Abstract
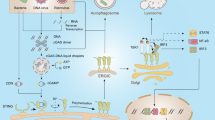
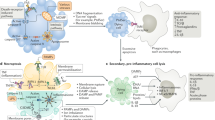
The modulation of host cell death pathways by bacteria has been recognized as a major pathogenicity mechanism. Among other strategies, bacterial pathogens can hijack the cell death machinery of host cells by influencing the signalling pathways that converge on the mitochondria. In particular, many bacterial proteins have evolved to interact in a highly specific manner with host mitochondria, thereby modulating the decision between cell life and death. In this Review, we explore the intimate interactions between bacterial pathogens and mitochondrial cell death pathways.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zychlinsky, A., Prevost, M. C. & Sansonetti, P. J. Shigella flexneri induces apoptosis in infected macrophages. Nature 358, 167–169 (1992). First publication describing programmed cell death of human cells (macrophages) in response to bacterial infection.
Muller, A. et al. Neisserial porin (PorB) causes rapid calcium influx in target cells and induces apoptosis by the activation of cysteine proteases. EMBO J. 18, 339–352 (1999).
Fan, T. et al. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J. Exp. Med. 187, 487–496 (1998). Describes the inhibition of apoptosis in Chlamydia -infected cells. First indication of a block in apoptosis signalling upstream of mitochondria.
Fischer, S. F., Schwarz, C., Vier, J. & Hacker, G. Characterization of antiapoptotic activities of Chlamydia pneumoniae in human cells. Infect. Immun. 69, 7121–7129 (2001).
Rajalingam, K. et al. Epithelial cells infected with Chlamydophila pneumoniae (Chlamydia pneumoniae) are resistant to apoptosis. Infect. Immun. 69, 7880–7888 (2001).
Bohme, L. & Rudel, T. Host cell death machinery as a target for bacterial pathogens. Microbes Infect. 11, 1063–1070 (2009).
Gray, M. W., Burger, G. & Lang, B. F. Mitochondrial evolution. Science 283, 1476–1481 (1999).
Lucattini, R., Likic, V. A. & Lithgow, T. Bacterial proteins predisposed for targeting to mitochondria. Mol. Biol. Evol. 21, 652–658 (2004).
Golstein, P. & Kroemer, G. Cell death by necrosis: towards a molecular definition. Trends Biochem. Sci. 32, 37–43 (2007).
Kroemer, G., Galluzzi, L. & Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 87, 99–163 (2007). Describes mitochondrial cell death.
Levine, B. & Kroemer, G. Autophagy in the pathogenesis of disease. Cell 132, 27–42 (2008).
Galluzzi, L. et al. To die or not to die: that is the autophagic question. Curr. Mol. Med. 8, 78–91 (2008).
Kerr, J. F., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972). In this groundbreaking work, Kerr and colleagues first described the morphological phenotype of apoptosis.
Zitvogel, L., Kepp, O. & Kroemer, G. Decoding cell death signals in inflammation and immunity. Cell 140, 798–804.
Savill, J. & Fadok, V. Corpse clearance defines the meaning of cell death. Nature 407, 784–788 (2000).
Yu, S. W. et al. Mediation of poly(ADP-ribose) polymerase-1-dependent cell death by apoptosis-inducing factor. Science 297, 259–263 (2002).
Leist, M. & Jaattela, M. Four deaths and a funeral: from caspases to alternative mechanisms. Nature Rev. Mol. Cell Biol. 2, 589–598 (2001).
Scott, F. L. et al. XIAP inhibits caspase-3 and -7 using two binding sites: evolutionarily conserved mechanism of IAPs. EMBO J. 24, 645–655 (2005).
Cullen, S. P. & Martin, S. J. Mechanisms of granule-dependent killing. Cell Death Differ. 15, 251–262 (2008).
Danial, N. N. & Korsmeyer, S. J. Cell death: critical control points. Cell 116, 205–219 (2004).
Scaffidi, C. et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17, 1675–1687 (1998).
Wang, X. The expanding role of mitochondria in apoptosis. Genes Dev. 15, 2922–2933 (2001).
Green, D. R. Apoptotic pathways: ten minutes to dead. Cell 121, 671–674 (2005).
