Key Points
-
Hantaviruses are negative-sense single-stranded RNA viruses that infect many species of rodents, shrews, moles and bats.
-
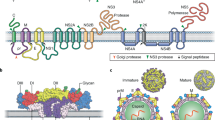
Hantaviruses primarily replicate in the endothelium and might use integrins to enter cells. Following entry, the virus synthesizes viral mRNA to produce viral proteins and replicate the genome (comprising small, medium and large segments), although the exact details of these processes remain unclear. The viral genome is encapsided by nucleocapsid protein to form ribonucleoproteins, which interact with viral glycoprotein to form virus particles.
-
The three segments of viral RNA encode nucleocapsid protein, glycoprotein and RNA-dependent RNA polymerase.
-
Infection of reservoir hosts is asymptomatic, possibly owing to immunosuppression.
-
Infection of humans causes either haemorrhagic fever with renal syndrome or hantavirus cardiopulmonary syndrome.
-
Disease pathology is characterized by increased permeability of the endothelial cells lining capillaries and is also thought to be mediated by enhanced immune responses, such as increased production of cytokines and expansion of cytotoxic T cells.
Abstract
Hantaviruses are negative-sense single-stranded RNA viruses that infect many species of rodents, shrews, moles and bats. Infection in these reservoir hosts is almost asymptomatic, but some rodent-borne hantaviruses also infect humans, causing either haemorrhagic fever with renal syndrome (HFRS) or hantavirus cardiopulmonary syndrome (HCPS). In this Review, we discuss the basic molecular properties and cell biology of hantaviruses and offer an overview of virus-induced pathology, in particular vascular leakage and immunopathology.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vaheri, A., Mills, J. N., Spiropoulou, C. F. & Hjelle, B. in Oxford Textbook of Zoonoses: Biology, Clinical Practice and Public Health Control (eds Palmer, S. R., Lord Soulsby, Torgerson, P. R. & Brown, D. W. G.) 307–322 (Oxford Univ. Press, 2011).
Vaheri, A. et al. Hantavirus infections in Europe and their impact on public health. Rev. Med. Virol. 23, 35–49 (2013).
Hjelle, B. & Torres-Pérez, F. Hantaviruses in the Americas and their role as emerging pathogens. Viruses 2, 2259–2286 (2010).
Jonsson, C. B., Figueiredo, L. T. & Vapalahti, O. A global perspective on hantavirus ecology, epidemiology, and disease. Clin. Microbiol. Rev. 23, 412–441 (2010).
CDC. Hantavirus pulmonary syndrome in visitors to a national park – Yosemite Valley, California, 2012. Morb. Mortal. Wkly Rep. 61, 952 (2012).
Schmaljohn, C. S. et al. Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome. Science 227, 1041–1044 (1985). This pioneering article establishes hantaviruses as a separate genus in the Bunyaviridae family.
Schmaljohn, C. S. & Nichol, S. T. in Fields Virology (eds Knipe, D. M. & Howley, P. M.) 1741–1790 (Lippincott Williams & Wilkins, 2007).
Spiropoulou, C. F. in Bunyaviridae. Molecular and Cellular Biology (eds Plyusnin, A. & Elliott, R. M.) 41–60 (Caister Academic Press, 2011).
Hussein, I. T., Haseeb, A. Haque, A. & Mir, M. A. Recent advances in hantavirus molecular biology and disease. Adv. Appl. Microbiol. 74, 35–75 (2011).
Jääskeläinen, K. M. et al. Tula and Puumala hantavirus NSs ORFs are functional and the products inhibit activation of the interferon-β promoter. J. Med. Virol. 79, 1527–1536 (2007).
Virtanen, J. O., Jääskeläinen, K. M., Djupsjöbacka, J., Vaheri, A. & Plyusnin, A. Tula hantavirus NSs protein accumulates in the perinuclear area in infected and transfected cells. Arch. Virol. 155, 117–121 (2010).
