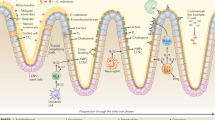
Key Points
-
The mouse pathogen Citrobacter rodentium is a useful model to investigate important human intestinal diseases, including enteropathogenic Escherichia coli (EPEC) and enterohaemorrhagic E. coli (EHEC) infections, Crohn's disease, ulcerative colitis and colon tumorigenesis.
-
Whole-genome sequencing of multiple pathogenic attaching and effacing (A/E) bacteria has led to the identification of many genes that are involved in pathogenesis, including gene families that encode effector proteins of the type III secretion system (T3SS). The functions of many putative virulence genes have been evaluated in the C. rodentium model, which has improved our understanding of pathogenesis and the corresponding host responses.
-
C. rodentium elicits robust inflammasome-dependent responses in a caspase 1- and caspase 11-dependent manner. Type I interferon signalling is a key factor that regulates inflammasome activation in C. rodentium infection.
-
The intestinal microbiota is crucial for coordinating mucosal immune responses to C. rodentium infection, including the development of IgA+ plasma cells, group 3 innate lymphoid cells (ILC3s; also known as inducible T helper (iTH) cells), TH17 cells and TH22 cells.
-
Defined dietary components, such as vitamin D, vitamin E, selenium, ligands from cruciferous vegetables and polyunsaturated fatty acids (PUFAs), as well as the intestinal microbiota, directly modify mucosal immune responses and epithelial barrier function in response to C. rodentium infection.
-
Future research using the C. rodentium model will focus on quantitative proteomics, metabolomics and four-dimensional (4D) imaging studies to unravel pathogen–host–microbiota interactions in unprecedented detail.
Abstract
Citrobacter rodentium is a mucosal pathogen of mice that shares several pathogenic mechanisms with enteropathogenic Escherichia coli (EPEC) and enterohaemorrhagic E. coli (EHEC), which are two clinically important human gastrointestinal pathogens. Thus, C. rodentium has long been used as a model to understand the molecular basis of EPEC and EHEC infection in vivo. In this Review, we discuss recent studies in which C. rodentium has been used to study mucosal immunology, including the deregulation of intestinal inflammatory responses during bacteria-induced colitis and the role of the intestinal microbiota in mediating resistance to colonization by enteric pathogens. These insights should help to elucidate the roles of mucosal inflammatory responses and the microbiota in the virulence of enteric pathogens.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schauer, D. B. & Falkow, S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect. Immun. 61, 4654–4661 (1993).
Schauer, D. B. & Falkow, S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect. Immun. 61, 2486–2492 (1993).
Deng, W., Vallance, B. A., Li, Y., Puente, J. L. & Finlay, B. B. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol. Microbiol. 48, 95–115 (2003).
Frankel, G. & Phillips, A. D. Attaching effacing Escherichia coli and paradigms of Tir-triggered actin polymerization: getting off the pedestal. Cell. Microbiol. 10, 549–556 (2008).
Frankel, G. et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30, 911–921 (1998).
Nataro, J. P. & Kaper, J. B. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11, 142–201 (1998).
Petty, N. K. et al. The Citrobacter rodentium genome sequence reveals convergent evolution with human pathogenic Escherichia coli. J. Bacteriol. 192, 525–538 (2010).
Wong, A. R. et al. Enteropathogenic and enterohaemorrhagic Escherichia coli: even more subversive elements. Mol. Microbiol. 80, 1420–1438 (2011).
Mundy, R. et al. Identification of a novel Citrobacter rodentium type III secreted protein, EspI, and roles of this and other secreted proteins in infection. Infect. Immun. 72, 2288–2302 (2004).
Arbeloa, A. et al. Distribution of espM and espT among enteropathogenic and enterohaemorrhagic Escherichia coli. J. Med. Microbiol. 58, 988–995 (2009).