Willis, S. N. & Adams, J. M. Life in the balance: how BH3-only proteins induce apoptosis. Curr. Opin. Cell Biol. 17, 617–625 (2005).
Huang, D. C. & Strasser, A. BH3-only proteins-essential initiators of apoptotic cell death. Cell 103, 839–842 (2000).
Youle, R. J. & Strasser, A. The BCL-2 protein family: opposing activities that mediate cell death. Nature Rev. Mol. Cell Biol. 9, 47–59 (2008).
Wei, M. C. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292, 727–730 (2001). Demonstrates a redundant but essential role of BAK and BAX in mitochondrial apoptosis pathways.
Xie, Z. & Klionsky, D. J. Autophagosome formation: core machinery and adaptations. Nature Cell Biol. 9, 1102–1109 (2007).
Qu, X. et al. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell 128, 931–946 (2007).
Espert, L. et al. Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J. Clin. Invest. 116, 2161–2172 (2006).
Tsujimoto, Y. & Shimizu, S. Another way to die: autophagic programmed cell death. Cell Death Differ. 12 (Suppl. 2), 1528–1534 (2005).
Kroemer, G. & Levine, B. Autophagic cell death: the story of a misnomer. Nature Rev. Mol. Cell Biol. 9, 1004–1010 (2008).
Khwaja, A. & Tatton, L. Resistance to the cytotoxic effects of tumor necrosis factor a can be overcome by inhibition of a FADD/caspase-dependent signaling pathway. J. Biol. Chem. 274, 36817–36823 (1999).
Matsumura, H. et al. Necrotic death pathway in Fas receptor signaling. J. Cell Biol. 151, 1247–1256 (2000).
Vercammen, D. et al. Inhibition of caspases increases the sensitivity of L929 cells to necrosis mediated by tumor necrosis factor. J. Exp. Med. 187, 1477–1485 (1998).
Vercammen, D. et al. Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188, 919–930 (1998).
Chan, F. K. et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J. Biol. Chem. 278, 51613–51621 (2003).
Degterev, A. et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nature Chem. Biol. 1, 112–119 (2005).
Holler, N. et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nature Immunol. 1, 489–495 (2000).
Kawahara, A., Ohsawa, Y., Matsumura, H., Uchiyama, Y. & Nagata, S. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 143, 1353–1360 (1998).
Festjens, N., Vanden Berghe, T. & Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta 1757, 1371–1387 (2006).
Tsujimoto, Y. & Shimizu, S. Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12, 835–840 (2007).
Kim, Y. S., Morgan, M. J., Choksi, S. & Liu, Z. G. TNF-induced activation of the Nox1 NADPH oxidase and its role in the induction of necrotic cell death. Mol. Cell 26, 675–687 (2007).
Zong, W. X., Ditsworth, D., Bauer, D. E., Wang, Z. Q. & Thompson, C. B. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 18, 1272–1282 (2004).
Xu, Y., Huang, S., Liu, Z. G. & Han, J. Poly(ADP-ribose) polymerase-1 signaling to mitochondria in necrotic cell death requires RIP1/TRAF2-mediated JNK1 activation. J. Biol. Chem. 281, 8788–8795 (2006).
Hitomi, J. et al. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell 135, 1311–1323 (2008).
Galluzzi, L. & Kroemer, G. Necroptosis: a specialized pathway of programmed necrosis. Cell 135, 1161–1163 (2008).
He, S. et al. Receptor interacting protein kinase-3 determines cellular necrotic response to TNF-α. Cell 137, 1100–1111 (2009).
Cho, Y. S. et al. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell 137, 1112–1123 (2009).
Zhang, D. W. et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science 325, 332–336 (2009).
Zhivotovsky, B., Galluzzi, L., Kepp, O. & Kroemer, G. Adenine nucleotide translocase: a component of the phylogenetically conserved cell death machinery. Cell Death Differ. 16, 1419–1425 (2009).
Kozjak-Pavlovic, V., Ross, K. & Rudel, T. Import of bacterial pathogenicity factors into mitochondria. Curr. Opin. Microbiol. 11, 9–14 (2008).
Cornelis, G. R. The type III secretion injectisome. Nature Rev. Microbiol. 4, 811–825 (2006).