Rönnberg, T. S. et al. Searching for cellular partners of hantaviral nonstructural protein NSs: the yeast two-hybrid screening of mouse cDNA library and analysis of cellular interactome. PLoS ONE 7, e34307 (2012).
Van Knippenberg, I., Fragkoudis, R. & Elliott, R. M. The transient nature of Bunyamwera orthobunyavirus NSs protein expression: effects of increased stability of NSs protein on virus replication. PLoS ONE 8, e64137 (2013).
Hepojoki, J., Strandin, T., Lankinen, H. & Vaheri, A. Hantavirus structure – molecular interactions behind the scene. J. Gen. Virol. 93, 1631–1644 (2012).
Huiskonen, J. T. et al. Electron cryo-tomography of Tula hantavirus suggests a unique assembly paradigm for enveloped viruses. J. Virol. 84, 4889–4897 (2010). This work obtains the first solved structure of the hantavirus spike.
Kallio, E. R. et al. Prolonged survival of Puumala hantavirus outside the host: evidence for indirect transmission via the environment. J. Gen. Virol. 87, 2127–2134 (2006).
Mackow, E. R. & Gavrilovskaya, I. N. Hantavirus regulation of endothelial cell functions. Thromb. Haemost. 102, 1030–1041 (2009). This report summarizes the concept that disrupted endothelial function is a crucial factor in disease mediated by hantaviruses.
Takagi, J. & Springer, T. A. Integrin activation and structural rearrangement. Immunol. Rev. 186, 141–163 (2002).
Gavrilovskaya, I., Shepley, M., Shaw, R., Ginsberg, M. & Mackow E. β3 integrins mediate the cellular entry of hantaviruses that cause respiratory failure. Proc. Natl Acad. Sci. USA 95, 7074–7079 (1998).
Mackow, E. R. & Gavrilovskaya, I. N. Cellular receptors and hantavirus pathogenesis. Curr. Top. Microbiol. Immunol. 256, 91–115 (2001).
Krautkrämer, E. & Zeier, M. Hantavirus causing hemorrhagic fever with renal syndrome enters from the apical surface and requires decay-accelerating factor (DAF/CD55). J. Virol. 82, 4257–4264 (2008).
Choi, Y. et al. A hantavirus causing hemorrhagic fever with renal syndrome requires gC1qR/p32 for efficient cell binding and infection. Virology 381, 178–183 (2008).
Jin, M. et al. Hantaan virus enters cells by clathrin-dependent receptor-mediated endocytosis. Virology 294, 60–69 (2002).
Ramanathan, H. N. & Jonsson, C. B. New and Old World hantaviruses differentially utilized host cytoskeletal components during their life cycles. Virology 374, 138–150 (2008).
Lozach, P. Y. et al. Entry of bunyaviruses into mammalian cells. Cell Host Microbe 7, 488–499 (2009).
Ramanathan, H. N. et al. Dynein-dependent transport of the Hantaan virus nucleocapsid protein to the endoplasmic reticulum-Golgi intermediate compartment. J. Virol. 81, 8634–8647 (2007).
Flick, K. et al. Rescue of Hantaan virus minigenomes. Virology 306, 219–224 (2003).
Boyloy, M., Plotch, S. J. & Krug, R. M. Globin mRNAs are primers for the transcription of influenza viral RNA in vitro. Proc. Natl Acad. Sci. USA 75, 4886–4890 (1978).
Mir, M. A., Duran, W. A., Hjelle, B. L., Ye, C. & Panganiban, A. T. Storage of cellular 5′ mRNA caps in P bodies for viral cap-snatching. Proc. Natl Acad. Sci. USA 105, 19294–19299 (2008).
Heinemann, P., Schmidt-Chanasit, J. & Günther, S. The N terminus of Andes virus L protein suppresses mRNA and protein expression in mammalian cells. J. Virol. 87, 6975–6985 (2013).
Reguera, J., Weber, F. & Cusack, S. Bunyaviridae RNA polymerases (L-protein) have an N-terminal, influenza-like endonuclease domain, essential for viral cap-dependent transcription. PLoS Pathog. 6, e1001101 (2010).
