functional in malaria parasites. Nucleic Acids Res. 37, 3788–3798 (2009).
Gilson, P. et al. MSP119 miniproteins can serve as targets for invasion inhibitory antibodies in Plasmodium falciparum provided they contain the correct domains for cell surface trafficking. Mol. Microbiol. 68, 124–138 (2008).
Balaji, S., Babu, M. M., Iyer, L. M. & Aravind, L. Discovery of the principal specific transcription factors of Apicomplexa and their implication for the evolution of the AP2-integrase DNA binding domains. Nucleic Acids Res. 33, 3994–4006 (2005).
Pino, P. et al. A tetracycline-repressible transactivator system to study essential genes in malaria parasites. Cell Host Microbe 12, 824–834 (2012). This is a powerful demonstration of the use of tetracycline inducible gene expression in Plasmodium spp..
Elsworth, B. et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 511, 587–591 (2014). This is a powerful demonstration of the use of inducible ribozyme expression in P. falciparum.
Agop-Nersesian, C., Pfahler, J., Lanzer, J. & Meissner, M. Functional expression of ribozymes in Apicomplexa: towards exogenous control of gene expression by inducible RNA-cleavage. Int. J. Parasitol. 38, 673–681 (2008).
Prommana, P. et al. Inducible knockdown of Plasmodium gene expression using the glmS ribozyme. PLoS ONE 8, e73783 (2013).
Banaszynski, L. A., Chen, L. C., Maynard-Smith, L. A., Ooi, A. G. & Wandless, T. J. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 126, 995–1004 (2006).
Armstrong, C. & Goldberg, D. An FKBP destabilization domain modulates protein levels in Plasmodium falciparum. Nature Methods 4, 1007–1009 (2007). This article describes a FKBP DD approach to regulate protein expression in P. falciparum that proved to be a substantial advance in inducible gene expression.
Muralidharan, V., Oksman, A., Iwamoto, M., Wandless, T. J. & Goldberg, D. E. Asparagine repeat function in a Plasmodium falciparum protein assessed via a regulatable fluorescent affinity tag. Proc. Natl Acad. Sci. USA 108, 4411–4416 (2011).
de Azevedo, M. F. et al. Systematic analysis of FKBP inducible degradation domain tagging strategies for the human malaria parasite Plasmodium falciparum. PLoS ONE 7, e40981 (2012).
Russo, I., Oksman, A., Vaupel, B. & Goldberg, D. E. A calpain unique to alveolates is essential in Plasmodium falciparum and its knockdown reveals an involvement in pre-S-phase development. Proc. Natl Acad. Sci. USA 106, 1554–1559 (2009).
Dvorin, J. D. et al. A plant-like kinase in Plasmodium falciparum regulates parasite egress from erythrocytes. Science 328, 910–912 (2010). This is one of the best demonstrations of conditional mutagenesis in Plasmodium spp., highlighting the power of the FKBP approach.
Azevedo, M. F. et al. Inhibition of Plasmodium falciparum CDPK1 by conditional expression of its J-domain demonstrates a key role in schizont development. Biochem. J. 452, 433–441 (2013).
Farrell, A. et al. A DOC2 protein identified by mutational profiling is essential for apicomplexan parasite exocytosis. Science 335, 218–221 (2012).
Jain, S. et al. The prokaryotic ClpQ protease plays a key role in growth and development of mitochondria in Plasmodium falciparum. Cell. Microbiol. 15, 1660–1673 (2013).
Muralidharan, V., Oksman, A., Pal, P., Lindquist, S. & Goldberg, D. E. Plasmodium falciparum heat shock protein 110 stabilizes the asparagine repeat-rich parasite proteome during malarial fevers. Nature Commun. 3, 1310 (2012).
Beck, J. R., Muralidharan, V., Oksman, A. & Goldberg, D. E. HSP101/PTEX mediates export of diverse malaria effector proteins into the host erythrocyte. Nature 511, 592–595 (2014).