Backert, S. & Meyer, T. F. Type IV secretion systems and their effectors in bacterial pathogenesis. Curr. Opin. Microbiol. 9, 207–217 (2006).
Bolender, N., Sickmann, A., Wagner, R., Meisinger, C. & Pfanner, N. Multiple pathways for sorting mitochondrial precursor proteins. EMBO Rep. 9, 42–49 (2008).
Nougayrede, J. P. & Donnenberg, M. S. Enteropathogenic Escherichia coli EspF is targeted to mitochondria and is required to initiate the mitochondrial death pathway. Cell. Microbiol. 6, 1097–1111 (2004). Describes targeting of EspF to mitochondria and the effects this has on cell survival.
Papatheodorou, P. et al. The enteropathogenic Escherichia coli (EPEC) Map effector is imported into the mitochondrial matrix by the TOM/Hsp70 system and alters organelle morphology. Cell. Microbiol. 8, 677–689 (2006).
Crane, J. K., McNamara, B. P. & Donnenberg, M. S. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell. Microbiol. 3, 197–211 (2001).
Nagai, T., Abe, A. & Sasakawa, C. Targeting of enteropathogenic Escherichia coli EspF to host mitochondria is essential for bacterial pathogenesis: critical role of the 16th leucine residue in EspF. J. Biol. Chem. 280, 2998–3011 (2005).
Kenny, B. & Jepson, M. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2, 579–590 (2000).
Ma, C. et al. Citrobacter rodentium infection causes both mitochondrial dysfunction and intestinal epithelial barrier disruption in vivo: role of mitochondrial associated protein (Map). Cell. Microbiol. 8, 1669–1686 (2006).
Kenny, B. & Valdivia, R. Host-microbe interactions: bacteria. Curr. Opin. Microbiol. 12, 1–3 (2009).
Salama, N. R., Otto, G., Tompkins, L. & Falkow, S. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69, 730–736 (2001).
Cover, T. L. & Blaser, M. J. Purification and characterization of the vacuolating toxin from Helicobacter pylori. J. Biol. Chem. 267, 10570–10575 (1992).
Nguyen, V. Q., Caprioli, R. M. & Cover, T. L. Carboxy-terminal proteolytic processing of Helicobacter pylori vacuolating toxin. Infect. Immun. 69, 543–546 (2001).
Ye, D. & Blanke, S. R. Functional complementation reveals the importance of intermolecular monomer interactions for Helicobacter pylori VacA vacuolating activity. Mol. Microbiol. 43, 1243–1253 (2002).
Torres, V. J., McClain, M. S. & Cover, T. L. Interactions between p-33 and p-55 domains of the Helicobacter pylori vacuolating cytotoxin (VacA). J. Biol. Chem. 279, 2324–2331 (2004).
Reyrat, J. M. et al. 3D imaging of the 58 kDa cell binding subunit of the Helicobacter pylori cytotoxin. J. Mol. Biol. 290, 459–470 (1999).
de Bernard, M. et al. Identification of the Helicobacter pylori VacA toxin domain active in the cell cytosol. Infect. Immun. 66, 6014–6016 (1998).
Galmiche, A. et al. The N terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. EMBO J. 19, 6361–6370 (2000). Provides first evidence for the mitochondrial translocation of the bacterial protein VacA and its pro-apoptotic function in mitochondria.
Yamasaki, E. et al. Helicobacter pylori vacuolating cytotoxin induces activation of the proapoptotic proteins Bax and Bak, leading to cytochrome c release and cell death, independent of vacuolation. J. Biol. Chem. 281, 11250–11259 (2006).
Domanska, G. et al. Helicobacter pylori VacA toxin/subunit p34: targeting of an anion channel to the inner mitochondrial membrane. PLoS Pathog. 6, e1000878.
Bantel, H. et al. α-Toxin is a mediator of Staphylococcus aureus-induced cell death and activates caspases via the intrinsic death pathway independently of death receptor signaling. J. Cell Biol. 155, 637–648 (2001).
Essmann, F. et al. Staphylococcus aureus α-toxin-induced cell death: predominant necrosis despite apoptotic caspase activation. Cell Death Differ. 10, 1260–1272 (2003).