Garcin, D. et al. The 5′ ends of Hantaan virus (Bunyaviridae) RNAs suggest a prime-and-realign mechanism for the initiation of RNA synthesis. J. Virol. 69, 5754–5766 (1995). This article describes the prime-and-realign mechanism used in the initiation of hantavirus RNA synthesis.
Fontana, J., López-Montero, N., Elliott, R. M., Fernández, J. J. & Risco, C. The unique architecture of Bunyamwera virus factories around the Golgi complex. Cell. Microbiol. 10, 2012–2028 (2008).
Ravkov, E. V. & Compans, R. W. Hantavirus nucleocapsid protein is expressed as a membrane-associated protein in the perinuclear region. J. Virol. 75, 1808–1815 (2001).
Kukkonen, S. K. J., Vaheri, A. & Plyusnin, A. Tula hantavirus L protein is a 250 kDa peripheral membrane-associated protein. J. Gen. Virol. 85, 1181–1189 (2004).
Kaukinen, P., Vaheri, A. & Plyusnin, A. Hantavirus nucleocapsid protein: a multifunctional molecule with both house-keeping and ambassadorial duties. Arch. Virol. 150, 1693–1713 (2005).
Ariza, A. et al. Nucleocapsid protein structures from orthobunyaviruses reveal insight into ribonucleoprotein architecture and RNA polymerization. Nucleic Acids Res. 17, 1–15 (2013).
Ferron, F. et al. The hexamer structure of the Rift Valley Fever virus nucleoprotein suggests a mechanism for its assembly into ribonucleoprotein complexes. PLoS Pathog. 20117, e1002030 (2011).
Hepojoki, J., Strandin, T., Vaheri, A. & Lankinen, H. Interactions and oligomerization of hantavirus glycoproteins. J. Virol. 84, 227–242 (2010).
Hepojoki, J. et al. Cytoplasmic tails of hantavirus glycoproteins interact with the nucleocapsid protein. J. Gen. Virol. 92, 1189–1198 (2010).
Strandin, T. et al. The cytoplasmic tail of hantavirus Gn interacts with RNA. Virology 418, 12–20 (2011).
Piper, M. E., Sorenson, D. R. & Gerrard, S. R. Efficient cellular release of Rift Valley Fever virus requires genomic RNA. PLoS ONE 6, e18070 (2011).
Strandin, T., Hepojoki, J. & Vaheri, A. Cytoplasmic tails of bunyavirus Gn glycoproteins—could they act as matrix protein surrogates? Virology 437, 73–80 (2013).
Zheng, W. & Tao, Y. J. Structure and assembly of influenza A virus ribonucleoprotein complex. FEBS Lett. 587, 1206–1214 (2013).
Terasaki, K., Murakami, S., Lokugamage, K. G. & Makino, S. Mechanism of tripartite RNA genome packaging in Rift Valley fever virus. Proc. Natl Acad. Sci. USA 108, 804–809 (2011).
Rowe, R. K., Suszko, J. W. & Pekosz, A. Roles for the recycling endosome, Rab8, and Rab11 in hatavirus release from epithelial cells. Virology 382, 239–249 (2008).
Meyer, B. J. & Schmaljohn, C. S. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 8, 61–67 (2000).
Henttonen, H. et al. Recent discoveries of new hantaviruses widen their range and question their origins. Ann. N. Y. Acad. Sci. 1149, 84–89 (2008).
Kallio, E. R. et al. Endemic hantavirus infection impairs the winter survival of its rodent host. Ecology 88, 1911–1916 (2007).
Luis, A. D., Douglass, R. J., Hudson, P. J., Mills, J. N. & Bjørnstad, O. N. Sin Nombre hantavirus decreases survival of male deer mice. Oecologia 169, 431–439 (2012).
Spengler, J. R. et al. Experimental Andes virus infection in deer mice: characteristics of infection and clearance in a heterologous rodent host. PLoS ONE 8, e55310 (2013).
