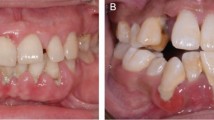
Key Points
-
Periodontitis is intimately associated with a characterized polymicrobial dental-plaque community. However, the species and mechanisms that result in disease remain unclear.
-
The main protective mechanism of the periodontium is the orchestrated expression of select innate host defence mediators. These mediators reduce the microbial load on the epithelial-cell surface by both neutralization and bacterial killing.
-
The main mechanism of bone loss in periodontitis seems to be mediated by the host response to a unique microbial consortium. However, most of the bacteria found in the dental-plaque biofilm are capable of initiating inflammatory cytokine responses, rendering identification of key indicators of disease difficult.
-
Examination of gingival crevicular fluid from clinically healthy sites revealed that this fluid contains cytokines that may induce inflammation. However, the levels of these cytokines in healthy sites are lower than levels in diseased sites, indicating that a disruption of host homeostasis contributes to disease.
-
Extensive analysis of dental-plaque bacteria associated with disease has revealed three bacterial species that display strong associations both with each other and diseased sites. Evidence indicates that these bacteria, designated the 'red-complex' species, may interfere with the protective barrier of the host innate defence response.
-
Red-complex bacteria maybe key species in the pathogenic dental-plaque biofilm. By modulating the innate host defence barrier, they facilitate the growth of other members of the dental-plaque biofilm. The increase in the number and types of bacterial species that occupy the gingival crevice present multiple opportunities to disrupt host homeostasis programmes.
-
Physical removal of the dental-plaque biofilm remains the most effective treatment. However, vaccine strategies targeting red-complex bacteria have also shown efficacy in animal models of disease. The ability to attenuate bone loss by the inhibition of a single bacterial species is consistent with the key species concept.
-
A novel pro-resolving mediator shows promise as an effective periodontitis treatment in pre-clinical studies. A compound that restores host homeostasis is consistent with the theory that an important component of the disease is disruption of host homeostasis programmes.
Abstract
Periodontitis, or gum disease, affects millions of people each year. Although it is associated with a defined microbial composition found on the surface of the tooth and tooth root, the contribution of bacteria to disease progression is poorly understood. Commensal bacteria probably induce a protective response that prevents the host from developing disease. However, several bacterial species found in plaque (the 'red-complex' bacteria: Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola) use various mechanisms to interfere with host defence mechanisms. Furthermore, disease may result from 'community-based' attack on the host. Here, I describe the interaction of the host immune system with the oral bacteria in healthy states and in diseased states.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Socransky, S. S. & Haffajee, A. D. Evidence of bacterial etiology: a historical perspective. Periodontol. 2000 5, 7–25 (1994).
Socransky, S. S., Haffajee, A. D., Cugini, M. A., Smith, C. & Kent, R. L. Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 25, 134–144 (1998). This landmark work describes the different bacterial complexes associated with periodontal disease. It greatly influenced the field as other investigators sought bacterial virulence properties that could be associated with red-complex bacteria.
Ximenez-Fyvie, L. A., Haffajee, A. D. & Socransky, S. S. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J. Clin. Periodontol. 27, 648–657 (2000).
Darveau, R. P., Tanner, A. & Page, R. C. The microbial challenge in periodontitis. Periodontol. 2000 14, 12–32 (1997).
Tanner, A., Kent, R., Maiden, M. F. & Taubman, M. A. Clinical, microbiological and immunological profile of healthy, gingivitis and putative active periodontal subjects. J. Periodont. Res. 31, 195–204 (1996).
Huyghe, A. et al. Novel microarray design strategy to study complex bacterial communities. Appl. Environ. Microbiol. 74, 1876–1885 (2008).
Kroes, I., Lepp, P. W. & Relman, D. A. Bacterial diversity within the human subgingival crevice. Proc. Natl Acad. Sci. USA 96, 14547–14552 (1999).
Berglundh, T., Liljenberg, B. & Lindhe, J. Some effects of periodontal therapy on local and systemic immunological parameters. J. Clin. Periodontol. 26, 91–98 (1999).
Yoshinari, N. et al. Effects of scaling and root planing on the amounts of interleukin-1 and interleukin-1 receptor antagonist and the mRNA expression of interleukin-1β in gingival crevicular fluid and gingival tissues. J. Periodont. Res. 39, 158–167 (2004).
Roberts, F. A., Hockett, R. D. Jr, Bucy, R. P. & Michalek, S. M. Quantitative assessment of inflammatory cytokine gene expression in chronic adult periodontitis. Oral Microbiol. Immunol. 12, 336–344 (1997).
