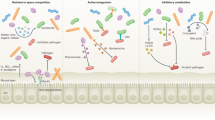
Key Points
-
Many infections self-resolve, but in most cases the mechanism of this resolution is unknown.
-
There are several theories concerning how homeostasis might be restored in the oral cavity, skin, and intestinal and urogenital tracts, the sites that collectively contain the bulk of the human microbiome.
-
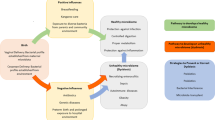
The shifts in microbial abundance and diversity are often linked with host immunity, and together these influence whether we remain healthy or become sick.
-
By correlating changes in the microbiota with a diseased state, it should be possible to develop novel interventions that help restore health.
-
Mechanisms that seem to be involved in the restoration of homeostasis include: bacterial co-aggregation; production of biosurfactants, antimicrobials and signalling molecules that target the host or pathogens; competitive exclusion of pathogens; immunomodulation; and factors that increase tight junction barrier function on the host epithelia.
Abstract
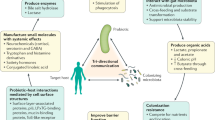
In a healthy host, a balance exists between members of the microbiota, such that potential pathogenic and non-pathogenic organisms can be found in apparent harmony. During infection, this balance can become disturbed, leading to often dramatic changes in the composition of the microbiota. For most bacterial infections, nonspecific antibiotics are used, killing the non-pathogenic members of the microbiota as well as the pathogens and leading to a substantial delay in the restoration of a healthy microbiota. However, in some cases, infections can self-resolve without the intervention of antibiotics. In this Review, we explore the mechanisms underlying microbiota restoration following insult (antibiotic or otherwise) to the skin, oral cavity, and gastrointestinal and urogenital tracts, highlighting recovery by natural processes and after probiotic administration.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dominguez-Bello, M. G. et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl Acad. Sci. USA 107, 11971–11975 (2010).
Nakamura, N. et al. Molecular ecological analysis of faecal bacterial populations from term infants fed formula supplemented with selected blends of prebiotics. Appl. Environ. Microbiol. 75, 1121–1128 (2009).
Magne, F. et al. A longitudinal study of infant faecal microbiota during weaning. FEMS Microbiol. Ecol. 58, 563–571 (2006).
Turnbaugh, P. J. et al. A core gut microbiome in obese and lean twins. Nature 457, 480–484 (2009). The first insight into microbial differences and similarities between twins, and how organisms might influence obesity.
Lodinová-Zádníková, R., Cukrowska, B. & Tlaskalova-Hogenova, H. Oral administration of probiotic Escherichia coli after birth reduces frequency of allergies and repeated infections later in life (after 10 and 20 years). Int. Arch. Allergy Immunol. 131, 209–211 (2003).
Kalliomäki, M. et al. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of allergic diseases by probiotics. J. Nutr. 140, S713–S721 (2010).
Aumeunier, A. et al. Systemic Toll-like receptor stimulation suppresses experimental allergic asthma and autoimmune diabetes in NOD mice. PLoS ONE 5, e11484 (2010).
Donskey, C. J. The role of the intestinal tract as a reservoir and source for transmission of nosocomial pathogens. Clin. Infect. Dis. 39, 219–226 (2004).
Nowrouzian, F. L., Adlerberth, I. & Wold, A. E. Enhanced persistence in the colonic microbiota of Escherichia coli strains belonging to phylogenetic group B2: role of virulence factors and adherence to colonic cells. Microbes Infect. 8, 834–840 (2006).
Stebbings, S. et al. Comparison of the faecal microflora of patients with ankylosing spondylitis and controls using molecular methods of analysis. Rheumatology (Oxford) 41, 1395–1401 (2002).
Turnbaugh, P. J. et al. The human microbiome project. Nature 449, 804–810 (2007).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009). A comprehensive analysis of the human microbiota and documentation of its variability across sites and between individuals.
Gao, Z., Tseng, C. H., Pei, Z. & Blaser, M. J. Molecular analysis of human forearm superficial skin bacterial biota. Proc. Natl Acad. Sci. USA 104, 2927–2932 (2007).
