Key Points
-
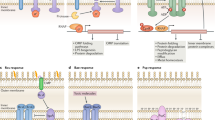
The unfolded protein response (UPR) is a cytoprotective response that is aimed at restoring cellular homeostasis following physiological stress exerted on the endoplasmic reticulum (ER). The UPR is also invoked in innate immune signalling in response to invading microorganisms.
-
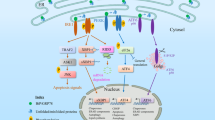
Several intracellular bacterial pathogens exploit the host secretory pathway to generate replication-permissive vacuoles that are derived from, or that closely interact with, the ER. Their resulting proliferation in the ER can elicit the UPR.
-
Intracellular bacteria can either induce or downregulate the UPR to promote their survival, possibly via the concerted action of various effector molecules.
-
ER stress and innate immune signalling pathways intersect in the control of inflammatory responses to invading microorganisms. In this context, the UPR can directly detect bacterial toxins or effectors, or can sense and respond to cellular alterations that are caused by these pathogenic molecules, which leads to the activation of pro-inflammatory responses that provide protection against bacterial infections.
-
Bacterial pathogens have evolved molecular mechanisms that mitigate or exploit the UPR to promote their intracellular survival and proliferation.
Abstract
The unfolded protein response (UPR) is a cytoprotective response that is aimed at restoring cellular homeostasis following physiological stress exerted on the endoplasmic reticulum (ER), which also invokes innate immune signalling in response to invading microorganisms. Although it has been known for some time that the UPR is modulated by various viruses, recent evidence indicates that it also has multiple roles during bacterial infections. In this Review, we describe how bacteria interact with the ER, including how bacteria induce the UPR, how subversion of the UPR promotes bacterial proliferation and how the UPR contributes to innate immune responses against invading bacteria.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Walter, P. & Ron, D. The unfolded protein response: from stress pathway to homeostatic regulation. Science 334, 1081–1086 (2011). This is an excellent current review of the UPR.
Cho, J. A. et al. The unfolded protein response element IRE1α senses bacterial proteins invading the ER to activate RIG-I and innate immune signaling. Cell Host Microbe 13, 558–569 (2013). This paper shows that generation of RNA fragments by IRE1 in response to toxin translocation to the ER activates innate immune responses.
He, B. Viruses, endoplasmic reticulum stress, and interferon responses. Cell Death Differ. 13, 393–403 (2006).
Schroder, M. & Kaufman, R. J. The mammalian unfolded protein response. Annu. Rev. Biochem. 74, 739–789 (2005).
Zhang, K. & Kaufman, R. J. From endoplasmic-reticulum stress to the inflammatory response. Nature 454, 455–462 (2008). This is an excellent investigation of the links between ER stress and inflammation.
Gardner, B. M. & Walter, P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333, 1891–1894 (2011).
Olzmann, J. A., Kopito, R. R. & Christianson, J. C. The mammalian endoplasmic reticulum-associated degradation system. Cold Spring Harb. Perspect. Biol. 5, a013185 (2013).
Bertolotti, A., Zhang, Y., Hendershot, L. M., Harding, H. P. & Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nature Cell Biol. 2, 326–332 (2000).
Kimata, Y., Oikawa, D., Shimizu, Y., Ishiwata-Kimata, Y. & Kohno, K. A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J. Cell Biol. 167, 445–456 (2004).
Pincus, D. et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 8, e1000415 (2010).
Gardner, B. M., Pincus, D., Gotthardt, K., Gallagher, C. M. & Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 5, a013169 (2013). This is an excellent current review of how the UPR is initiated.
Lee, K. et al. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev. 16, 452–466 (2002).
Lee, A. H., Iwakoshi, N. N. & Glimcher, L. H. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol. Cell. Biol. 23, 7448–7459 (2003).
Hollien, J. et al. Regulated IRE1-dependent decay of messenger RNAs in mammalian cells. J. Cell Biol. 186, 323–331 (2009).