Champagne, C. M. et al. Potential for gingival crevice fluid measures as predictors of risk for periodontal diseases. Periodontol. 2000 31, 167–180 (2003).
Giannobile, W. V. Crevicular fluid biomarkers of oral bone loss. Curr. Opin. Periodontol. 4, 11–20 (1997).
Kamma, J., Mombelli, A., Tsinidou, K., Vasdekis, V. & Giannopoulou, C. Cytokines in gingival crevicular fluid of adolescents and young adults. Oral Microbiol. Immunol. 24, 7–10 (2009).
Thunell, D. H. et al. A multiplex immunoassay demonstrates reductions in gingival crevicular fluid cytokines following initial periodontal therapy. J. Periodont. Res. 45, 148–152 (2009).
Page, R. C. & Kornman, K. S. The pathogenesis of human periodontitis: an introduction. Periodontol. 2000 14, 9–11 (1997).
Van Dyke, T. E. The management of inflammation in periodontal disease. J. Periodontol. 79, 1601–1608 (2008).
O'Neill, L. A. The interleukin-1 receptor/Toll-like receptor superfamily: 10 years of progress. Immunol. Rev. 226, 10–18 (2008).
Beutler, B., Hoebe, K., Du, X. & Ulevitch, R. J. How we detect microbes and respond to them: the Toll-like receptors and their transducers. J. Leukoc. Biol. 74, 479–485 (2003).
Kolenbrander, P. E. et al. Bacterial interactions and successions during plaque development. Periodontol. 2000 42, 47–79 (2006). This manuscript describes the ecological succession that occurs in dental-plaque formation and details the known adhesive interactions that occur among different oral species and that facilitate their highly ordered structure.
Frank, D. N. et al. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl Acad. Sci. USA 104, 13780–13785 (2007).
Oakley, B. B., Fiedler, T. L., Marrazzo, J. M. & Fredricks, D. N. Diversity of human vaginal bacterial communities and associations with clinically defined bacterial vaginosis. Appl. Environ. Microbiol. 74, 4898–4909 (2008).
Yang, L. et al. Inflammation and intestinal metaplasia of the distal esophagus are associated with alterations in the microbiome. Gastroenterology 137, 588–597 (2009).
Marsh, P. D. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 8, 263–271 (1994). This article describes the microbial shift that occurs in periodontitis.
Paster, B. J. et al. Bacterial diversity in human subgingival plaque. J. Bacteriol. 183, 3770–3783 (2001). One of the most extensive analyses of dental plaque, carried out by 16s RNA identification. This work notably increased the number of identified species in dental plaque by identifying non-cultivatable species.
Kumar, P. S., Griffen, A. L., Moeschberger, M. L. & Leys, E. J. Identification of candidate periodontal pathogens and beneficial species by quantitative 16S clonal analysis. J. Clin. Microbiol. 43, 3944–3955 (2005).
Brinig, M. M., Lepp, P. W., Ouverney, C. C., Armitage, G. C. & Relman, D. A. Prevalence of bacteria of division TM7 in human subgingival plaque and their association with disease. Appl. Environ. Microbiol. 69, 1687–1694 (2003).
Lepp, P. W. et al. Methanogenic Archaea and human periodontal disease. Proc. Natl Acad. Sci. USA 101, 6176–6181 (2004).
Vianna, M. E., Holtgraewe, S., Seyfarth, I., Conrads, G. & Horz, H. P. Quantitative analysis of three hydrogenotrophic microbial groups, methanogenic archaea, sulfate-reducing bacteria, and acetogenic bacteria, within plaque biofilms associated with human periodontal disease. J. Bacteriol. 190, 3779–3785 (2008).
Kumar, P. S. et al. Changes in periodontal health status are associated with bacterial community shifts as assessed by quantitative 16S cloning and sequencing. J. Clin. Microbiol. 44, 3665–3673 (2006).
Bosshardt, D. D. & Lang, N. P. The junctional epithelium: from health to disease. J. Dent. Res. 84, 9–20 (2005).
Duerkop, B. A., Vaishnava, S. & Hooper, L. V. Immune responses to the microbiota at the intestinal mucosal surface. Immunity 31, 368–376 (2009).