Grice, E. A. et al. Topographical and temporal diversity of the human skin microbiome. Science 324, 1190–1192 (2009).
Caporaso, J. G. et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl Acad. Sci. USA 3 Jun 2010 (doi:10.1073/pnas.1000080107).
Brotman, R. M., Ravel, J., Cone, R. A. & Zenilman, J. M. Rapid fluctuation of the vaginal microbiota measured by Gram stain analysis. Sex. Transm. Infect. 86, 297–302 (2010).
Hummelen, R. et al. Deep sequencing of the vaginal microbiota of women with HIV. PLoS ONE 5, e12078 (2010). First use of Illumina sequencing on vaginal samples and description of clusters associated with health and BV.
Kim, T. K. et al. Heterogeneity of vaginal microbial communities within individuals. J. Clin. Microbiol. 47, 1181–1189 (2009).
Ptacnik, R. et al. Diversity predicts stability and resource use efficiency in natural phytoplankton communities. Proc. Natl Acad. Sci. USA 105, 5134–5138 (2008).
Reid, G., Bruce, A. W., Cook, R. L. & Llano, M. Effect on urogenital flora of antibiotic therapy for urinary tract infection. Scand. J. Infect. Dis. 22, 43–47 (1990).
Vermeulen, G. M., van Zwet, A. A. & Bruinse, H. W. Changes in the vaginal flora after two percent clindamycin vaginal cream in women at high risk of spontaneous preterm birth. BJOG 108, 697–700 (2001).
Anukam, K. et al. Augmentation of antimicrobial metronidazole therapy of bacterial vaginosis with oral probiotic Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14: randomized, double-blind, placebo controlled trial. Microbes Infect. 8, 1450–1454 (2006).
Martinez, R. C. et al. Improved cure of bacterial vaginosis with single dose of tinidazole (2 g), Lactobacillus rhamnosus GR-1, and Lactobacillus reuteri RC-14: a randomized, double-blind, placebo-controlled trial. Can. J. Microbiol. 55, 133–138 (2009).
Wolvers, D. et al. Guidance for substantiating the evidence for beneficial effects of probiotics: prevention and management of infections by probiotics. J. Nutr. 140, S698–S712 (2010).
Bouwstra, J. A. & Ponec, M. The skin barrier in healthy and diseased state. Biochim. Biophys. Acta. 1758, 2080–2095 (2006).
Callard, R. E. & Harper, J. I. The skin barrier, atopic dermatitis and allergy: a role for Langerhans cells? Trends Immunol. 28, 294–298 (2007).
Yao, D. et al. Molecular characterization of Staphylococcus aureus isolates causing skin and soft tissue infections (SSTIs). BMC Infect. Dis. 10, 133 (2010).
Iwatsuki, K., Yamasaki, O., Morizane, S. & Oono, T. Staphylococcal cutaneous infections invasion, evasion and aggression. J. Dermatol. Sci. 42, 203–214 (2006).
Grice, E. A. et al. Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc. Natl Acad. Sci. USA 107, 14799–14804 (2010). An important study showing a selective shift in the microbiota of the skin in association with immune responses to wounds.
Howell-Jones, R. S. et al. A review of the microbiology, antibiotic usage and resistance in chronic skin wounds. J. Antimicrob. Chemother. 55, 143–149 (2005).
Guggenheim, M. et al. Changes in bacterial isolates from burn wounds and their antibiograms: a 20-year study (1986–2005). Burns 35, 53–60 (2009).
Altoparlak, U., Erol, S., Akcay, M. N., Celebi, F. & Kadanali, A. The time-related changes of antimicrobial resistance patterns and predominant bacterial profiles of burn wounds and body flora of burned patients. Mol. Biol. Rep. 30, 660–664 (2004).
Wolcott, R. D. et al. Chronic wounds and the medical biofilm paradigm. J. Wound Care 19, 45–53 (2010).
Baisley, K. et al. Bacterial vaginosis in female facility workers in north-western Tanzania: prevalence and risk factors. Sex. Transm. Infect. 85, 370–375 (2009).
Ramjee, G., Karim, S. S. & Sturm, A. W. Sexually transmitted infections among sex workers in KwaZulu-Natal, South Africa. Sex. Transm. Dis. 25, 346–349 (1998).