Haslinger, B., Strangfeld, K., Peters, G., Schulze-Osthoff, K. & Sinha, B. Staphylococcus aureus α-toxin induces apoptosis in peripheral blood mononuclear cells: role of endogenous tumour necrosis factor-a and the mitochondrial death pathway. Cell. Microbiol. 5, 729–741 (2003).
Genestier, A. L. et al. Staphylococcus aureus Panton-Valentine leukocidin directly targets mitochondria and induces Bax-independent apoptosis of human neutrophils. J. Clin. Invest. 115, 3117–3127 (2005).
Braun, J. S. et al. Pneumolysin causes neuronal cell death through mitochondrial damage. Infect. Immun. 75, 4245–4254 (2007).
He, D. et al. Clostridium difficile toxin A causes early damage to mitochondria in cultured cells. Gastroenterology 119, 139–150 (2000).
Matarrese, P. et al. Clostridium difficile toxin B causes apoptosis in epithelial cells by thrilling mitochondria. Involvement of ATP-sensitive mitochondrial potassium channels. J. Biol. Chem. 282, 9029–9041 (2007).
Petit, P. et al. Lethal toxin from Clostridium sordellii induces apoptotic cell death by disruption of mitochondrial homeostasis in HL-60 cells. Cell. Microbiol. 5, 761–771 (2003).
Choi, C. H. et al. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell. Microbiol. 7, 1127–1138 (2005).
Muller, A. et al. Targeting of the pro-apoptotic VDAC-like porin (PorB) of Neisseria gonorrhoeae to mitochondria of infected cells. EMBO J. 19, 5332–5343 (2000). First example of bacterial outer membrane protein localizing to mitochondria and inducing cell death.
Massari, P., Ho, Y. & Wetzler, L. M. Neisseria meningitidis porin PorB interacts with mitochondria and protects cells from apoptosis. Proc. Natl Acad. Sci. USA 97, 9070–9075 (2000).
Kozjak-Pavlovic, V. et al. Bacterial porin disrupts mitochondrial membrane potential and sensitizes host cells to apoptosis. PLoS Pathog. 5, e1000629 (2009). Delineates the import route of PorB into mitochondria and its mode of action in mitochondrial damage.
Walther, D. M., Papic, D., Bos, M. P., Tommassen, J. & Rapaport, D. Signals in bacterial b-barrel proteins are functional in eukaryotic cells for targeting to and assembly in mitochondria. Proc. Natl Acad. Sci. USA 106, 2531–2536 (2009).
Kepp, O. et al. Bim and Bmf synergize to induce apoptosis in Neisseria gonorrhoeae infection. PLoS Pathog. 5, e1000348 (2009).
Kepp, O., Rajalingam, K., Kimmig, S. & Rudel, T. Bak and Bax are non-redundant during infection- and DNA damage-induced apoptosis. EMBO J. 26, 825–834 (2007).
Sisko, J. L., Spaeth, K., Kumar, Y. & Valdivia, R. H. Multifunctional analysis of Chlamydia-specific genes in a yeast expression system. Mol. Microbiol. 60, 51–66 (2006).
Niu, H., Kozjak-Pavlovic, V., Rudel, T. & Rikihisa, Y. Anaplasma phagocytophilum Ats-1 is imported into host cell mitochondria and interferes with apoptosis induction. PLoS Pathog. 6, e1000774 (2010). The first example of a secreted bacterial protein that targets mitochondria and inhibits apoptosis.
Baines, C. P., Kaiser, R. A., Sheiko, T., Craigen, W. J. & Molkentin, J. D. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nature Cell Biol. 9, 550–555 (2007).
Block, A. et al. The Pseudomonas syringae type III effector HopG1 targets mitochondria, alters plant development and suppresses plant innate immunity. Cell. Microbiol. 12, 318–330 (2010).
Bergsbaken, T., Fink, S. L. & Cookson, B. T. Pyroptosis: host cell death and inflammation. Nature Rev. Microbiol. 7, 99–109 (2009).
Boise, L. H. & Collins, C. M. Salmonella-induced cell death: apoptosis, necrosis or programmed cell death? Trends Microbiol. 9, 64–67 (2001).