Mundy, R. et al. Identification of a novel type IV pilus gene cluster required for gastrointestinal colonization of Citrobacter rodentium. Mol Microbiol 48, 795–809 (2003).
Mallick, E. M. et al. A novel murine infection model for Shiga toxin-producing Escherichia coli. J. Clin. Invest. 122, 4012–4024 (2012). This article describes the generation of a C. rodentium strain that produces Stx, which can be used as a more realistic model of EHEC infection.
Chandrakesan, P. et al. Utility of a bacterial infection model to study epithelial–mesenchymal transition, mesenchymal–epithelial transition or tumorigenesis. Oncogene 33, 2639–2654 (2013).
Higgins, L. M., Frankel, G., Douce, G., Dougan, G. & MacDonald, T. T. Citrobacter rodentium infection in mice elicits a mucosal TH1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infection Immun. 67, 3031–3039 (1999).
Mundy, R., MacDonald, T. T., Dougan, G., Frankel, G. & Wiles, S. Citrobacter rodentium of mice and man. Cell. Microbiol. 7, 1697–1706 (2005).
Lupp, C. et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 204 (2007).
Hoffmann, C. et al. Community-wide response of the gut microbiota to enteropathogenic Citrobacter rodentium infection revealed by deep sequencing. Infect. Immun. 77, 4668–4678 (2009).
Clare, S. et al. Enhanced susceptibility to Citrobacter rodentium infection in microRNA-155-deficient mice. Infect. Immun. 81, 723–732 (2013).
Wiles, S. et al. Organ specificity, colonization and clearance dynamics in vivo following oral challenges with the murine pathogen Citrobacter rodentium. Cell. Microbiol. 6, 963–972 (2004). This study uses bioluminescence imaging to characterize the C. rodentium infection cycle in mice.
Papapietro, O. et al. R-spondin 2 signalling mediates susceptibility to fatal infectious diarrhoea. Nature Commun. 4, 1898 (2013). This study was the first to identify the molecular basis of genetic susceptibility to C. rodentium infection.
Borenshtein, D., McBee, M. E. & Schauer, D. B. Utility of the Citrobacter rodentium infection model in laboratory mice. Curr. Opin. Gastroenterol. 24, 32–37 (2008).
Borenshtein, D., Nambiar, P. R., Groff, E. B., Fox, J. G. & Schauer, D. B. Development of fatal colitis in FVB mice infected with Citrobacter rodentium. Infect. Immun. 75, 3271–3281 (2007).
Willing, B. P., Vacharaksa, A., Croxen, M., Thanachayanont, T. & Finlay, B. B. Altering host resistance to infections through microbial transplantation. PLoS ONE 6, e26988 (2011). This study shows that increased IL-22 production by the intestinal microbiota is associated with host resistance to C. rodentium infection.
Ivanov, I. I. et al. Induction of intestinal TH17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009). This study shows that SFB of the microbiota can stimulate CD4+ T helper cells to release IL-17 and IL-22, which results in resistance to C. rodentium infection.
Ghosh, S. et al. Colonic microbiota alters host susceptibility to infectious colitis by modulating inflammation, redox status, and ion transporter gene expression. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G39–G49 (2011).
Borenshtein, D. et al. Decreased expression of colonic Slc26a3 and carbonic anhydrase iv as a cause of fatal infectious diarrhea in mice. Infect. Immun. 77, 3639–3650 (2009).
Wiles, S., Pickard, K. M., Peng, K., MacDonald, T. T. & Frankel, G. In vivo bioluminescence imaging of the murine pathogen Citrobacter rodentium. Infect. Immun. 74, 5391–5396 (2006).
Luperchio, S. A. & Schauer, D. B. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3, 333–340 (2001).
Collins, J. W., Meganck, J., Kuo, C., Francis, K. P. & Frankel, G. 4D multimodality imaging of Citrobacter rodentium infections in mice. J. Vis. Exp. http://dx.doi.org/10.3791/50450 (2013).