Hollien, J. & Weissman, J. S. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science 313, 104–107 (2006).
Harding, H. P., Zhang, Y. & Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397, 271–274 (1999).
Harding, H. P. et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell 11, 619–633 (2003).
Urra, H., Dufey, E., Lisbona, F., Rojas-Rivera, D. & Hetz, C. When ER stress reaches a dead end. Biochim. Biophys. Acta 1833, 3507–3517 (2013).
Haze, K., Yoshida, H., Yanagi, H., Yura, T. & Mori, K. Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol. Biol. Cell 10, 3787–3799 (1999).
Adachi, Y. et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct. Function 33, 75–89 (2008).
Wu, J. et al. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev. Cell 13, 351–364 (2007).
Yamamoto, K. et al. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev. Cell 13, 365–376 (2007).
Bernales, S., McDonald, K. L. & Walter, P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biol. 4, e423 (2006).
Bommiasamy, H. et al. ATF6α induces XBP1-independent expansion of the endoplasmic reticulum. J. Cell Sci. 122, 1626–1636 (2009).
Schuck, S., Prinz, W. A., Thorn, K. S., Voss, C. & Walter, P. Membrane expansion alleviates endoplasmic reticulum stress independently of the unfolded protein response. J. Cell Biol. 187, 525–536 (2009).
Ogata, M. et al. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol. Cell. Biol. 26, 9220–9231 (2006).
Ding, W. X. et al. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem. 282, 4702–4710 (2007).
Urano, F. et al. Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287, 664–666 (2000). This paper shows that IRE1 links ER stress to JNK, which is an activator of AP-1.
Detilleux, P. G., Deyoe, B. L. & Cheville, N. F. Entry and intracellular localization of Brucella spp. in Vero cells: fluorescence and electron microscopy. Vet. Pathol. 27, 317–328 (1990).
Detilleux, P. G., Deyoe, B. L. & Cheville, N. F. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 58, 2320–2328 (1990).
Horwitz, M. A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J. Exp. Med. 158, 1319–1331 (1983).
Pizarro-Cerdá, J. et al. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 66, 5711–5724 (1998).
Pizarro-Cerdá, J., Moreno, E., Sanguedolce, V., Mege, J. L. & Gorvel, J. P. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 66, 2387–2392 (1998).
Tilney, L. G., Harb, O. S., Connelly, P. S., Robinson, C. G. & Roy, C. R. How the parasitic bacterium Legionella pneumophila modifies its phagosome and transforms it into rough ER: implications for conversion of plasma membrane to the ER membrane. J. Cell. Sci. 114, 4637–4650 (2001).
Celli, J. et al. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198, 545–556 (2003). This paper provides evidence that Brucella spp. exploits the ER via its VirB T4SS to evade killing by macrophages.
Robinson, C. G. & Roy, C. R. Attachment and fusion of endoplasmic reticulum with vacuoles containing Legionella pneumophila. Cell. Microbiol. 8, 793–805 (2006).
Asare, R. & Abu Kwaik, Y. Early trafficking and intracellular replication of Legionella longbeachaea within an ER-derived late endosome-like phagosome. Cell. Microbiol. 9, 1571–1587 (2007).
Derré, I., Swiss, R. & Agaisse, H. The lipid transfer protein CERT interacts with the Chlamydia inclusion protein IncD and participates to ER-Chlamydia inclusion membrane contact sites. PLoS Pathog. 7, e1002092 (2011).
Dumoux, M., Clare, D. K., Saibil, H. R. & Hayward, R. D. Chlamydiae assemble a pathogen synapse to hijack the host endoplasmic reticulum. Traffic 13, 1612–1627 (2012).
Mehlitz, A. et al. The chlamydial organism Simkania negevensis forms ER vacuole contact sites and inhibits ER-stress. Cell. Microbiol. 16, 1224–1243 (2014). This paper identifies a new bacterial pathogen that associates with the ER.