Olsson, G. E., Leirs, H. & Henttonen, H. Hantaviruses and their hosts in Europe: reservoirs here and there, but not everywhere? Vector Borne Zoonotic Dis. 10, 546–561 (2010).
Gavrilovskaya, I. N. et al. Features of circulation of hemorrhagic fever with renal syndrome (HFRS) virus among small mammals in the European U.S.S.R. Arch. Virol. 75, 313–316 (1983).
Hardestam, J. et al. Puumala hantavirus excretion kinetics in bank voles (Myodes glareolus). Emerg. Infect. Dis. 14, 1209–1215 (2008).
Botten, J. et al. Shedding and intracage transmission of Sin Nombre hantavirus in the deer mouse (Peromyscus maniculatus) model. J. Virol. 76, 7587–7594 (2002).
Voutilainen, L. Interactions between Puumala hantavirus and its host, the bank vole, in the boreal zone. Thesis, Univ. Helsinki (2013).
Easterbrook, J. D. & Klein, S. L. Immunological mechanisms mediating hantavirus persistence in rodent reservoirs. PLoS Pathog. 4, e1000172 (2008).
Easterbrook, J. D., Zink, M. C. & Klein, S. L. Regulatory T cells enhance persistence of the zoonotic pathogen Seoul virus in its reservoir host. Proc. Natl Acad. Sci. USA 104, 15502–15507 (2007).
Schountz, T. et al. Regulatory T cell-like responses in deer mice persistently infected with Sin Nombre virus. Proc. Natl Acad. Sci. USA 104, 15496–15501 (2007). References 58 and 59 are the first to describe the role of T cells in the reservoir hosts of hantaviruses.
Guivier, E. et al. TNF-α expression and promoter sequences reflect the balance of tolerance/resistance to Puumala hantavirus infection in European bank vole populations. Infect. Genet. Evol. 10, 1208–1217 (2010).
Au, R. Y., Jedlicka, A. E., Li, W., Pekosz, A. & Klein, S. L. Seoul virus suppresses NF-κB-mediated inflammatory responses of antigen presenting cells from Norway rats. Virology 400, 115–127 (2010).
Li, W. & Klein, S. L. Seoul virus-infected rat lung endothelial cells and alveolar macrophages differ in their ability to support virus replication and induce regulatory T cell phenotypes. J. Virol. 86, 11845–11855 (2012).
Matthys, V. & Mackow, E. R. Hantavirus regulation of type I interferon responses. Adv. Virol. 2012, 524024 (2012).
Martinez, V. P. et al. Person-to-person transmission of Andes virus. Emerg. Infect. Dis. 11, 1848–1853 (2005).
Sinisalo, M. et al. Headache and low platelets in a patient with acute leukemia. J. Clin. Virol. 48, 159–161 (2010).
Van Loock, F., Thomas, I., Clement, J., Ghoos, S. & Colson, P. A case-control study after a hantavirus infection outbreak in the south of Belgium: who is at risk? Clin. Infect. Dis. 28, 834–839 (1999).
Vapalahti, K., Virtala, A. M., Vaheri, A. & Vapalahti, O. Case-control study on Puumala virus infection: smoking is a risk factor. Epidemiol. Infect. 138, 576–584 (2010).
Vapalahti, O. et al. Hantavirus infections in Europe. Lancet Infect. Dis. 3, 653–661 (2003).
Peters, C. J. & Khan, A. S. Hantavirus pulmonary syndrome: the new American hemorrhagic fever. Clin. Infect. Dis. 34, 1224–1231 (2002).
Bi, Z., Formenty, P. B. H. & Roth, C. E. Hantavirus infection: a review and global update. J. Infect. Dev. Ctries 2, 3–23 (2008).
Kruger, D. H., Schönrich, G. & Klempa, B. Human pathogenic hantaviruses and prevention of infection. Hum. Vaccin. 7, 685–693 (2011).
Duchin, J. S. et al. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330, 949–955 (1994).
MacNeil, A., Nichol, S. T. & Spiropoulou, C. F. Hantavirus pulmonary syndrome. Virus Res. 162, 138–147 (2011).