Wiles, S., Dougan, G. & Frankel, G. Emergence of a 'hyperinfectious' bacterial state after passage of Citrobacter rodentium through the host gastrointestinal tract. Cell. Microbiol. 7, 1163–1172 (2005).
Wickham, M. E., Brown, N. F., Boyle, E. C., Coombes, B. K. & Finlay, B. B. Virulence is positively selected by transmission success between mammalian hosts. Curr. Biol. 17, 783–788 (2007).
Bishop, A. L., Wiles, S., Dougan, G. & Frankel, G. Cell attachment properties and infectivity of host-adapted and environmentally adapted Citrobacter rodentium. Microbes Infect. 9, 1316–1324 (2007).
Deng, W., Li, Y., Vallance, B. A. & Finlay, B. B. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69, 6323–6335 (2001).
Deng, W. et al. Dissecting virulence: systematic and functional anlyses of a pathogenicity island. Proc. Natl Acad. Sci. USA 101, 3597–3602 (2004).
Lai, Y., Rosenshine, I., Leong, J. M. & Frankel, G. Intimate host attachment: enteropathogenic and enterohaemorrhagic Escherichia coli. Cell Microbiol. 15, 1796–1808 (2013).
Crepin, V. F. et al. Dissecting the role of the Tir:Nck and Tir:IRTKS/IRSp53 signalling pathways in vivo. Mol. Microbiol. 75, 308–323 (2010). This study shows that the Tir signalling pathways that are triggered in cultured epithelial cells in vitro by EPEC, EHEC and C. rodentium are not required for A/E lesion formation in vivo.
Kelly, M. et al. Essential role of the type III secretion system effector NleB in colonization of mice by Citrobacter rodentium. Infect. Immun. 74, 2328–2337 (2006).
Guttman, J. A., Samji, F. N., Li, Y., Vogl, A. W. & Finlay, B. B. Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect. Immun. 74, 6075–6084 (2006).
Guttman, J. A. et al. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell. Microbiol. 8, 634–645 (2006).
Hemrajani, C. et al. NleH effectors interact with Bax inhibitor-1 to block apoptosis during enteropathogenic Escherichia coli infection. Proc. Natl Acad. Sci. USA 107, 3129–3134 (2010).
Selyunin, A. S., Reddick, L. E., Weigele, B. A. & Alto, N. M. Selective protection of an ARF1–GTP signaling axis by a bacterial scaffold induces bidirectional trafficking arrest. Cell Rep. 6, 878–891 (2014).
Marches, O. et al. EspJ of enteropathogenic and enterohaemorrhagic Escherichia coli inhibits opsono-phagocytosis. Cell. Microbiol. 10, 1104–1115 (2008).
Sham, H. P. et al. Attaching and effacing bacterial effector NleC suppresses epithelial inflammatory responses by inhibiting NF-кB and p38 mitogen-activated protein kinase activation. Infect. Immun. 79, 3552–3562 (2011).
Kim, M. et al. Bacterial interactions with the host epithelium. Cell Host Microbe 8, 20–35 (2010).
Kamada, N. et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329 (2012). This study suggests that temporal expression of the virulence factor Ler controls the ability of C. rodentium to outcompete the gut microbiota.
Gruenheid, S. et al. Identification and characterization of NleA, a non-LEE-encoded type III translocated virulence factor of enterohaemorrhagic Escherichia coli O157:H7. Mol. Microbiol. 51, 1233–1249 (2004).
Hart, E. et al. RegA, an AraC-like protein, is a global transcriptional regulator that controls virulence gene expression in Citrobacter rodentium. Infect. Immun. 76, 5247–5256 (2008).
Gueguen, E. & Cascales, E. Promoter swapping unveils the role of the Citrobacter rodentium CTS1 type VI secretion system in interbacterial competition. Appl. Environ. Microbiol. 79, 32–38 (2013).
Yang, J., Tauschek, M., Hart, E., Hartland, E. L. & Robins-Browne, R. M. Virulence regulation in Citrobacter rodentium: the art of timing. Microb. Biotechnol. 3, 259–268 (2010).