Kagan, J. C. & Roy, C. R. Legionella phagosomes intercept vesicular traffic from endoplasmic reticulum exit sites. Nature Cell Biol. 4, 945–954 (2002). This paper shows that Legionella spp. recruit early secretory vesicles after internalization by phagocytic cells.
Starr, T. et al. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 11, 33–45 (2012).
Celli, J., Salcedo, S. P. & Gorvel, J.-P. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc. Natl Acad. Sci. USA 102, 1673–1678 (2005). This paper provides evidence that B. abortus intercepts traffic from ERESs to promote its intracellular replication.
Porte, F., Liautard, J. P. & Köhler, S. Early acidification of phagosomes containing Brucella suis is essential for intracellular survival in murine macrophages. Infect. Immun. 67, 4041–4047 (1999).
Starr, T., Ng, T. W., Wehrly, T. D., Knodler, L. A. & Celli, J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 9, 678–694 (2008).
Robertson, D. K., Gu, L., Rowe, R. K. & Beatty, W. L. Inclusion biogenesis and reactivation of persistent Chlamydia trachomatis requires host cell sphingolipid biosynthesis. PLoS Pathog. 5, e1000664 (2009).
van Ooij, C. et al. Host cell-derived sphingolipids are required for the intracellular growth of Chlamydia trachomatis. Cell. Microbiol. 2, 627–637 (2000).
Machner, M. P. & Isberg, R. R. Targeting of host Rab GTPase function by the intravacuolar pathogen Legionella pneumophila. Dev. Cell 11, 47–56 (2006).
Murata, T. et al. The Legionella pneumophila effector protein DrrA is a Rab1 guanine nucleotide-exchange factor. Nature Cell Biol. 8, 971–977 (2006). References 48 and 49 show that Legionella spp. translocates a GEF into infected cells to manipulate ER-to-Golgi traffic.
Ingmundson, A., Delprato, A., Lambright, D. G. & Roy, C. R. Legionella pneumophila proteins that regulate Rab1 membrane cycling. Nature 450, 365–369 (2007).
Arasaki, K. & Roy, C. R. Legionella pneumophila promotes functional interactions between plasma membrane syntaxins and Sec22b. Traffic 11, 587–600 (2010).
Arasaki, K., Toomre, D. K. & Roy, C. R. The Legionella pneumophila effector DrrA is sufficient to stimulate SNARE-dependent membrane fusion. Cell Host Microbe 11, 46–57 (2012).
Nagai, H. A. Bacterial guanine nucleotide exchange factor activates ARF on Legionella phagosomes. Science 295, 679–682 (2002).
Dorer, M. S., Kirton, D., Bader, J. S. & Isberg, R. R. RNA interference analysis of Legionella in Drosophila cells: exploitation of early secretory apparatus dynamics. PLoS Pathog. 2, e34 (2006).
Comerci, D. J., Lorenzo, M. M., Sieira, R., Gorvel, J.-P. & Ugalde, R. A. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3, 159–168 (2001).
Boschiroli, M. L. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl Acad. Sci. 99, 1544–1549 (2002).
de Jong, M. F., Sun, Y. H., den Hartigh, A. B., van Dijl, J. M. & Tsolis, R. M. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol. Microbiol. 70, 1378–1396 (2008).
de Barsy, M. et al. Identification of a Brucella spp. secreted effector specifically interacting with human small GTPase Rab2. Cell. Microbiol. 13, 1044–1058 (2011).
Marchesini, M. I., Herrmann, C. K., Salcedo, S. P., Gorvel, J.-P. & Comerci, D. J. In search of Brucella abortus type IV secretion substrates: screening and identification of four proteins translocated into host cells through VirB system. Cell. Microbiol. 13, 1261–1274 (2011).
Myeni, S. et al. Brucella modulates secretory trafficking via multiple type IV secretion effector proteins. PLoS Pathog. 9, e1003556 (2013). This paper identifies several novel T4SS effectors as well as a potential role for these effectors in induction of the UPR.