Aboud, S. et al. Genital tract infections among HIV-infected pregnant women in Malawi, Tanzania and Zambia. Int. J. STD AIDS 19, 824–832 (2008).
Amsel, R. et al. Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am. J. Med. 74, 14–22 (1983).
Nugent, R. P., Krohn, M. A. & Hillier, S. L. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of Gram stain interpretation. J. Clin. Microbiol. 29, 297–301 (1991).
Gómez, L. M. et al. Evidence of a gene-environment interaction that predisposes to spontaneous preterm birth: a role for asymptomatic bacterial vaginosis and DNA variants in genes that control the inflammatory response. Am. J. Obstet. Gynecol. 202, 386.e1–386.e6 (2010).
Stock, S. J. et al. Elafin (SKALP/Trappin-2/proteinase inhibitor-3) is produced by the cervix in pregnancy and cervicovaginal levels are diminished in bacterial vaginosis. Reprod. Sci. 16, 1125–1134 (2009).
Iqbal, S. M. et al. Elevated elafin/trappin-2 in the female genital tract is associated with protection against HIV acquisition. AIDS 23, 1669–1677 (2009).
Ness, R. B. et al. A cluster analysis of bacterial vaginosis-associated microflora and pelvic inflammatory disease. Am. J. Epidemiol. 162, 585–590 (2005).
Zozaya-Hinchliffe, M., Lillis, R., Martin, D. H. & Ferris, M. J. Quantitative PCR assessments of bacterial species in women with and without bacterial vaginosis. J. Clin. Microbiol. 48, 1812–1819 (2010).
Wolrath, H., Borén, H., Hallén, A. & Forsum, U. Trimethylamine content in vaginal secretion and its relation to bacterial vaginosis. APMIS 110, 819–824 (2002).
Livengood, C. H. 3rd et al. Effectiveness of two tinidazole regimens in treatment of bacterial vaginosis: a randomized controlled trial. Obstet. Gynecol. 110, 302–309 (2007).
Schwebke, J. R. & Desmond, R. A. A randomized trial of the duration of therapy with metronidazole plus or minus azithromycin for treatment of symptomatic bacterial vaginosis. Clin. Infect. Dis. 44, 213–219 (2007).
Anukam, K. C. et al. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes Infect. 8, 2772–2776 (2006).
Reid, G. et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunol. Med. Microbiol. 35, 131–134 (2003).
Schippa, S. et al. A distinctive 'microbial signature' in celiac pediatric patients. BMC Microbiol. 10, 175 (2010). This work implies that treatment of Bacteroides spp. and E. coli with antibiotics or probiotics might ameliorate coeliac disease.
Sanz, Y. et al. Differences in faecal bacterial communities in coeliac and healthy children as detected by PCR and denaturing gradient gel electrophoresis. FEMS Immunol. Med. Microbiol. 51, 562–568 (2007).
Candela, M. et al. High taxonomic level fingerprint of the human intestinal microbiota by ligase detection reaction – universal array approach. BMC Microbiol. 10, 116 (2010).
Hartman, A. L. Human gut microbiome adopts an alternative state following small bowel transplantation. Proc. Natl Acad. Sci. USA 106, 17187–17192 (2009).
Rickard, A. H., Gilbert, P., High, N. J., Kolenbrander, P. E. & Handley, P. S. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 11, 94–100 (2003).
Macklaim, J. et al. At the crossroads of vaginal health and disease, the genome sequence of Lactobacillus iners AB-1. Proc. Natl Acad. Sci. USA 8 Nov 2010 (doi:10.1073/pnas.1000086107).
Darveau, R. P. Periodontitis: a polymicrobial disruption of host homeostasis. Nature Rev. Microbiol. 8, 481–490 (2010).
Kolenbrander, P. E., Palmer, R. J. Jr, Periasamy, S. & Jakubovics, N. S. Oral multispecies biofilm development and the key role of cell–cell distance. Nature Rev. Microbiol. 8, 471–480 (2010).