Rutherford, S. T. & Bassler, B. L. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2, a012427 (2012).
Coulthurst, S. J. et al. Quorum sensing has an unexpected role in virulence in the model pathogen Citrobacter rodentium. EMBO Rep. 8, 698–703 (2007).
Lawhon, S. D., Maurer, R., Suyemoto, M. & Altier, C. Intestinal short-chain fatty acids alter Salmonella Typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 46, 1451–1464 (2002).
Kawai, T. & Akira, S. Toll-like receptor and RIG-I-like receptor signaling. Ann. NY Acad. Sci. 1143, 1–20 (2008).
Gibson, D. et al. MyD88 signalling plays a critical role in host defence by controlling pathogen burden and promoting epithelial cell homeostasis during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10, 618–631 (2008).
Lebeis, S. L., Bommarius, B., Parkos, C. A., Sherman, M. A. & Kalman, D. TLR signaling mediated by MyD88 is required for a protective innate immune response by neutrophils to Citrobacter rodentium. J. Immunol. 179, 566–577 (2007).
Bergstrom, K. S., Sham, H. P., Zarepour, M. & Vallance, B. A. Innate host responses to enteric bacterial pathogens: a balancing act between resistance and tolerance. Cell. Microbiol. 14, 475–484 (2012).
Gibson, D. L. et al. Toll-like receptor 2 plays a critical role in maintaining mucosal integrity during Citrobacter rodentium-induced colitis. Cell. Microbiol. 10, 388–403 (2008).
Khan, M. A. et al. Toll-like receptor 4 contributes to colitis development but not to host defense during Citrobacter rodentium infection in mice. Infection Immun. 74, 2522–2536 (2006).
Geddes, K. et al. Identification of an innate T helper type 17 response to intestinal bacterial pathogens. Nature Med. 17, 837–844 (2011).
Zaki, M. H., Lamkanfi, M. & Kanneganti, T.-D. The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol. 32, 171–179 (2011).
Franchi, L., Eigenbrod, T., Munoz-Planillo, R. & Nunez, G. The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nature Immunol. 10, 241–247 (2009).
Rathinam, V. A., Vanaja, S. K. & Fitzgerald, K. A. Regulation of inflammasome signaling. Nature Immunol. 13, 333–332 (2012).
Kayagaki, N. et al. Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 (2011). This study describes an essential role for caspase 11 in driving IL-1 responses against C. rodentium.
Gurung, P. et al. Toll or interleukin-1 receptor (TIR) domain-containing adaptor inducing interferon-β (TRIF)-mediated caspase-11 protease production integrates Toll-like receptor 4 (TLR4) protein-and Nlrp3 inflammasome-mediated host defense against enteropathogens. J. Biol. Chem. 287, 34474–34483 (2012).
Broz, P. et al. Caspase-11 increases susceptibility to Salmonella infection in the absence of caspase-1. Nature 490, 288–291 (2012).
Rathinam, V. A. et al. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by Gram-negative bacteria. Cell 150, 606–619 (2012).
Kayagaki, N. et al. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341, 1246–1249 (2013).
Liu, Z. et al. Role of inflammasomes in host defense against Citrobacter rodentium infection. J. Biol. Chem. 287, 16955–16964 (2012). References 66, 67 and 68 show that the TRIF–type I IFN axis is essential for inflammasome-mediated responses to C. rodentium.
Khan, M. A. et al. Flagellin-dependent and -independent inflammatory responses following infection by enteropathogenic Escherichia coli and Citrobacter rodentium. Infect. Immun. 76, 1410–1422 (2008).
Simmons, C. P. et al. Central role for B lymphocytes and CD4+ T cells in immunity to infection by the attaching and effacing pathogen Citrobacter rodentium. Infection Immun. 71, 5077–5086 (2003).