Knust, B., Macnell, A. & Rollin, P. E. Hantavirus pulmonary syndrome clinical findings: evaluation a surveillance case definition. Vector Borne Zoonotic Dis. 12, 393–399 (2012).
Simpson, S. Q., Spikes, L., Patel, S. & Faruqi, I. Hantavirus pulmonary syndrome. Infect. Dis. North Am. 24, 159–173 (2010).
Rasmuson, J. et al. Time to revise the paradigm of hantavirus pulmonary syndrome caused by European hantavirus. Eur. J. Clin. Microbiol. Infect. Dis. 30, 685–690 (2011). This discussion points out that HCPS and HFRS seem to cause the same disease.
Klempa, B. et al. Complex evolution and epidemiology of Dobrava-Belgrade hantavirus: definition of genotypes and their characteristics. Arch. Virol. 158, 521–529 (2013).
Vaheri, A., Vapalahti, O. & Plyusnin, A. How to diagnose hantavirus infections and detect them in rodents and insectivores. Rev. Med. Virol. 18, 277–288 (2008).
Outinen, T. K. et al. Plasma cell-free DNA levels are elevated in acute Puumala hantavirus infection. PLoS ONE 7, e31455 (2012).
Kanerva, M., Mustonen, J. & Vaheri, A. Pathogenesis of Puumala and other hantavirus infections. Rev. Med. Virol. 8, 67–86 (1998).
Zaki, S. R. et al. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146, 552–579 (1995).
Kanerva, M. et al. Pulmonary involvement in nephropathia epidemica: radiological findings and their clinical correlations. Clin. Nephrol. 46, 369–378 (1996).
Shrivastava-Ranjan, P., Rollin, P. E. & Spiropoulou, C. F. Andes virus disrupts the endothelial cell barrier by induction of vascular endothelial growth factor and downregulation of VE-cadherin. J. Virol. 84, 11227–11234 (2010).
Gavrilovskaya, I., Gorbunova, E., Koster, F. & Mackow, E. Elevated VEGF levels in pulmonary edema fluid and PBMCs from patients with acute hantavirus pulmonary syndrome. Adv. Virol. 2012, 674360 (2012).
Gavrilovskaya, I. N., Gorbunova, E. E. & Mackow, E. R. Pathogenic hantaviruses direct the adherence of quiescent platelets to infected endothelial cells. J. Virol. 84, 4832–4839 (2010).
Laine, O. et al. Platelet ligands and ADAMTS13 during Puumala hantavirus infection and associated thrombocytopenia. Blood Coagul. Fibrinolysis 22, 468–472 (2011).
Laine, O. et al. Enhanced thrombin formation and fibrinolysis during acute Puumala hantavirus infection. Thromb. Res. 126, 154–158 (2010).
Krautkrämer, E., Grouls, S., Stein, N., Reiser, J. & Zeier, M. Pathogenic Old World hantaviruses infect renal glomerular and tubular cells and induce disassembling of cell-to-cell contacts. J. Virol. 85, 9811–9823 (2011).
Valdivieso, F. et al. Neutralizing antibodies in survivors of Sin Nombre and Andes hantavirus infection. Emerg. Infect. Dis. 12, 166–168 (2006).
Terajima, M. & Ennis, F. A. T cells and pathogenesis of hantavirus cardiopulmonary syndrome and hemorrhagic fever with renal syndrome. Viruses 3, 1059–1073 (2011).
Rasmuson, J. et al. Presence of activated airway T lymphocytes in human Puumala hantavirus disease. Chest 140, 715–722 (2011).
Tuuminen, T. et al. Human CD8+ T cell memory generation in Puumala hantavirus infection occurs after the acute phase and is associated with boosting of EBV-specific CD8+ memory T cells. J. Immunol. 179, 1988–1995 (2007).
Van Epps, H. L. et al. Long-lived memory T lymphocyte responses after hantavirus infection. J. Exp. Med. 196, 579–588 (2002).