Pei, Y. et al. Plasmodium yoelii inhibitor of cysteine proteases is exported to exomembrane structures and interacts with yoelipain-2 during asexual blood-stage development. Cell. Microbiol. 15, 1508–1526 (2013).
Saridaki, T., Sanchez, C. P., Pfahler, J. & Lanzer, M. A conditional export system provides new insights into protein export in Plasmodium falciparum-infected erythrocytes. Cell. Microbiol. 10, 2483–2495 (2008).
Goldfless, S. J., Wagner, J. C. & Niles, J. C. Versatile control of Plasmodium falciparum gene expression with an inducible protein–RNA interaction. Nature Commun. 5, 5329 (2014).
Voorberg-van der Wel, A. et al. Transgenic fluorescent Plasmodium cynomolgi liver stages enable live imaging and purification of malaria hypnozoite-forms. PLoS ONE 8, e54888 (2013).
Boyle, M. J. et al. Isolation of viable Plasmodium falciparum merozoites to define erythrocyte invasion events and advance vaccine and drug development. Proc. Natl Acad. Sci. USA 107, 14378–14383 (2010).
Ghorbal, M. et al. Genome editing in the human malaria parasite Plasmodium falciparum using the CRISPR–Cas9 system. Nature Biotech. 32, 819–821 (2014).
Nkrumah, L. J. et al. Efficient site-specific integration in Plasmodium falciparum chromosomes mediated by mycobacteriophage Bxb1 integrase. Nature Methods 3, 615–621 (2006).
Straimer, J. et al. Site-specific genome editing in Plasmodium falciparum using engineered zinc-finger nucleases. Nature Methods 9, 993–998 (2012).
McNamara, C. W. et al. Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 (2013).
Straimer, J. et al. Drug resistance. K13-propeller mutations confer artemisinin resistance in Plasmodium falciparum clinical isolates. Science 347, 428–431 (2015).
Mali, P. et al. RNA-guided human genome engineering via Cas9. Science 339, 823–826 (2013).
Wagner, J. C., Platt, R. J., Goldfless, S. J., Zhang, F. & Niles, J. C. Efficient CRISPR-Cas9-mediated genome editing in Plasmodium falciparum. Nature Methods 11, 915–918 (2014).
Zhang, C. et al. Efficient editing of malaria parasite genome using the CRISPR/Cas9 system. mBio 5, e01414-14 (2014).
Pfander, C. et al. A scalable pipeline for highly effective genetic modification of a malaria parasite. Nature Methods 8, 1078–1082 (2011).
Schwach, F. et al. PlasmoGEM, a database supporting a community resource for large-scale experimental genetics in malaria parasites. Nucleic Acids Res. 43, D1176–D1182 (2015).
Zhang, Y., Buchholz, F., Muyrers, J. P. & Stewart, A. F. A new logic for DNA engineering using recombination in Escherichia coli. Nature Genet. 20, 123–128 (1998).
Wang, J. et al. An improved recombineering approach by adding RecA to λ Red recombination. Mol. Biotechnol. 32, 43–53 (2006).
Gomes, A. R. et al. A genome-scale vector resource enables high-throughput reverse genetic screening in a malaria parasite. Cell Host Microbe 17, 404–413 (2015).
Tewari, R. et al. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe 8, 377–387 (2010).
Balu, B., Shoue, D. A., Fraser, M. J. Jr & Adams, J. H. High-efficiency transformation of Plasmodium falciparum by the lepidopteran transposable element piggyBac. Proc. Natl Acad. Sci. USA 102, 16391–16396 (2005). This article describes a key first step toward saturation mutagenesis, and hence non-chemical forward genetics, in Plasmodium spp..
Balu, B., Singh, N., Maher, S. P. & Adams, J. H. A genetic screen for attenuated growth identifies genes crucial for intraerythrocytic development of Plasmodium falciparum. PLoS ONE 5, e13282 (2010).