Moughal, N. A., Adonogianaki, E., Thornhill, M. H. & Kinane, D. F. Endothelial cell leukocyte adhesion molecule-1 (ELAM-1) and intercellular adhesion molecule-1 (ICAM-1) expression in gingival tissue during health and experimentally-induced gingivitis. J. Periodont. Res. 27, 623–630 (1992).
Nylander, K., Danielsen, B., Fejerskov, O. & Dabelsteen, E. Expression of the endothelial leukocyte adhesion molecule-1 (ELAM-1) on endothelial cells in experimental gingivitis in humans. J. Periodontol. 64, 355–357 (1993).
Gemmell, E., Walsh, L. J., Savage, N. W. & Seymore, G. J. Adhesion molecule expression in chronic inflammatory periodontal disease tissue. J. Periodont. Res. 29, 46–53 (1994).
Tonetti, M. S. Molecular factors associated with compartmentalization of gingival immune responses and transepithelial neutrophil migration. J. Periodont. Res. 32, 104–109 (1997).
Tonetti, M. S. et al. Localized expression of mRNA for phagocyte-specific hemotactic cytokines in human periodontal infections. Infect. Immun. 62, 4005–4014 (1994).
Tonetti, M. S., Imboden, M. A. & Lang, N. P. Neutrophil migration into the gingival sulcus is associated with transepithelial gradients of interleukin-8 and ICAM-1. J. Periodontol. 69, 1139–1147 (1998). This study provides a mechanism for the transmigration of neutrophils from the vasculature to the gingival crevice. This work initiated further studies aiming to better understand the orchestrated expression of select innate defence mediators in clinically healthy periodontal tissue.
Schiott, C. R. & Loe, H. The origin and variation in number of leukocytes in the human saliva. J. Periodont. Res. 5, 36–41 (1970).
Hart, T. C., Shapira, L. & Van Dyke, T. E. Neutrophil defects as risk factors for periodontal diseases. J. Periodontol. 65, 521–529 (1994).
Carrassi, A., Abati, S., Santarelli, G. & Vogel, G. Periodontitis in a patient with chronic neutropenia. J. Periodontol. 60, 352–357 (1989).
Page, R. C., Beatty, P. & Waldrop, T. C. Molecular basis for the functional abnormality in neutrophils from patients with generalized prepubertal periodontitis. J. Periodont. Res. 22, 182–183 (1987).
Waldrop, T. C., Anderson, D. C., Hallmon, W. W., Schmalstieg, F. C. & Jacobs, R. L. Periodontal manifestations of the heritable Mac-1, LFA-1, deficiency syndrome. Clinical, histopathologic and molecular characteristics. J. Periodontol. 58, 400–416 (1987).
Attström, R. & Schroeder, H. E. Effect of experimental neutropenia on initial gingivitis in dogs. Scand. J. Dent. Res. 87, 7–23 (1979).
Sallay, K., Listgarten, M., Sanavi, F., Ring, I. & Nowotny, A. Bacterial invasion of oral tissues of immunosuppressed rats. Infect. Immun. 43, 1091–1093 (1984).
Hemmerle, J. & Frank, R. M. Bacterial invasion of periodontal tissues after experimental immunosuppression in rats. J. Biol. Buccale 19, 271–282 (1991).
Yoshinari, N., Kameyama, Y., Aoyama, Y., Nishiyama, H. & Noguchi, T. Effect of long-term methotrexate-induced neutropenia on experimental periodontal lesion in rats. J. Periodont. Res. 29, 393–400 (1994).
Lu, Q., Jin, L., Darveau, R. P. & Samaranayake, L. P. Expression of human β-defensins-1 and -2 peptides in unresolved chronic periodontitis. J. Periodont. Res. 39, 221–227 (2004).
Lu, Q., Samaranayake, L. P., Darveau, R. P. & Jin, L. Expression of human β-defensin-3 in gingival epithelia. J. Periodont. Res. 40, 474–481 (2005).
Jin, L. & Darveau, R. P. Soluble CD14 levels in gingival crevicular fluid of subjects with untreated adult periodontitis. J. Periodontol. 72, 634–640 (2001).
Jin, L., Ren, L., Leung, W. K. & Darveau, R. P. The in vivo expression of membrane-bound CD14 in periodontal health and disease. J. Periodontol. 75, 578–585 (2004).
Ren, L., Jin, L. & Leung, W. K. Local expression of lipopolysaccharide-binding protein in human gingival tissues. J. Periodont. Res. 39, 242–248 (2004).
Mahanonda, R. & Pichyangkul, S. Toll-like receptors and their role in periodontal health and disease. Periodontol. 2000 43, 41–55 (2007).