Salcedo, S. P. et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front. Cell. Infect. Microbiol. 3, 28 (2013).
de Jong, M. F. et al. Sensing of bacterial type IV secretion via the unfolded protein response. mBio 4, e00418-12 (2013). This paper shows that elements of the UPR sense localization of a Brucella spp. effector protein to the ER.
Barlowe, C. et al. COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77, 895–907 (1994).
Kuge, O. et al. SAR1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J. Cell Biol. 125, 51–65 (1994).
Fugier, E. et al. The glyceraldehyde-3-phosphate dehydrogenase and the small GTPase Rab 2 are crucial for Brucella replication. PLoS Pathog. 5, e1000487 (2009).
Seimon, T. A. et al. Induction of ER stress in macrophages of tuberculosis granulomas. PLoS ONE 5, e12772 (2010).
Baird, M. et al. The unfolded protein response is activated in Helicobacter-induced gastric carcinogenesis in a non-cell autonomous manner. Lab. Invest. 93, 112–122 (2012).
Akazawa, Y. et al. Endoplasmic reticulum stress contributes to Helicobacter pylori VacA-induced apoptosis. PLoS ONE 8, e82322 (2013).
Smith, J. A. et al. Brucella induces an unfolded protein response via TcpB that supports intracellular replication in macrophages. PLoS Pathog. 9, e1003785 (2013). This paper implicates a bacterial TIR domain-containing protein in induction of the UPR in B. abortus -infected macrophages.
Pillich, H., Loose, M., Zimmer, K.-P. & Chakraborty, T. Activation of the unfolded protein response by Listeria monocytogenes. Cell. Microbiol. 14, 949–964 (2012).
Qin, Q. M. et al. RNAi screen of endoplasmic reticulum-associated host factors reveals a role for IRE1α in supporting Brucella replication. PLoS Pathog. 4, e1000110 (2008). This paper is the first to report a UPR-associated factor in promoting bacterial replication.
Gekara, N. O., Groebe, L., Viegas, N. & Weiss, S. Listeria monocytogenes desensitizes immune cells to subsequent Ca2+ signaling via listeriolysin O-induced depletion of intracellular Ca2+ stores. Infect. Immun. 76, 857–862 (2008).
Price, C. T., Al-Quadan, T., Santic, M., Rosenshine, I. & Abu Kwaik, Y. Host proteasomal degradation generates amino acids essential for intracellular bacterial growth. Science 334, 1553–1557 (2011).
Bischof, L. J. et al. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 4, e1000176 (2008). This paper shows that the UPR functions as a defence mechanism against the action of bacterial pore-forming toxins.
Morinaga, N., Yahiro, K., Matsuura, G., Moss, J. & Noda, M. Subtilase cytotoxin, produced by Shiga-toxigenic Escherichia coli, transiently inhibits protein synthesis of Vero cells via degradation of BiP and induces cell cycle arrest at G1 by downregulation of cyclin D1. Cell. Microbiol. 10, 921–929 (2008).
Paton, A. W. et al. AB5 subtilase cytotoxin inactivates the endoplasmic reticulum chaperone BiP. Nature 443, 548–552 (2006). This paper shows that a toxin of enterohaemorrhagic E. coli can cleave BiP.
Wolfson, J. J. et al. Subtilase cytotoxin activates PERK, IRE1 and ATF6 endoplasmic reticulum stress-signalling pathways. Cell. Microbiol. 10, 1775–1786 (2008).
Chaudhary, A. et al. The Brucella TIR-like protein TcpB interacts with the death domain of MyD88. Biochem. Biophys. Res. Commun. 417, 299–304 (2012).
Cirl, C. et al. Subversion of Toll-like receptor signaling by a unique family of bacterial Toll/interleukin-1 receptor domain-containing proteins. Nature Med. 14, 399–406 (2008).