Fonager, J. et al. Development of the piggyBac transposable system for Plasmodium berghei and its application for random mutagenesis in malaria parasites. BMC Genomics 12, 155 (2011).
Crabb, B. S., de Koning-Ward, T. F. & Gilson, P. R. Toward forward genetic screens in malaria-causing parasites using the piggyBac transposon. BMC Biol. 9, 21 (2011).
Noulin, F., Borlon, C., Van Den Abbeele, J., D'Alessandro, U. & Erhart, A. 1912–2012: a century of research on Plasmodium vivax in vitro culture. Trends Parasitol. 29, 286–294 (2013).
Hillenmeyer, M. E. et al. The chemical genomic portrait of yeast: uncovering a phenotype for all genes. Science 320, 362–365 (2008).
Giaever, G. et al. Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391 (2002).
Flannery, E. L., Chatterjee, A. K. & Winzeler, E. A. Antimalarial drug discovery — approaches and progress towards new medicines. Nature Rev. Microbiol. 11, 849–862 (2013).
Tilley, L., McFadden, G., Cowman, A. & Klonis, N. Illuminating Plasmodium falciparum-infected red blood cells. Trends Parasitol. 23, 268–277 (2007).
Waller, R. F., Reed, M. B., Cowman, A. F. & McFadden, G. I. Protein trafficking to the plastid of Plasmodium falciparum is via the secretory pathway. EMBO J. 19, 1794–1802 (2000).
McMillan, P. J. et al. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell. Microbiol. 15, 1401–1418 (2013).
Menard, R. et al. Looking under the skin: the first steps in malarial infection and immunity. Nature Rev. Microbiol. 11, 701–712 (2013).
Frevert, U. & Nacer, A. Immunobiology of Plasmodium in liver and brain. Parasite Immunol. 35, 267–282 (2013).
Ferrer, M. et al. Imaging of the spleen in malaria. Parasitol. Int. 63, 195–205 (2014).
de Moraes, L. V., Tadokoro, C. E., Gomez-Conde, I., Olivieri, D. N. & Penha-Goncalves, C. Intravital placenta imaging reveals microcirculatory dynamics impact on sequestration and phagocytosis of Plasmodium-infected erythrocytes. PLoS Pathog. 9, e1003154 (2013).
Kimura, K. et al. CD8+ T cells specific for a malaria cytoplasmic antigen form clusters around infected hepatocytes and are protective at the liver stage of infection. Infect. Immun. 81, 3825–3834 (2013).
Pai, S. et al. Real-time imaging reveals the dynamics of leukocyte behaviour during experimental cerebral malaria pathogenesis. PLoS Pathog. 10, e1004236 (2014).
Franke-Fayard, B., Waters, A. P. & Janse, C. J. Real-time in vivo imaging of transgenic bioluminescent blood stages of rodent malaria parasites in mice. Nature Protoc. 1, 476–485 (2006).
Braks, J. et al. Bioluminescence imaging of P. berghei schizont sequestration in rodents. Methods Mol. Biol. 923, 353–368 (2013).
Miller, J. L. et al. Quantitative bioluminescent imaging of pre-erythrocytic malaria parasite infection using luciferase-expressing Plasmodium yoelii. PLoS ONE 8, e60820 (2013).
Ploemen, I. H. et al. Visualisation and quantitative analysis of the rodent malaria liver stage by real time imaging. PLoS ONE 4, e7881 (2009).
Ramakrishnan, C. et al. Salivary gland-specific P. berghei reporter lines enable rapid evaluation of tissue-specific sporozoite loads in mosquitoes. PLoS ONE 7, e36376 (2012).
Annoura, T., Chevalley, S., Janse, C. J., Franke-Fayard, B. & Khan, S. M. Quantitative analysis of Plasmodium berghei liver stages by bioluminescence imaging. Methods Mol. Biol. 923, 429–443 (2013).