Vallance, B. A., Deng, W., Knodler, L. A. & Finlay, B. B. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infection Immun. 70, 2070–2081 (2002).
Simmons, C. P. et al. Impaired resistance and enhanced pathology during infection with a noninvasive, attaching-effacing enteric bacterial pathogen, Citrobacter rodentium, in mice lacking IL-12 or IFN-γ. J. Immunol. 168, 1804–1812 (2002).
Shiomi, H. et al. Gamma interferon produced by antigen-specific CD4+ T cells regulates the mucosal immune responses to Citrobacter rodentium infection. Infection Immun. 78, 2653–2666 (2010).
O'Quinn, D. B., Palmer, M. T., Lee, Y. K. & Weaver, C. T. Emergence of the TH17 pathway and its role in host defense. Adv. Immunol. 99, 115–163 (2008).
Spits, H. et al. Innate lymphoid cells — a proposal for uniform nomenclature. Nature Rev. Immunol. 13, 145–149 (2013).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Torchinsky, M. B., Garaude, J., Martin, A. P. & Blander, J. M. Innate immune recognition of infected apoptotic cells directs TH17 cell differentiation. Nature 458, 78–82 (2009).
Lawley, T. D. et al. Targeted restoration of the intestinal microbiota with a simple, defined bacteriotherapy resolves relapsing Clostridium difficile disease in mice. PLoS Pathog. 8, e1002995 (2012).
Fritz, J. H. et al. Acquisition of a multifunctional IgA+ plasma cell phenotype in the gut. Nature 481, 199–203 (2012).
Rodrigues, D. M., Sousa, A. J., Johnson-Henry, K. C., Sherman, P. M. & Gareau, M. G. Probiotics are effective for the prevention and treatment of Citrobacter rodentium-induced colitis in mice. J. Infect. Dis. 206, 99–109 (2012).
Fanning, S. et al. Bifidobacterial surface-exopolysaccharide facilitates commensal–host interaction through immune modulation and pathogen protection. Proc. Natl Acad. Sci. USA 109, 2108–2113 (2012).
Collins, J. W. et al. Fermented dairy products modulate C. rodentium induced colonic hyperplasia. J. Infect. Dis. http://dx.doi.org/10.1093/infdis/jiu205 (2014).
Zenewicz, L. A. et al. IL-22 deficiency alters colonic microbiota to be transmissible and colitogenic. J. Immunol. 190, 5306–5312 (2013).
Kiss, E. A. et al. Natural aryl hydrocarbon receptor ligands control organogenesis of intestinal lymphoid follicles. Science 334, 1561–1565 (2011).
Ryz, N. R. et al. Active vitamin D (1,25-dihydroxyvitamin D3) increases host susceptibility to Citrobacter rodentium by suppressing mucosal TH17 responses. Am. J. Physiol. Gastrointest. Liver Physiol. 303, G1299–G1311 (2012).
Smith, A. D., Botero, S., Shea-Donohue, T. & Urban, J. F. Jr. The pathogenicity of an enteric Citrobacter rodentium infection is enhanced by deficiencies in the antioxidants selenium and vitamin E. Infect. Immun. 79, 1471–1478 (2011).
Ghosh, S. et al. Fish oil attenuates omega-6 polyunsaturated fatty acid-induced dysbiosis and infectious colitis but impairs LPS dephosphorylation activity causing sepsis. PLoS ONE 8, e55468 (2013). This study shows that polyunsaturated fatty acids (omega-3 PUFA and omega-6 PUFA) modulate C. rodentium translocation and mortality in mice.
Duck, L. W. et al. Isolation of flagellated bacteria implicated in Crohn's disease. Inflamm. Bowel Dis. 13, 1191–1201 (2007).
Mondot, S. et al. Highlighting new phylogenetic specificities of Crohn's disease microbiota. Inflamm. Bowel Dis. 17, 185–192 (2011).
Monteleone, I., Pallone, F. & Monteleone, G. TH17-related cytokines: new players in the control of chronic intestinal inflammation. BMC Med. 9, 122 (2011).