Cadieux, P. A., Burton, J., Devillard, E. & Reid, G. Lactobacillus by-products inhibit the growth and virulence of uropathogenic Escherichia coli. J. Physiol. Pharmacol. 60 (Suppl. 6), 13–18 (2009).
Keijser, B. J. F. et al. Pyrosequencing analysis of the oral microflora in healthy adults. J. Dent. Res. 87, 1016–1020 (2008).
Zaura, E., Kijser, B. J. F., Huse, S. M. & Crielaard, W. Defining the healthy core microbiome of oral microbial communities. BMC Microbiol. 9, 259 (2009). This study reveals unexpected diversity in the microbiota in the oral cavity.
Van Hoogmoed, C. G., Van der Kuijl-Booii, M., Van der Mei, H. C. & Busscher, H. J. Inhibition of Streptococcus mutans NS adhesion to glass with and without a salivary conditioning film by biosurfactant-releasing Streptococcus mitis strains. Appl. Environ. Microbiol. 66, 659–663 (2000).
Van Hoogmoed, C. G., Van der Mei, H. C. & Busscher, H. J. The influence of biosurfactants released by S. mitis BMS on the adhesion of pioneer strains and cariogenic bacteria. Biofouling 20, 261–267 (2004).
Velraeds, M. M., Van de Belt-Gritter, B., Van der Mei, H. C., Reid, G. & Busscher, H. J. Interference in initial adhesion of uropathogenic bacteria and yeasts to silicone rubber by a Lactobacillus acidophilus biosurfactant. J. Med. Microbiol. 47, 1081–1085 (1998).
Reid, G. & Tieszer, C. Preferential adhesion of bacteria from a mixed population to a urinary catheter. Cells Mater. 3, 171–176 (1993).
Saunders, S. G., Bocking, A., Challis, J. & Reid, G. Disruption of Gardnerella vaginalis biofilms by Lactobacillus. Colloids Surf. B Biointerfaces 55, 138–142 (2007).
Banat, I. M. et al. Microbial biosurfactants production, applications and future potential. Appl. Microbiol. Biotechnol. 87, 27–44 (2010).
Asaduzzaman, S. M. & Sonomoto, K. Lantibiotics: diverse activities and unique modes of action. J. Biosci. Bioeng. 107, 475–487 (2009).
Tabasco, R., García-Cayuela, T., Peláez, C. & Requena, T. Lactobacillus acidophilus La-5 increases lactacin B production when it senses live target bacteria. Int. J. Food Microbiol. 132, 109–116 (2009).
Kjos, M., Snipen, L., Salehian, Z., Nes, I. F. & Diep, D. B. The Abi proteins and their involvement in bacteriocin self-immunity. J. Bacteriol. 192, 2068–2076 (2010).
Hasper, H. E. et al. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science 313, 1636–1637 (2006).
Corr, S. C. et al. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc. Natl Acad. Sci. USA 104, 7617–2761 (2007). An elegant illustration of bacteriocin helping an organism to protect the gut from infection.
Xu, H. Y. et al. Antagonistic potential against pathogenic microorganisms and hydrogen peroxide production of indigenous lactobacilli isolated from vagina of Chinese pregnant women. Biomed. Environ. Sci. 21, 365–371 (2008).
Martín, R. & Suárez, J. E. Biosynthesis and degradation of H2O2 by vaginal lactobacilli. Appl. Environ. Microbiol. 76, 400–405 (2010).
Voltan, S. et al. Lactobacillus crispatus M247-derived H2O2 acts as a signal transducing molecule activating peroxisome proliferator activated receptor-γ in the intestinal mucosa. Gastroenterology 135, 1216–1227 (2008).
Ferry, S. A., Holm, S. E., Stenlund, H., Lundholm, R. & Monsen, T. J. The natural course of uncomplicated lower urinary tract infection in women illustrated by a randomized placebo controlled study. Scand. J. Infect. Dis. 36, 296–301 (2004). An interesting paper describing the spontaneous resolution of a urinary tract infection.
Nicolle, L. E., Zhanel, G. G. & Harding, G. K. Microbiological outcomes in women with diabetes and untreated asymptomatic bacteriuria. World J. Urol. 24, 61–65 (2006).