Hernandez, L. D., Pypaert, M., Flavell, R. A. & Galan, J. E. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 163, 1123–1131 (2003).
Fischer, S. F. et al. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J. Exp. Med. 200, 905–916 (2004).
Dong, F. et al. Degradation of the proapoptotic proteins Bik, Puma, and Bim with Bcl-2 domain 3 homology in Chlamydia trachomatis-infected cells. Infect. Immun. 73, 1861–1864 (2005).
Paschen, S. A. et al. Cytopathicity of Chlamydia is largely reproduced by expression of a single chlamydial protease. J. Cell Biol. 182, 117–127 (2008).
Pirbhai, M., Dong, F., Zhong, Y., Pan, K. Z. & Zhong, G. The secreted protease factor CPAF is responsible for degrading pro-apoptotic BH3-only proteins in Chlamydia trachomatis-infected cells. J. Biol. Chem. 281, 31495–31501 (2006).
Verbeke, P. et al. Recruitment of BAD by the Chlamydia trachomatis vacuole correlates with host-cell survival. PLoS Pathog. 2, e45 (2006).
Rajalingam, K. et al. Mcl-1 is a key regulator of apoptosis resistance in Chlamydia trachomatis-infected cells. PLoS ONE 3, e3102 (2008).
Howie, H. L., Shiflett, S. L. & So, M. Extracellular signal-regulated kinase activation by Neisseria gonorrhoeae downregulates epithelial cell proapoptotic proteins Bad and Bim. Infect. Immun. 76, 2715–2721 (2008).
Hauck, C. R. & Meyer, T. F. 'Small' talk: Opa proteins as mediators of Neisseria-host-cell communication. Curr. Opin. Microbiol. 6, 43–49 (2003).
Nogueira, C. V. et al. Rapid pathogen-induced apoptosis: a mechanism used by dendritic cells to limit intracellular replication of Legionella pneumophila. PLoS Pathog. 5, e1000478 (2009).
Banga, S. et al. Legionella pneumophila inhibits macrophage apoptosis by targeting pro-death members of the Bcl2 protein family. Proc. Natl Acad. Sci. USA 104, 5121–5126 (2007).
Berghe, T. V. et al. Necroptosis, necrosis and secondary necrosis converge on similar cellular disintegration features. Cell Death Differ. 17, 922–930 (2009).
Laguna, R. K., Creasey, E. A., Li, Z., Valtz, N. & Isberg, R. R. A Legionella pneumophila-translocated substrate that is required for growth within macrophages and protection from host cell death. Proc. Natl Acad. Sci. USA 103, 18745–18750 (2006).
Carneiro, L. A. et al. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host Microbe 5, 123–136 (2009). Demonstrates mitochondrion-dependent necrotic cell death in epithelial cells as a consequence of Shigella spp. infection. Cell death was potently counterbalanced by a signalling pathway activated by the pathogen early during infection.
Zychlinsky, A. & Sansonetti, P. J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 5, 201–204 (1997).
Goldmann, O., Sastalla, I., Wos-Oxley, M., Rohde, M. & Medina, E. Streptococcus pyogenes induces oncosis in macrophages through the activation of an inflammatory programmed cell death pathway. Cell. Microbiol. 11, 138–155 (2009).
Porcelli, S. A. & Jacobs, W. R. Jr. Tuberculosis: unsealing the apoptotic envelope. Nature Immunol. 9, 1101–1102 (2008).
Winau, F. et al. Apoptotic vesicles crossprime CD8 T cells and protect against tuberculosis. Immunity 24, 105–117 (2006).
Schaible, U. E. et al. Apoptosis facilitates antigen presentation to T lymphocytes through MHC-I and CD1 in tuberculosis. Nature Med. 9, 1039–1046 (2003).
Chen, M., Gan, H. & Remold, H. G. A mechanism of virulence: virulent Mycobacterium tuberculosis strain H37Rv, but not attenuated H37Ra, causes significant mitochondrial inner membrane disruption in macrophages leading to necrosis. J. Immunol. 176, 3707–3716 (2006).
Chen, M. et al. Lipid mediators in innate immunity against tuberculosis: opposing roles of PGE2 and LXA4 in the induction of macrophage death. J. Exp. Med. 205, 2791–2801 (2008).