Sekirov, I. et al. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 76, 4726–4736 (2008).
Bergstrom, K. S. et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog. 6, e1000902 (2010).
Wlodarska, M. et al. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect. Immun. 79, 1536–1545 (2011). This study shows that metronidazole treatment alters the microbiota and supresses goblet cell function, thereby enhancing C. rodentium colonization.
Burger-van Paassen, N. et al. The regulation of intestinal mucin MUC2 expression by short-chain fatty acids: implications for epithelial protection. Biochem. J. 420, 211–219 (2009).
Wlodarska, M. et al. NLRP6 inflammasome orchestrates the colonic host–microbial interface by regulating goblet cell mucus secretion. Cell 156, 1045–1059 (2014). This study is the first to demonstrate that innate immune signalling pathways modulate goblet cell function and defence against C. rodentium infection.
Croxen, M. A. & Finlay, B. B. Molecular mechanisms of Escherichia coli pathogenicity. Nature Rev. Microbiol. 8, 26–38 (2010).
Raymond, B. et al. Subversion of trafficking, apoptosis, and innate immunity by type III secretion system effectors. Trends Microbiol. 21, 430–441 (2013).
Law, R. J., Gur-Arie, L., Rosenshine, I. & Finlay, B. B. In vitro and in vivo model systems for studying enteropathogenic Escherichia coli infections. Cold Spring Harb. Perspect. Med. 3, a009977 (2013).
Basu, R. et al. TH22 cells are an important source of IL-22 for host protection against enteropathogenic bacteria. Immunity 37, 1061–1075 (2012).
Gibson, D. L. et al. Interleukin-11 reduces TLR4-induced colitis in TLR2-deficient mice and restores intestinal STAT3 signaling. Gastroenterology 139, 1277–1288 (2010).
Ota, N. et al. IL-22 bridges the lymphotoxin pathway with the maintenance of colonic lymphoid structures during infection with Citrobacter rodentium. Nature Immunol. 12, 941–948 (2011).
Tumanov, A. V. et al. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe 10, 44–53 (2011).
Kim, M. H., Kang, S. G., Park, J. H., Yanagisawa, M. & Kim, C. H. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology 145, 396–406 (2013).
Weinstock, G. M. Genomic approaches to studying the human microbiota. Nature 489, 250–256 (2012).
Pham, T. A. & Lawley, T. D. Emerging insights on intestinal dysbiosis during bacterial infections. Curr. Opin. Microbiol. 17, 67–74 (2014).
Winter, S. E. et al. Gut inflammation provides a respiratory electron acceptor for Salmonella. Nature 467, 426–429 (2010).
Spees, A. M. et al. Streptomycin-induced inflammation enhances Escherichia coli gut colonization through nitrate respiration. mBio 4, e00430–13 (2013).
Barker, N., van de Wetering, M. & Clevers, H. The intestinal stem cell. Genes Dev. 22, 1856–1864 (2008).
Diez, E. et al. Identification and characterization of Cri1, a locus controlling mortality during Citrobacter rodentium infection in mice. Genes Immun. 12, 280–290 (2011).
Chandrakesan, P. et al. Novel changes in NF-кB activity during progression and regression phases of hyperplasia: role of MEK, ERK, and p38. J. Biol. Chem. 285, 33485–33498 (2010).
Higgins, L. M. et al. Role of bacterial intimin in colonic hyperplasia and inflammation. Science 285, 588–591 (1999).
Brown, J. B. et al. Epithelial phosphatidylinositol-3-kinase signaling is required for β-catenin activation and host defense against Citrobacter rodentium infection. Infect. Immun. 79, 1863–1872 (2011).
Zheng, Y. et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nature Med. 14, 282–289 (2008).
Deng, W. et al. A comprehensive proteomic analysis of the type III secretome of Citrobacter rodentium. J. Biol. Chem. 285, 6790–6800 (2010).