Manigold, T. et al. Highly differentiated, resting Gn-specific memory CD8+ T cells persist years after infection by Andes hantavirus. PLoS Pathog. 6, e1000779 (2010).
Akira, S., Hirano, T. Taga, T. & Kishimoto, T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 4, 2860–2867 (1990).
Borges, A. A. et al. Role of mixed Th1 and Th2 serum cytokines on pathogenesis and prognosis of hantavirus pulmonary syndrome. Microbes Infect. 10, 1150–1157 (2008).
Linderholm, M., Ahlm, C., Settergren, B., Waage, A. & Tärnvik, A. Elevated plasma levels of tumor necrosis factor (TNF)-α, soluble TNF receptors, interleukin (IL)-6, and IL-10 in patients with hemorrhagic fever with renal syndrome. J. Infect. Dis. 173, 38–43 (1996).
Outinen, T. K. et al. The severity of Puumala hantavirus induced nephropathia epidemica can be better evaluated using plasma interleukin-6 than C-reactive protein determinations. BMC Infect. Dis. 10, 132 (2010).
Sadeghi, M. et al. Cytokine expression during early and late phase of acute Puumala hantavirus infection. BMC Immunol. 12, 65 (2011).
Mäkelä, S. et al. Urinary excretion of interleukin-6 correlates with proteinuria in acute Puumala hantavirus-induced nephritis. Am. J. Kidney Dis. 43, 809–816 (2004).
Prescott, J. et al. The adaptive immune response does not influence hantavirus disease or persistence in Syrian hamster. Immunology http://dx.doi.org/10.1111/imm.12116 (2013).
Tsukada, H. et al. Ligation of endothelial αvβ3 integrin increases capillary hydraulic conductivity in single microvessels of rat lung. J. Clin. Invest. 91, 103–109 (1995).
Bossi, F. et al. Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J. Immunol. 173, 6921–6927 (2004).
Paakkala, A., Mustonen, J., Viander, M., Huhtala, H. & Pasternack, A. Complement activation in nephropathia epidemica caused by Puumala hantavirus. Clin. Nephrol. 53, 424–431 (2000).
Sane, J. et al. Complement activation in Puumala hantavirus infection correlates with disease severity. Ann. Med. 44, 468–475 (2012).
Mantovani, A., Garlanda, C. & Bottazzi, B. Pentraxin 3, a non-redundant soluble pattern recognition receptor involved in innate immunity. Vaccine 21 (Suppl. 2), S43–S47 (2003).
Outinen, T. K. et al. High pentraxin-3 plasma levels associate with thrombocytopenia in acute Puumala hantavirus-induced nephropathia epidemica. Eur. J. Clin. Microbiol. Infect. Dis. 31, 957–963 (2012).
Hepojoki, J. et al. Acute hantavirus infection induces the production of galectin-3 binding protein (90K/Mac-2BP). Abstract O3-6. IX International Conference on HFRS, HPS & Hantaviruses. (2013).
Mellor, A. L. & Munn, D. H. IDO expression by dendritic cells: tolerance and tryphophan catabolism. Nature Rev. Immunol. 4, 762–774 (2004).
Outinen, T. K. et al. High activity of indoleamine 2,3-dioxygenase is associated with renal insufficiency in Puumala hantavirus induced nephropathia epidemica. J. Med. Virol. 83, 731–737 (2011).
Klingström, J. et al. Loss of cell membrane integrity in Puumala hantavirus-infected patients correlates with levels of epithelial cell apoptosis and perforin. J. Virol. 80, 8279–8282 (2006).
Groen, J. et al. Hantavirus antigen detection in kidney biopsies from patients with nephropathia epidemica. Clin. Nephrol. 46, 379–383 (1996).
Ala-Houhala, I. et al. Increased glomerular permeability in patients with nephropathia epidemica caused by Puumala hantavirus. Nephrol. Dial. Transplant. 17, 246–252 (2002).
Gupta, S. et al. Hantavirus-infection confers resistance to cytotoxic lymphocyte-mediated apoptosis. PLoS Pathog. 9, e1003272 (2013).