Ren, L., Leung, W. K., Darveau, R. P. & Jin, L. The expression profile of lipopolysaccharide-binding protein, membrane-bound CD14, and Toll-like receptors 2 and 4 in chronic periodontitis. J. Periodontol. 76, 1950–1959 (2005).
Sugawara, Y. et al. Toll-like receptors, NOD1, and NOD2 in oral epithelial cells. J. Dent. Res. 85, 524–529 (2006).
Otte, J. M., Cario, E. & Podolsky, D. K. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology 126, 1054–1070 (2004).
Eskan, M. A., Hajishengallis, G. & Kinane, D. F. Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae. Infect. Immun. 75, 892–898 (2007).
Dixon, D. R., Bainbridge, B. W. & Darveau, R. P. Modulation of the innate immune response within the periodontium. Periodontol. 2000 35, 53–74 (2004).
Darveau, R. P., Belton, C. M., Reife, R. A. & Lamont, R. J. Local chemokine paralysis, a novel pathogenic mechanism for Porphyromonas gingivalis. Infect. Immun. 66, 1660–1665 (1998). The first observation that P. gingivalis inhibits IL-8 secretion from gingival epithelial cells, and the proposal that P. gingivalis could induce a local chemokine paralysis as a mechanism of virulence.
Hasegawa, Y. et al. Gingival epithelial cell transcriptional responses to commensal and opportunistic oral microbial species. Infect. Immun. 75, 2540–2547 (2007).
Huang, G. T., Zhang, H. B., Dang, H. N. & Haake, S. K. Differential regulation of cytokine genes in gingival epithelial cells challenged by Fusobacterium nucleatum and Porphyromonas gingivalis. Microbiol. Pathog. 37, 303–312 (2004).
Chung, W. O., Dommisch, H., Yin, L. & Dale, B. A. Expression of defensins in gingiva and their role in periodontal health and disease. Curr. Pharm. Des. 13, 3073–3083 (2007).
Krisanaprakornkit, S. et al. Inducible expression of human β-defensin 2 by Fusobacterium nucleatum in oral epithelial cells: multiple signaling pathways and role of commensal bacteria in innate immunity and the epithelial barrier. Infect. Immun. 68, 2907–2915 (2000).
Crawford, J. M., Taubman, M. A. & Smith, D. J. The natural history of periodontal bone loss in germfree and gnotobiotic rats infected with periodontopathic microorganisms. J. Periodont. Res. 13, 316–325 (1978).
Dixon, D. R., Reife, R. A., Cebra, J. J. & Darveau, R. P. Commensal bacteria influence innate status within gingival tissues: a pilot study. J. Periodontol. 75, 1486–1492 (2004).
Stappenbeck, T. S., Hooper, L. V. & Gordon, J. I. Developmental regulation of intestinal angiogenesis by indigenous microbes via Paneth cells. Proc. Natl Acad. Sci. USA 99, 15451–15455 (2002).
Xu, J. & Gordon, J. I. Inaugural Article: Honor thy symbionts. Proc. Natl Acad. Sci. USA 100, 10452–10459 (2003).
Umesaki, Y. & Setoyama, H. Structure of the intestinal flora responsible for development of the gut immune system in a rodent model. Microbes Infect. 2, 1343–1351 (2000).
Gordon, H. A. & Pesti, L. The gnotobiotic animal as a tool in the study of host microbial relationships. Bacteriol. Rev. 35, 390–429 (1971).
Duncan, H. E. & Edberg, S. C. Host-microbe interaction in the gastrointestinal tract. Crit. Rev. Microbiol. 21, 85–100 (1995).
Falk, P. G., Hooper, L. V., Midtvedt, T. & Gordon, J. I. Creating and maintaining the gastrointestinal ecosystem: what we know and need to know from gnotobiology. Microbiol. Mol. Biol. Rev. 62, 1157–1170 (1998).
Macpherson, A. J. & Harris, N. L. Interactions between commensal intestinal bacteria and the immune system. Nature Rev. Immunol. 4, 478–485 (2004).
Cebra, J. J. Influences of microbiota on intestinal immune system development. Am. J. Clin. Nutr. 69, S1046–S1051 (1999).
Chadwick, V. S. & Anderson, R. P. in Inflammatory Bowel Disease (eds MacDermott, R. P. & Stenson, W. F.) 241–258 (Elsevier Science, Amsterdam, 1992).