Hardwidge, P. R. et al. Proteomic analysis of the intestinal epithelial cell response to enteropathogenic Escherichia coli. J. Biol. Chem. 279, 20127–20136 (2004).
Hartland, E. L. et al. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol. Microbiol. 32, 151–158 (1999).
Kenny, B. Phosphorylation of tyrosine 474 of the enteropathogenic Escherichia coli (EPEC) Tir receptor molecule is essential for actin nucleating activity and is preceded by additional host modifications. Mol. Microbiol. 31, 1229–1241 (1999).
Gruenheid, S. et al. Enteropathogenic E. coli Tir binds Nck to initiate actin pedestal formation in host cells. Nature Cell Biol. 3, 856–859 (2001).
Wong, A. R., Raymond, B., Collins, J. W., Crepin, V. F. & Frankel, G. The enteropathogenic E. coli effector EspH promotes actin pedestal formation and elongation via WASP-interacting protein (WIP). Cell. Microbiol. 14, 1051–1070 (2012).
Campellone, K. G. & Leong, J. M. Nck-independent actin assembly is mediated by two phosphorylated tyrosines within enteropathogenic Escherichia coli Tir. Mol. Microbiol. 56, 416–432 (2005).
Brady, M. J., Campellone, K. G., Ghildiyal, M. & Leong, J. M. Enterohaemorrhagic and enteropathogenic Escherichia coli Tir proteins trigger a common Nck-independent actin assembly pathway. Cell. Microbiol. 9, 2242–2253 (2007).
Vingadassalom, D. et al. Insulin receptor tyrosine kinase substrate links the E. coli O157:H7 actin assembly effectors Tir and EspF(U) during pedestal formation. Proc. Natl Acad. Sci. USA 106, 6754–6759 (2009).
Weiss, S. M. et al. IRSp53 links the enterohemorrhagic E. coli effectors Tir and EspFU for actin pedestal formation. Cell Host Microbe 5, 244–258 (2009).
Acknowledgements
The authors are supported by the Canadian Institutes for Health (CIHR) operating grants (to K.M.K. and B.B.F.), US National Institutes of Health (NIH) grant AI083713 (to K.A.F.), NIH grant AI085761 (to K.M.K.), the Wellcome Trust and the UK Medical Research Council (MRC) (to G.F.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Crohn's disease
-
A chronic inflammatory disease of the gastrointestinal tract; it primarily affects the ileum and colon and causes recurrent abdominal pain, fatigue, weight loss, blood and mucus in the faeces, and diarrhoea.
- Ulcerative colitis
-
A chronic inflammatory disease of the colon and rectum that is characterized by recurrent abdominal pain, chills, fever, colitis and diarrhoea.
- Sterilizing immunity
-
An immune response that completely prevents an infection.
- Coprophagy
-
The consumption of faeces.
- H-NS family
-
(Histone-like nucleoid-structuring family). A family of DNA-binding proteins that bind to AT-rich double-stranded DNA and are involved in transcriptional silencing and bacterial chromosome organization.
- Integration host factor
-
(IHF). A histone-like DNA-binding protein that binds to consensus sites and bends the DNA to form a nucleoprotein complex that promotes transcription.
- AHL-type quorum sensing system
-
(N-Acyl-homoserine lactone quorum sensing system). A dedicated communication system that is present in Gram-negative bacteria and is used to regulate specific genes in response to population density via the production of autoinducer 1.
- LuxS quorum sensing system
-
A communication system that is found in both Gram-positive and Gram-negative bacteria; it controls the expression of virulence genes in a cell density-dependent manner via the production of the signalling molecule autoinducer 2 by luxS.
- C3H/HeJ mice
-
A substrain of the widely used laboratory mouse strain C3H; they carry a mutation in Tlr4 and are resistant to endotoxin exposure.