Radhakrishnan, G. K., Harms, J. S. & Splitter, G. A. Modulation of microtubule dynamics by a TIR domain protein from the intracellular pathogen Brucella melitensis. Biochem. J. 439, 79–83 (2011).
Sengupta, D. et al. Subversion of innate immune responses by Brucella through the targeted degradation of the TLR signaling adapter, MAL. J. Immunol. 184, 956–964 (2010).
Snyder, G. A. et al. Crystal structures of the Toll/interleukin-1 receptor (TIR) domains from the Brucella protein TcpB and host adaptor TIRAP reveal mechanisms of molecular mimicry. J. Biol. Chem. 289, 669–679 (2014).
Salcedo, S. P. et al. Brucella control of dendritic cell maturation is dependent on the TIR-containing protein btp1. PLoS Pathog. 4, e21 (2008).
Hasnain, S. Z., Lourie, R., Das, I., Chen, A. C. & McGuckin, M. A. The interplay between endoplasmic reticulum stress and inflammation. Immunol. Cell Biol. 90, 260–270 (2012).
Uehara, T. et al. S-nitrosylated protein-disulphide isomerase links protein misfolding to neurodegeneration. Nature 441, 513–517 (2006).
Martinon, F., Chen, X., Lee, A. H. & Glimcher, L. H. TLR activation of the transcription factor XBP1 regulates innate immune responses in macrophages. Nature Immunol. 11, 411–418 (2010).
Pahl, H. L. & Baeuerle, P. A. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-κB. EMBO J. 14, 2580–2588 (1995). This paper provides the first evidence of a link between ER stress and NF-κB signalling.
Pahl, H. L. & Baeuerle, P. A. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J. Virol. 69, 1480–1484 (1995).
Malathi, K., Dong, B., Gale, M. Jr & Silverman, R. H. Small self-RNA generated by RNase L amplifies antiviral innate immunity. Nature 448, 816–819 (2007).
Malathi, K. et al. RNase L releases a small RNA from HCV RNA that refolds into a potent PAMP. RNA 16, 2108–2119 (2010).
Yoneyama, M. et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nature Immunol. 5, 730–737 (2004).
Zeng, L. et al. XBP-1 couples endoplasmic reticulum stress to augmented IFN-ß induction via a cis-acting enhancer in macrophages. J. Immunol. 185, 2324–2330 (2010).
Lerner, A. G. et al. IRE1α induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell. Metab. 16, 250–264 (2012).
Menu, P. et al. ER stress activates the NLRP3 inflammasome via an UPR-independent pathway. Cell Death Dis. 3, e261 (2012).
Oslowski, C. M. et al. Thioredoxin-interacting protein mediates ER stress-induced β cell death through initiation of the inflammasome. Cell. Metab. 16, 265–273 (2012). References 93–95 demonstrate a link between ER stress and activation of the NLRP3 inflammasome.
Yamazaki, H. et al. Activation of the Akt–NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 183, 1480–1487 (2009).
Vecchi, C. et al. ER stress controls iron metabolism through induction of hepcidin. Science 325, 877–880 (2009).
Zhang, K. et al. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124, 587–599 (2006).
Barquero-Calvo, E. et al. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE 2, e631 (2007).
Lapaque, N. et al. Differential inductions of TNF-α and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell. Microbiol. 8, 401–413 (2006).
Roux, C. M. et al. Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell. Microbiol. 9, 1851–1869 (2007).
Chang, W. L. et al. Influenza virus infection causes global respiratory tract B cell response modulation via innate immune signals. J. Immunol. 178, 1457–1467 (2007).
Lee, S. Y., Lee, M. S., Cherla, R. P. & Tesh, V. L. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell. Microbiol. 10, 770–780 (2008).
Lee, M. S., Kim, M. H. & Tesh, V. L. Shiga toxins expressed by human pathogenic bacteria induce immune responses in host cells. J. Microbiol. 51, 724–730 (2013).