Björkström, N. K. et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 208, 13–21 (2010).
Gavrilovskaya, I. N., Gorbunova, E. E., Mackow, N. A. & Mackow, E. R. Hantaviruses direct endothelial cell permeability by sensitizing cells to the vascular permeability factor VEGF, while angiopoietin 1 and sphingosine 1-phosphate inhibit hantavirus-directed permeability. J. Virol. 82, 5797–5806 (2008).
Gorbunova, E. E., Gavrilovskaya, I. N., Pepini, T. & Mackow, E. R. VEGFR2 and Src kinase inhibitors suppress Andes virus-induced endothelial cell permeability. J. Virol. 85, 2296–2303 (2010).
Antonen, J. et al. A severe case of Puumala hantavirus infection successfully treated with bradykinin receptor antagonist icatibant. Scand. J. Infect. Dis. 45, 494–496 (2013). This is the first report on the treatment of hantavirus infection by inhibition of vascular leakage.
Sironen, T. & Plyusnin, A. in Bunyaviridae: Molecular and Cellular Biology (eds Plyusnin, A. & Elliott, R. M.) 61–94 (Caister Academic, 2011).
Ramsden, C., Holmes, E. C. & Charleston, M. A. Hantavirus evolution in relation to its rodent and insectivore hosts: no evidence for codivergence. Mol. Biol. Evol. 26, 143–153 (2009).
Blasdell, K., Henttonen, H. & Buchy, P. in New Frontiers of Molecular Epidemiology of Infectious Diseases (eds Morand, S., Beaudeau, F. & Cabaret, J.) 179–216 (Springer, 2012).
Kanerva, M., Vaheri, A., Mustonen, J. & Partanen, J. High-producer allele of tumour necrosis factor-α is part of the susceptibility MHC haplotype in severe Puumala virus-induced nephropathia epidemica. Scand. J. Infect. Dis. 30, 532–534 (1998).
Guo, W. P. et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 9, e1003159 (2013). The phylogenetic analysis presented in this article suggests that hantaviruses first appeared in bats or in moles and shrews before emerging in rodents.
Klempa, B. et al. Occurrence of renal and pulmonary syndrome in a region of Northeast Germany where Tula hantavirus circulates. J. Clin. Microbiol. 41, 4894–4897 (2003).
Deter, J. et al. Association between the DQA MHC class II gene and Puumala virus infection in Myodes glareolus, the bank vole. Infect. Genet. Evol. 8, 450–458 (2008).
Mustonen, J. et al. Genetic susceptibility to severe course of nephropathia epidemica caused by Puumala hantavirus. Kidney Int. 49, 217–221 (1996). This is the first work to relate the severity of an acute viral infection to a given HLA haplotype.
Wang, M. L. et al. Genetic susceptibility to haemorrhagic fever with renal syndrome caused by Hantaan virus in Chinese Han population. Int. J. Immunogenet. 36, 227–229 (2009).
Korva, M., Saksida, A., Kunilo, S., Vidan Jeras, B. & Avsic-Zupanc, T. HLA-associated hemorrhagic fever with renal syndrome disease progression in Slovenian patients. Clin. Vaccine Immunol. 18, 1435–1440 (2011). This large study shows that the severities of DOBV-mediated HFRS and PUUV-mediated HFRS are associated with different HLA haplotypes.
Mäkelä, S. et al. Human leucocyte antigen-B8-DR3 is a more important risk factor for severe Puumala hantavirus infection than the tumor necrosis factor-α(−308) G/A polymorphism. J. Infect. Dis. 186, 843–846 (2002).
Maes, P., et al. Tumor necrosis factor-α genetic predisposing factors can influence clinical severity in nephropathia epidemica. Viral Immunol. 19, 558–564 (2006).
Borges, A. A. et al. Association of −308G/A polymorphism in the tumor necrosis factor-α gene promoter with susceptibility to development of hantavirus cardiopulmonary syndrome in the Ribeirão Preto region, Brazil. Arch. Virol. 155, 971–975 (2010).