Amante, F. H. et al. Immune-mediated mechanisms of parasite tissue sequestration during experimental cerebral malaria. J. Immunol. 185, 3632–3642 (2010).
Ploemen, I. et al. Evaluation of immunity against malaria using luciferase-expressing Plasmodium berghei parasites. Malar. J. 10, 350 (2011).
Lin, J. W. et al. Screening inhibitors of P. berghei blood stages using bioluminescent reporter parasites. Methods Mol. Biol. 923, 507–522 (2013).
Zuzarte-Luis, V., Sales-Dias, J. & Mota, M. M. Simple, sensitive and quantitative bioluminescence assay for determination of malaria pre-patent period. Malar. J. 13, 15 (2014).
Noulin, F. et al. Cryopreserved reticulocytes derived from hematopoietic stem cells can be invaded by cryopreserved Plasmodium vivax isolates. PLoS ONE 7, e40798 (2012).
Dembele, L. et al. Persistence and activation of malaria hypnozoites in long-term primary hepatocyte cultures. Nature Med. 20, 307–312 (2014).
Waters, A. P., Thomas, A. W., van Dijk, M. R. & Janse, C. J. Transfection of malaria parasites. Methods 13, 134–147 (1997).
Menard, R. et al. Circumsporozoite protein is required for development of malaria sporozoites in mosquitoes. Nature 385, 336–340 (1997).
VanWye, J. D. & Haldar, K. Expression of green fluorescent protein in Plasmodium falciparum. Mol. Biochem. Parasitol. 87, 225–229 (1997).
de Koning-Ward, T. F., Thomas, A. W., Waters, A. P. & Janse, C. J. Stable expression of green fluorescent protein in blood and mosquito stages of Plasmodium berghei. Mol. Biochem. Parasitol. 97, 247–252 (1998).
de Koning-Ward, T. F., Sperança, M. A., Waters, A. P. & Janse, C. J. Analysis of stage specificity of promoters in Plasmodium berghei using luciferase as a reporter. Mol. Biochem. Parasitol. 100, 141–146 (1999).
Mamoun, C. B., Gluzman, I. Y., Goyard, S. K. Beverley, S. M. & Goldberg, D. E. A set of independent selectable markers for transfection of the human malaria parasite Plasmodium falciparum. Proc. Natl Acad. Sci. USA 96, 8716–8720 (1999).
Sultan, A. A., Thathy, V., de Koning-Ward, T. F. & Nussenzweig, V. Complementation of Plasmodium berghei TRAP knockout parasites using human dihydrofolate reductase gene as a selectable marker. Mol. Biochem. Parasitol. 113, 151–156 (2001).
de Koning-Ward T. F., Waters, A. P. & Crabb, B. S. Puromycin-N-acetyltransferase as a selectable marker for use in Plasmodium falciparum. Mol. Biochem. Parasitol. 117, 155–160 (2001).
Duraisingh, M. T., Triglia, T. & Cowman, A. F. Negative selection of Plasmodium falciparum reveals targeted gene deletion by double crossover recombination. Int. J. Parasitol. 32, 81–89 (2002).
Carvalho, T. G., Thiberge, S., Sakamoto, H. & Menard, R. Conditional mutagenesis using site-specific recombination in Plasmodium berghei. Proc. Natl Acad. Sci. USA 101, 14931–14936 (2004).
Ghanesan, S. M. et al. Yeast dihydroorate dehydrogenase as a new selectable marker for Plasmodium falciparum transfection. Mol. Biochem. Parasitol. 177, 29–34 (2011).
Acknowledgements
The authors apologize to those whose work could not be cited owing to length restrictions. They would like to acknowledge support from the National Health and Medical Research Council, Australia and from the Victorian State Government Operational Infrastructure Support Scheme, Australia.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Artemisinin
-
A rapid-acting antimalarial drug isolated from the plant Artemisia annua. Artemisinin-based combination therapies (ACTs) are recommended by the World Health Organisation as the first-line treatment for uncomplicated Plasmodium falciparum malaria.