Heyderman, R. S., Soriani, M. & Hirst, T. R. Is immune cell activation the missing link in the pathogenesis of post-diarrhoeal HUS? Trends Microbiol. 9, 262–266 (2001).
Zhao, Y. et al. Subtilase cytotoxin activates MAP kinases through PERK and IRE1 branches of the unfolded protein response. Toxicol. Sci. 120, 79–86 (2011).
Harama, D. et al. A subcytotoxic dose of subtilase cytotoxin prevents lipopolysaccharide-induced inflammatory responses, depending on its capacity to induce the unfolded protein response. J. Immunol. 183, 1368–1374 (2009).
Mimura, N. et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood 119, 5772–5781 (2012).
Watowich, S. S., Morimoto, R. I. & Lamb, R. A. Flux of the paramyxovirus hemagglutinin-neuraminidase glycoprotein through the endoplasmic reticulum activates transcription of the GRP78-BiP gene. J. Virol. 65, 3590–3597 (1991).
Isler, J. A., Skalet, A. H. & Alwine, J. C. Human cytomegalovirus infection activates and regulates the unfolded protein response. J. Virol. 79, 6890–6899 (2005).
Stahl, S. et al. Cytomegalovirus downregulates IRE1 to repress the unfolded protein response. PLoS Pathog. 9, e1003544 (2013).
Tirosh, B. et al. Human cytomegalovirus protein US11 provokes an unfolded protein response that may facilitate the degradation of class I major histocompatibility complex products. J. Virol. 79, 2768–2779 (2005).
Tardif, K. D., Mori, K., Kaufman, R. J. & Siddiqui, A. Hepatitis C virus suppresses the IRE1–XBP1 pathway of the unfolded protein response. J. Biol. Chem. 279, 17158–17164 (2004).
Chan, S. W. Unfolded protein response in hepatitis C virus infection. Front. Microbiol. 5, 233 (2014).
Trujillo-Alonso, V., Maruri-Avidal, L., Arias, C. F. & Lopez, S. Rotavirus infection induces the unfolded protein response of the cell and controls it through the nonstructural protein NSP3. J. Virol. 85, 12594–12604 (2011).
Zambrano, J. L. et al. Rotavirus infection activates the UPR but modulates its activity. Virol. J. 8, 359 (2011).
Smith, J. A. A new paradigm: innate immune sensing of viruses via the unfolded protein response. Front. Microbiol. 5, 222 (2014).
Fronzes, R., Christie, P. J. & Waksman, G. The structural biology of type IV secretion systems. Nature Rev. Microbiol. 7, 703–714 (2009).
Juhas, M., Crook, D. W. & Hood, D. W. Type IV secretion systems: tools of bacterial horizontal gene transfer and virulence. Cell. Microbiol. 10, 2377–2386 (2008).
Nagai, H. & Kubori, T. Type IVB secretion systems of Legionella and other Gram-negative bacteria. Front. Microbiol. 2, 136 (2011).
de Jong, M. F. & Tsolis, R. M. Brucellosis and type IV secretion. Future Microbiol. 7, 47–58 (2012).
Voth, D. E., Broederdorf, L. J. & Graham, J. G. Bacterial type IV secretion systems: versatile virulence machines. Future Microbiol. 7, 241–257 (2012).
Goldszmid, R. S. et al. Host ER-parasitophorous vacuole interaction provides a route of entry for antigen cross-presentation in Toxoplasma gondii-infected dendritic cells. J. Exp. Med. 206, 399–410 (2009).
Sinai, A. P., Webster, P. & Joiner, K. A. Association of host cell endoplasmic reticulum and mitochondria with the Toxoplasma gondii parasitophorous vacuole membrane: a high affinity interaction. J. Cell Sci. 110, 2117–2128 (1997).
Wang, T. et al. Toxoplasma gondii induce apoptosis of neural stem cells via endoplasmic reticulum stress pathway. Parasitology 141, 988–995 (2014).