Cegelski, L., Marshall, G. R., Eldridge, G. R. & Hultgren, S. J. The biology and future prospects of antivirulence therapies. Nature Rev. Microbiol. 6, 17–27 (2008).
Cegelski, L. et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nature Chem. Biol. 5, 913–919 (2009).
Laughton, J. M., Devillard, E., Heinrichs, D. E., Reid, G. & McCormick, J. K. Inhibition of expression of a staphylococcal superantigen-like protein by a soluble factor from Lactobacillus reuteri. Microbiology 152, 1155–1167 (2006).
Liu, C. H., Lee, S. M., Vanlare, J. M., Kasper, D. L. & Mazmanian, S. K. Regulation of surface architecture by symbiotic bacteria mediates host colonization. Proc. Natl Acad. Sci. USA 105, 3951–3956 (2008). This study shows the crucial role of capsular polysaccharides in host–symbiont mutualism.
Aas, J., Gessert, C. E. & Bakken, J. S. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin. Infect. Dis. 36, 580–585 (2003).
Nieuwdorp, M. et al. Treatment of recurrent Clostridium difficile-associated diarrhoea with a suspension of donor faeces. Ned. Tijdschr. Geneeskd. 152, 1927–1932 (2008).
Van Nood, E., Speelman, P., Kuijper, E. J. & Keller, J. J. Struggling with recurrent Clostridium difficile infections: is donor faeces the solution? Euro Surveill. 14, 19316 (2009).
Khoruts, A., Dicksved, J., Jansson, J. K. & Sadowsky, M. J. Changes in the composition of the human faecal microbiome after bacteriotherapy for recurrent Clostridium difficile-associated diarrhea. J. Clin. Gastroenterol. 44, 354–360 (2010).
Pamer, E. G. Immune responses to commensal and environmental microbes. Nature Immunol. 8, 1173–1178 (2007). An excellent description of how the host's immune system is faced with differentiating between microbial types that may or may not attack the host.
Ryu, J. H. et al. Innate immune homeostasis by the homeobox gene Caudal and commensal-gut mutualism in Drosophila. Science 319, 777–782 (2008).
Round, J. L., O'Connell, R. M. & Mazmanian, S. K. Coordination of tolerogenic immune responses by the commensal microbiota. J. Autoimmun. 34, J220–J225 (2010).
Mazmanian, S. K., Round, J. L. & Kasper, D. L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 453, 620–625 (2008).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Lai, Y. et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nature Med. 15, 1377–1382 (2009).
Blaser, M. J. & Falkow, S. What are the consequences of the disappearing human microbiota? Nature Rev. Microbiol. 7, 887–894 (2009). An excellent and topical review raising questions about microbial evolution and human survival.
Hafez, M. et al. The K5 capsule of Escherichia coli strain Nissle 1917 is important in mediating interactions with intestinal epithelial cells and chemokine induction. Infect. Immun. 77, 2995–3003 (2009).
Nazli, A. et al. Exposure to HIV-1 directly impairs mucosal epithelial barrier integrity allowing microbial translocation. PLoS Pathog. 6, e1000852 (2010).
Karczewski, J. et al. Regulation of human epithelial tight junction proteins by Lactobacillus plantarum in vivo and protective effects on the epithelial barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 298, G851–G859 (2010).
Qin, H., Zhang, Z., Hang, X. & Jiang. Y. L. plantarum prevents enteroinvasive Escherichia coli-induced tight junction proteins changes in intestinal epithelial cells. BMC Microbiol. 9, 63 (2009).
Garrett, W. S., Gordon, J. I. & Glimcher, L. H. Homeostasis and inflammation in the intestine. Cell 140, 859–870 (2010). An insightful paper about the different mechanisms by which the microorganisms and the host can influence the health–disease paradigm.
Reid, G. The importance of guidelines in the development and application of probiotics. Curr. Pharm. Des. 11, 11–16 (2005). An important outline of what is required in order to call a product 'probiotic'.
Shen, J., Ran, H. Z., Yin, M. H., Zhou, T. X. & Xiao, D. S. Meta-analysis: the effect and adverse events of lactobacilli versus placebo in maintenance therapy for Crohn disease. Intern. Med. J. 39, 103–109 (2009).