- Immunogens
-
Antigens that are capable of inducing an immune response.
- Transfection
-
The process of introducing nucleic acids into cells.
- Schizonts
-
Mature forms of malaria parasites that are present in the liver and blood, which in blood contain ∼12–16 individual merozoites.
- Single-crossover recombination
-
A homologous recombination event that leads to the insertion of the entire vector backbone and duplication of targeting sequences.
- Negative selection
-
A process used to deplete parasites that express a negative-selectable marker from a population, in order to enrich parasites that contain the desired genomic integration event.
- Double-crossover recombination
-
A homologous recombination event that results in the replacement of a DNA sequence that is flanked by two targeting sequences.
- Parasitophorous vacuole
-
A vacuole in the host cell, in which Plasmodium parasites reside and develop.
- Cryptic polyadenylation sites
-
A processing site that is not normally used for the addition of a polyadenylic acid tail to mRNA.
- Merosomes
-
Structures containing hundreds of infectious merozoites that are surrounded by a membrane that is derived from the hepatocyte host cell.
- Ubiquitylation
-
A post-translational enzymatic modification involving the attachment of ubiquitin to a protein substrate.
- Dominant negative transgenes
-
A gene that when expressed in trans causes an adverse effect on the normal, wild-type gene product that is expressed in the same cell.
- Maurer's cleft
-
Single-membrane-bound structure that is present in the cytoplasm of erythrocytes that are infected with Plasmodium falciparum and that functions in transport of proteins from the parasite to the surface of the erythrocyte.
- Viral 2A ribosomal skipping peptide
-
A peptide derived from foot-and-mouth disease virus 2A that, when introduced as a linker between two proteins, allows autonomous intraribosomal self-processing of the resulting polyproteins that are expressed from a single polycistronic mRNA transcript.
- Ring-form parasites
-
The feeding stages of blood-stage parasites that show a ring-like morphology in Giemsa-stained blood smears.
- Lambda Red recombination system
-
This tool enables targeted genetic changes to DNA in Escherichia coli expressing the lambda Red recombinase. This system has been used in conjunction with Gateway technology to convert Plasmodium berghei genomic DNA clones that have been maintained in E. coli into gene-targeting vectors.
- Gateway cloning system
-
A molecular methodology that enables the transfer of DNA fragments between plasmids using attP recombination sequences and a mixture of commercial clonase enzymes.
- Two-photon microscopy
-
A fluorescence imaging technique that absorbs two photons of infrared light to provide deeper tissue penetration, which enables living tissues to be imaged to a greater depth than conventional confocal microscopy.
- Non-essential genes
-
Genes that can be deleted in the parasite without causing parasite death under certain conditions. These include genes that do not impart moderate or severe growth defects when mutated. However, genes that are termed 'non-essential' may in fact be essential to parasite growth when tested under different environmental conditions.
- Hypnozoites
-
Dormant forms of Plasmodium spp. parasites that are present in the liver.
Rights and permissions
About this article
Cite this article
de Koning-Ward, T., Gilson, P. & Crabb, B. Advances in molecular genetic systems in malaria. Nat Rev Microbiol 13, 373–387 (2015). https://doi.org/10.1038/nrmicro3450
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3450
This article is cited by
-
Plasmodium vinckei genomes provide insights into the pan-genome and evolution of rodent malaria parasites
BMC Biology (2021)
-
Defining multiplicity of vector uptake in transfected Plasmodium parasites
Scientific Reports (2020)
-
Diverse target gene modifications in Plasmodium falciparum using Bxb1 integrase and an intronic attB
Parasites & Vectors (2018)
-
New rapid one-step PCR diagnostic assay for Plasmodium falciparum infective mosquitoes
Scientific Reports (2018)
-
Challenges and recent progress in drug discovery for tropical diseases
Nature (2018)