- BarA–SirA two-component regulatory system
-
A two-component system that is present in Salmonella enterica subsp. enterica serovar Typhimurium. BarA encodes a histidine kinase and SirA encodes the response regulator. Following activation, the system triggers a signalling cascade that results in increased expression of virulence genes and decreased expression of motility genes.
- iNOS
-
(Inducible nitric oxide synthase). A cytosolic enzyme that is found in multiple cell types and that produces nitrous oxide (NO) from L-arginine, following induction by lipopolysaccharide and pro-inflammatory cytokines. It is presumed that the production of NO, in conjunction with superoxide radicals, leads to the formation of antimicrobial reactive nitrogen intermediates, such as peroxynitrite and nitrosothiols, which restrict the growth of invading pathogens.
- Inflammasomes
-
Macromolecular complexes that are found in the cytosol of haematopoietic cells and are assembled in response to a range of microbial and endogenous danger signals, which leads to the proteolytic activation of the effector protein caspase 1. Inflammasomes typically consist of a receptor, an adaptor molecule (apoptosis-associated speck-like protein containing caspase activation and recruitment domain), and the effector caspase 1.
- Specific pathogen-free mice
-
Mice that are provided by laboratory animal vendors or are generated in a home laboratory, that have a guaranteed health status and are free of particular pathogens.
- Faecal microbiota transplantation
-
(FMT). A transplantation process in which the faecal material (including the faecal microbiota) from a healthy donor is transferred into a recipient. Patients are often treated by enema infusion or the consumption of capsules containing donor faeces.
- Intestinal lymphoid follicles
-
A type of lymphoid tissue that consists of aggregates of B cells, CD4+ T cells and IgA-producing plasma cells, which are found directly underneath the associated epithelium. These follicles are induced following environmental cues from the intestinal microbiota and dietary components.
- RegIII antimicrobial peptides
-
Secreted antimicrobial peptides that are produced in the gastrointestinal tract and pancreas and consist of a signal peptide and a single C-type lectin domain; they bind to peptidoglycan and are bactericidal for Gram-positive bacteria.
- Trefoil factor
-
A secretory protein that contains a trefoil motif and is produced by goblet cells in the gastrointestinal mucosa; it is thought to protect against mucosal damage by stabilizing the mucus layer.
- Resistin-like molecule B
-
A protein that is produced by intestinal goblet cells and is induced by microorganisms; it is thought to regulate innate mucosal immune responses, such as macrophage activation and antimicrobial lectin expression.
- Altered Schaedler flora
-
(ASF). A cocktail of eight culturable bacterial strains that is used to colonize the gastrointestinal tract of germ-free mice, thereby generating a defined low-complexity microbiota. Notably, different variations of ASF are commercially available.
- Monocolonized mice
-
Former germ-free mice that have been colonized with a single bacterial strain.
- Humanized microbiota
-
A term used to describe human microbiota colonizing the mouse gastrointestinal tract.
- Metabolome
-
The complete profile of small-molecule metabolites, such as amino acids, nucleotides, antioxidants, organic acids, vitamins, hormones, drugs and food components that are found within a cell, tissue, organ or entire organism.
Rights and permissions
About this article
Cite this article
Collins, J., Keeney, K., Crepin, V. et al. Citrobacter rodentium: infection, inflammation and the microbiota. Nat Rev Microbiol 12, 612–623 (2014). https://doi.org/10.1038/nrmicro3315
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3315
This article is cited by
-
Transcriptomic Response of Superworm in Facilitating Polyethylene Biodegradation
Journal of Polymers and the Environment (2024)
-
Fecal microbiota transplantation attenuates Escherichia coli infected outgrowth by modulating the intestinal microbiome
Microbial Cell Factories (2023)
-
The phylogenomic landscape of extended-spectrum β-lactamase producing Citrobacter species isolated from surface water
BMC Genomics (2023)
-
Modulating AHR function offers exciting therapeutic potential in gut immunity and inflammation
Cell & Bioscience (2023)
-
Quantitative dose-response analysis untangles host bottlenecks to enteric infection
Nature Communications (2023)