Lionetti, E. et al. Role of probiotics in pediatric patients with Helicobacter pylori infection: a comprehensive review of the literature. Helicobacter 15, 79–87 (2010).
Twetman, S. & Stecksen-Blicks, C. Probiotics and oral health effects in children. Int. J. Pediatr. Dent. 18, 3–10 (2008).
Nase, L. et al. Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 35, 412–420 (2001).
Caglar, E., Cildir, S. K., Ergeneli, S., Sandalli, N. & Twetman, S. Salivary mutans streptococci and lactobacilli levels after the ingestion of the probiotic bacterium Lactobacillus reuteri ATCC 55730 by straws or tablets. Acta Odontol. Scand. 64, 314–318 (2006).
Collado, M. C., Isolauri, E., Laitinen, K. & Salminen, S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am. J. Clin. Nutr. 88, 894–899 (2008).
Luoto, R., Laitinen, K., Nermes, M. & Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br. J. Nutr. 103, 1792–1799 (2010).
Laitinen, K., Poussa, T. & Isolauri, E. & the Nutrition, Allergy, Mucosal Immunology and Intestinal Microbiota Group. Probiotics and dietary counselling contribute to glucose regulation during and after pregnancy: a randomised controlled trial. Br. J. Nutr. 101, 1679–1687 (2009).
Peroni, D. G. et al. Prevalence and risk factors for atopic dermatitis in preschool children. Br. J. Dermatol. 158, 539–543 (2008).
Rybal'chenko, O. V., Gusleva, O. R., Orlova, O. G., Blinova, Yu. A & Bondarenko, V. M. [Microecological and electron microscopic study of skin microbiota in patients with atopic dermatitis]. Zh. Mikrobiol. Epidemiol. Immunobiol. 1, 67–72 (2010) (in Russian).
Kim, J. Y. et al. Effect of probiotic mix (Bifidobacterium bifidum, Bifidobacterium lactis, Lactobacillus acidophilus) in the primary prevention of eczema: a double-blind, randomized, placebo-controlled trial. Pediatr. Allergy Immunol. 21, e386–e393 (2010).
Woo, S. I., Kim, J. Y., Lee, Y. J., Kim, N. S. & Hahn, Y. S. Effect of Lactobacillus sakei supplementation in children with atopic eczema–dermatitis syndrome. Ann. Allergy Asthma Immunol. 104, 343–348 (2010).
Abrahamsson, T. R. et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J. Allergy Clin. Immunol. 119, 1174–1180 (2007).
Rose, M. A. et al. Efficacy of probiotic Lactobacillus GG on allergic sensitization and asthma in infants at risk. Clin. Exp. Allergy 40, 1398–1405 (2010).
Morelli, L., Zonenenschain, D., Del Piano, M. & Cognein, P. Utilization of the intestinal tract as a delivery system for urogenital probiotics. J. Clin. Gastroenterol. 38, S107–S110 (2004).
Chau, T. A. et al. Toll-like receptor 2 ligands on the staphylococcal cell wall downregulate superantigen-induced T cell activation and prevent toxic shock syndrome. Nature Med. 15, 641–648 (2009). This paper raises an important concept: that pathogens might prefer to become commensals.
NIH HMP Working Group. The NIH Human Microbiome Project. Genome Res. 19, 2317–2323 (2009).
Burton, J. P., Chilcott, C. N., Moore, C. J., Speiser, G. & Tagg, J. R. A preliminary study of the effect of probiotic Streptococcus salivarius K12 on oral malodour parameters. J. Appl. Microbiol. 100, 754–764 (2006).
Caglar, E. et al. Effect of yogurt with Bifidobacterium DN-173 010 on salivary mutans streptococci and lactobacilli in young adults. Acta Odontol. Scand. 63, 317–320 (2005).
Luoto, R., Laitinen, K., Nermes, M. & Isolauri, E. Impact of maternal probiotic-supplemented dietary counselling on pregnancy outcome and prenatal and postnatal growth: a double-blind, placebo-controlled study. Br. J. Nutr. 4, 1–8 (2010).