Xu, X. et al. Reactive oxygen species-triggered trophoblast apoptosis is initiated by endoplasmic reticulum stress via activation of caspase-12, CHOP, and the JNK pathway in Toxoplasma gondii infection in mice. Infect. Immun. 80, 2121–2132 (2012).
Yamamoto, M. et al. ATF6ß is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J. Exp. Med. 208, 1533–1546 (2011).
Acknowledgements
This work was supported by Washington State University funds and Public Health Service (PHS) grant AI112649 to J.C., and PHS grants AIAI050553, AI097107 and AI090387 to R.M.T.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Lysosomes
-
Membrane-enclosed organelles of eukaryotic cells that contain acid hydrolases capable of degrading biological polymers.
- Secretory pathway
-
A series of intracellular steps that eukaryotic cells use to transport proteins to specific cellular locations and also for extracellular secretion, which involves the endoplasmic reticulum and Golgi apparatus.
- Endocytic pathway
-
An array of intracellular membrane-bound compartments (including early endosomes, late endosomes and lysosomes) that internalize molecules at the plasma membrane and sort them for either degradation or recycling back to the plasma membrane.
- Autophagic pathway
-
A series of processes of recognition, capture and degradation of cytosolic content, such as protein aggregates, damaged organelles and invading microbes, which occur via engulfment into double-membrane bound autophagosomes and maturation along the endocytic pathway into degradative autolysosomes.
- ER-associated degradation
-
(ERAD). An essential component of endoplasmic reticulum (ER) quality control, the ERAD is a process of removal of misfolded or misassembled proteins in the lumen or membranes of the ER via polyubiquitination and targeting to the proteasome for degradation.
- Programmed cell death
-
A genetically regulated process of cellular suicide that involves specialized intracellular machineries and enables the elimination of specific cells.
- Retrotranslocation
-
The process by which the transport of misfolded proteins are transported from the endoplasmic reticulum lumen to the cytosol for degradation by the proteasome.
- Polyubiquitination
-
A post-translational modification of proteins that includes the covalent addition of chains of ubiquitin monomers that function as signals for proteasomal degradation of misfolded proteins.
- Proteasome
-
A large protein complex that degrades unneeded or misfolded proteins that have been tagged via polyubiquitination.
- SNARE proteins
-
(Soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins). Proteins that are involved in mediating intracellular membrane vesicle fusion in eukaryotic cells.
- Thioredoxin-interacting protein
-
(TXNIP). A protein that is induced via protein kinase R (PKR)-like ER kinase (PERK) and inositol-requiring enzyme 1 (IRE1), and that activates inflammatory processes and cell death following unabated endoplasmic reticulum stress.
- Hepcidin
-
A hormone that regulates iron homeostasis in mammals; it has a role in innate immunity as it limits iron availability to bacteria.
Rights and permissions
About this article
Cite this article
Celli, J., Tsolis, R. Bacteria, the endoplasmic reticulum and the unfolded protein response: friends or foes?. Nat Rev Microbiol 13, 71–82 (2015). https://doi.org/10.1038/nrmicro3393
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3393
This article is cited by
-
Ajugol's upregulation of TFEB-mediated autophagy alleviates endoplasmic reticulum stress in chondrocytes and retards osteoarthritis progression in a mouse model
Chinese Medicine (2023)
-
Staphylococcal virulence factor HlgB targets the endoplasmic-reticulum-resident E3 ubiquitin ligase AMFR to promote pneumonia
Nature Microbiology (2023)
-
Nod1-dependent NF-kB activation initiates hematopoietic stem cell specification in response to small Rho GTPases
Nature Communications (2023)
-
SEPTIN2 suppresses an IFN-γ-independent, proinflammatory macrophage activation pathway
Nature Communications (2023)
-
Control of immune cell function by the unfolded protein response
Nature Reviews Immunology (2023)