Mäkelä, S. et al. Polymorphism of the cytokine genes in hospitalized patients with Puumala hantavirus infection. Nephrol. Dial. Transplant. 16, 1368–1373 (2001).
Laine, O. et al. Polymorphism of PAI-1 and platelet GP Ia may associate with impairment of renal function and thrombocytopenia in Puumala hantavirus infection. Thromb. Res. 129, 611–615 (2012).
Acknowledgements
The authors' research was supported by European Commission Project QLK2-CT-2002-01358 (grant GOCE-CT-2003-010284 EDEN); the European Union (grant FP7-261504 EDENext; the project is catalogued as EDENext140 by the EDENext Steering Committee); The Academy of Finland; the Sigrid Jusélius Foundation; the Magnus Ehrnrooth Foundation; the Helsinki University Central Hospital, Finland; and Tampere University Hospital, Finland. The authors thank J. Antonen, P. Arstila, S. Butcher, F. Ennis, T. Hautala, J. Huiskonen, M. Hurme, J. Klingström, H. Lankinen, D. Libraty, Å. Lundkvist, A. Pasternack, A. Plyusnin, O. Vapalahti (as well as their former and current students and technicians) and the authors' former and current students and technicians for collaboration on research concerning hantavirus infections.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Thrombocytopenia
-
A low platelet count in the blood.
- Cytotoxic CD8+ T cells
-
A subpopulation of T cells that kills abnormal (infected, cancerous or damaged) cells as part of the adaptive immune system.
- Complement system
-
Serum proteins that can protect against infection as part of innate immunity. This efficiently regulated system has three major pathways, namely the classical, alternative and the lectin-dependent pathways.
- Clathrin-dependent endocytosis
-
A pathway for the internalization of plasma membrane proteins. Receptors cluster in membrane domains containing a polymeric clathrin coat and a complex of adaptor proteins and GTPases, leading to membrane invagination and scission to form a clathrin-coated vesicle containing the internalized receptors.
- Early endosomes
-
Intracellular vesicular structures that are precursors of mature endosomes and have an important role in endocytosis.
- P bodies
-
Cytoplasmic foci that are thought to store and degrade translationally repressed RNA.
- Regulatory T cells
-
A subpopulation of T cells that modulates the immune system. These are CD4+CD25+FOX3P+ cells that have suppressor activity towards other T cells either by cell–cell contact or by cytokine release.
- Pattern recognition receptors
-
A highly diverse group of soluble and surface-bound proteins that can detect specific molecular surface structures.
- Proteinuria
-
The presence of excess protein in the urine.
- Leukocytosis
-
A condition in which the number of white blood cells is above the normal range. Frequently associated with inflammation.
- Fibrinolysis
-
A process whereby fibrin clots (the products of blood coagulation) are broken down.
- Acute-phase proteins
-
A group of proteins, including C-reactive protein and fibrinogen, the blood concentration of which changes in response to trauma, inflammation or disease. These proteins can be inhibitors or mediators of inflammation.
- Caspases
-
A family of cysteine proteases that execute cell death events in the apoptotic pathway.
Rights and permissions
About this article
Cite this article
Vaheri, A., Strandin, T., Hepojoki, J. et al. Uncovering the mysteries of hantavirus infections. Nat Rev Microbiol 11, 539–550 (2013). https://doi.org/10.1038/nrmicro3066
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro3066
This article is cited by
-
Disparate macrophage responses are linked to infection outcome of Hantan virus in humans or rodents
Nature Communications (2024)
-
Therapeutic potential for P2Y2 receptor antagonism
Purinergic Signalling (2023)
-
SuPAR mediates viral response proteinuria by rapidly changing podocyte function
Nature Communications (2023)
-
Trends and focuses of hantavirus researches: a global bibliometric analysis and visualization from 1980 to 2020
Archives of Public Health (2022)
-
VKORC1 mutations in rodent populations of a tropical city-state as an indicator of anticoagulant rodenticide resistance
Scientific Reports (2022)