Cox, M. J. et al. Lactobacillus casei abundance is associated with profound shifts in the infant gut microbiome. PLoS ONE 5, e8745 (2010).
Gianotti, L. et al. A randomized double-blind trial on perioperative administration of probiotics in colorectal cancer patients. World J. Gastroenterol. 16, 167–175 (2010).
Acknowledgements
The authors acknowledge support from the Natural Sciences and Engineering Research Council of Canada and Danone Canada. Input from J. Macklaim is appreciated. They also thank E. K. Costello and M. G. Dominguez-Bello for input into figure 1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Gregor Reid has received research support from Danone (although he is not assessing any of their products and does not receive any consulting fees from the company) and retains an association with Chr. Hansen (although he no longer has any ownership interest in Lactobacillus rhamnosus str. GR-1, Lactobacillus reuteri str. RC-14 or patents pertaining to urogenital applications).
Related links
Glossary
- Microbiota
-
The microorganisms that inhabit a region of the body.
- Sebaceous
-
Pertaining to sebum, the substance secreted by glands of the skin.
- Dysbiosis
-
An imbalance in the bacterial population at a naturally occurring site of colonization, often resulting in health problems.
- Eubiosis
-
The state of having a healthy population of bacteria naturally occurring in a body site.
- Probiotic
-
A live microorganism that, when administered in adequate amounts, confer a health benefit on the host.
- Homeostasis
-
A condition of equilibrium or stability in the internal environment of the body, which is maintained by adjusting physiological processes to counteract external changes.
- Corneocyte
-
A dead keratin-filled squamous cell of the stratum corneum.
- Keratinocyte
-
An epithelial cell that produces keratin in the process of differentiating into the corneocytes of the stratum corneum.
- Impetigo
-
A contagious bacterial skin infection characterized by the eruption of superficial pustules and the formation of thick yellow crusts.
- Furuncle
-
A localized suppurative staphylococcal skin infection originating in a gland or hair follicle and characterized by pain, redness and swelling.
- Toxic shock syndrome
-
A rare, life-threatening complication of bacterial infection that has been most often associated with the use of superabsorbent tampons and occasionally linked with the use of contraceptive sponges. Often resulting from toxins produced by staphylococci, but also from those produced by group A Streptococcus spp.
- Biofilm
-
A microbial community that is enveloped by extracellular polymer matrices produced by the microorganisms and that adheres to a surface.
- Bacterial vaginosis
-
An aberrant condition in the vagina that is characterized by pH >4.5, malodour and depletion of normal microbiota, particularly lactobacilli.
- Planktonic
-
Referring to microscopic organisms that are suspended in a liquid (for instance, media or a buffer).
- Adhesin
-
An external bacterial product that enables adhesion to and colonization of a host.
- Rhamnolipid
-
A biosurfactant produced by Pseudomonas spp.
- Cariogenic
-
Producing or promoting the development of dental caries.
- Free radical
-
An atom or molecule that has at least one unpaired electron and is therefore unstable and highly reactive.
- Eczema
-
Non-contagious inflammation of the skin, characterized chiefly by redness, itching and the outbreak of lesions that may discharge serous fluid.
Rights and permissions
About this article
Cite this article
Reid, G., Younes, J., Van der Mei, H. et al. Microbiota restoration: natural and supplemented recovery of human microbial communities. Nat Rev Microbiol 9, 27–38 (2011). https://doi.org/10.1038/nrmicro2473
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2473
This article is cited by
-
Strain-specific alterations in gut microbiome and host immune responses elicited by tolerogenic Bifidobacterium pseudolongum
Scientific Reports (2023)
-
Pregnancy outcomes after vaginal probiotic supplementation before frozen embryo transfer: a randomized controlled study
Scientific Reports (2023)
-
Experimental evidence of shikonin as a novel intervention for anti-inflammatory effects
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
It's all relative: analyzing microbiome compositions, its significance, pathogenesis and microbiota derived biofilms: Challenges and opportunities for disease intervention
Archives of Microbiology (2023)
-
In silico prospection of Lactobacillus acidophilus strains with potential probiotic activity
Brazilian Journal of Microbiology (2023)