Enninga, J., Sansonetti, P. & Tournebize, R. Roundtrip explorations of bacterial infection: from single cells to the entire host and back. Trends Microbiol. 15, 483–490 (2007).
Brennan, M. A. & Cookson, B. T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 38, 31–40 (2000).
Fink, S. L. & Cookson, B. T. Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 73, 1907–1916 (2005).
Willingham, S. B. et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe 2, 147–159 (2007).
Majno, G. & Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 146, 3–15 (1995).
Andrabi, S. A., Dawson, T. M. & Dawson, V. L. Mitochondrial and nuclear cross talk in cell death: parthanatos. Ann. NY Acad. Sci. 1147, 233–241 (2008).
Rajalingam, K. et al. IAP-IAP complexes required for apoptosis resistance of C. trachomatis-infected cells. PLoS Pathog. 2, e114 (2006).
Paland, N. et al. NF-κB and inhibitor of apoptosis proteins are required for apoptosis resistance of epithelial cells persistently infected with Chlamydophila pneumoniae. Cell. Microbiol. 8, 1643–1655 (2006).
Acknowledgements
We thank L. Galluzzi for helpful advice during the preparation of the manuscript and L. Ogilvie for critically reading the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Calreticulin
-
Ca2+-binding endoplasmic reticulum chaperone that under cell stress conditions can translocate to the cell surface and serve as an 'eat me' signal for dendritic cells.
- Calpain
-
One of a group of Ca2+-activated cytoplasmic proteases that are found in many tissues and that hydrolyse various endogenous proteins.
- Granzyme
-
A secreted serine protease that enters target cells through perforin pores, then cleaves and activates intracellular caspases, leading to apoptosis.
- Perforin
-
A component of cytolytic granules that participates in the permeabilization of plasma membranes, allowing granzymes and other cytotoxic components to enter target cells.
- T3SS
-
A bacterial secretion system that consists of a needle-like apparatus that transports proteins from the bacterial cytoplasm across the bacterial cell envelope directly into the eukaryotic cell cytoplasm.
- T4SS
-
A multiprotein complex that mediates the translocation of macromolecules (proteins, DNA or DNA–protein) across the bacterial cell envelope into the extracellular medium or directly into recipient cells.
- T5SS
-
Include both two-partner secretion systems and autotransporters. Two-partner secretion systems comprise two distinct proteins: a transporter protein and a secreted effector protein. Autotransporters are multidomain proteins that are secreted as precursors across the inner membrane by the Sec machinery. The translocator domain of the protein then inserts into the outer membrane and facilitates surface localization of the passenger domain.
- TOM complex
-
A protein complex that recognizes nucleus-encoded mitochondrial pre-proteins and mediates their translocation across the membrane.
- SAM complex
-
A protein complex that facilitates the integration of imported proteins into the outer mitochondrial membrane.
- TIM complex
-
A protein complex in the inner membrane and intermembrane space of mitochondria that mediates transport of pre-proteins into and across the membrane.
- Type IV pilus
-
An elongated, flexible appendage that extends from the surface of Gram-negative bacterial cells and is used for adhesion and twitching motility.
Rights and permissions
About this article
Cite this article
Rudel, T., Kepp, O. & Kozjak-Pavlovic, V. Interactions between bacterial pathogens and mitochondrial cell death pathways. Nat Rev Microbiol 8, 693–705 (2010). https://doi.org/10.1038/nrmicro2421
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2421
This article is cited by
-
The survival of Amblyomma sculptum ticks upon blood-feeding depends on the expression of an inhibitor of apoptosis protein
Parasites & Vectors (2023)
-
The intracellular bacterium Rickettsia rickettsii exerts an inhibitory effect on the apoptosis of tick cells
Parasites & Vectors (2020)
-
Pseudomonas aeruginosa Induced Host Epithelial Cell Mitochondrial Dysfunction
Scientific Reports (2019)
-
Helicobacter pylori targets mitochondrial import and components of mitochondrial DNA replication machinery through an alternative VacA-dependent and a VacA-independent mechanisms
Scientific Reports (2017)
-
Targeting mitochondria: how intravacuolar bacterial pathogens manipulate mitochondria
Cell and Tissue Research (2017)