Hooper, L. V. et al. Molecular analysis of commensal host-microbial relationships in the intestine. Science 291, 881–884 (2001).
Rakoff-Nahoum, S., Paglino, J., Eslami-Varzaneh, F., Edberg, S. & Medzhitov, R. Recognition of commensal microflora by Toll-like receptors is required for intestinal homeostasis. Cell 118, 229–241 (2004).
Bartold, P. M., Walsh, L. J. & Narayanan, A. S. Molecular and cell biology of the gingiva. Periodontol. 2000 24, 28–55 (2000).
Mussig, E., Tomakidi, P. & Steinberg, T. Molecules contributing to the maintenance of periodontal tissues. Their possible association with orthodontic tooth movement. J. Orofac. Orthop. 66, 422–433 (2005).
Gorska, R. et al. Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J. Clin. Periodontol. 30, 1046–1052 (2003).
Page, R. C., Offenbacher, S., Schroeder, H. E., Seymour, G. J. & Kornman, K. S. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontol. 2000 14, 216–248 (1997). A summary of many of the current theories about periodontitis and the balance between periodontal health and disease, and an excellent review of how clinical markers have influenced our thinking about disease aetiology.
Cochran, D. L. Inflammation and bone loss in periodontal disease. J. Periodontol. 79, 1569–1576 (2008).
Nagasawa, T. et al. Roles of receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin in periodontal health and disease. Periodontol. 2000 43, 65–84 (2007).
Boyle, W. J., Simonet, W. S. & Lacey, D. L. Osteoclast differentiation and activation. Nature 423, 337–342 (2003).
Assuma, R., Oates, T., Cochran, D., Amar, S. & Graves, D. T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 160, 403–409 (1998).
Baker, P. J. et al. CD4+ T cells and the proinflammatory cytokines gamma interferon and interleukin-6 contribute to alveolar bone loss in mice. Infect. Immun. 67, 2804–2809 (1999).
Delima, A. J. et al. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J. Clin. Periodontol. 28, 233–240 (2001).
Graves, D. T. et al. Interleukin-1 and tumor necrosis factor antagonists inhibit the progression of inflammatory cell infiltration toward alveolar bone in experimental periodontitis. J. Periodontol. 69, 1419–1425 (1998).
Garlet, G. P. et al. The dual role of p55 tumour necrosis factor-α receptor in Actinobacillus actinomycetemcomitans-induced experimental periodontitis: host protection and tissue destruction. Clin. Exp. Immunol. 147, 128–138 (2007).
Niederman, R. et al. Infection-mediated early-onset periodontal disease in P/E-selectin-deficient mice. J. Clin. Periodontol. 28, 569–575 (2001).
Al-Rasheed, A., Scheerens, H., Rennick, D. M., Fletcher, H. M. & Tatakis, D. N. Accelerated alveolar bone loss in mice lacking interleukin-10. J. Dent. Res. 82, 632–635 (2003).
Dayan, S., Stashenko, P., Niederman, R. & Kupper, T. S. Oral epithelial overexpression of IL-1α causes periodontal disease. J. Dent. Res. 83, 786–790 (2004).
Janeway, C. J. The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol. Today 13, 11–16 (1992).
Kawai, T. & Akira, S. Pathogen recognition with Toll-like receptors. Curr. Opin. Immunol. 17, 338–344 (2005).
Yoshioka, H., Yoshimura, A., Kaneko, T., Golenbock, D. T. & Hara, Y. Analysis of the activity to induce toll-like receptor (TLR)2- and TLR4-mediated stimulation of supragingival plaque. J. Periodontol. 79, 920–928 (2008).
Burns, E., Bachrach, G., Shapira, L. & Nussbaum, G. Cutting Edge: TLR2 is required for the innate response to Porphyromonas gingivalis: activation leads to bacterial persistence and TLR2 deficiency attenuates induced alveolar bone resorption. J. Immunol. 177, 8296–8300 (2006).
Gibson, F. C. 3rd, Ukai, T., Genco, C. Engagement of specific innate immune signaling pathways during Porphyromonas givgivalis induced chronic inflammation and atherosclerosis. Front. Biosci. 13, 2041–2059 (2008).
Ji, S., Kim, Y., Min., B. M., Han, S. H. & Choi, Y. Innate immune responses of gingival epithelial cells to nonperiodontopathic and periodontopathic bacteria. J. Periodont. Res. 42, 503–510 (2007).
Huang, G. T., Kim, D., Lee, J. K., Kuramitsu, H. K. & Haake, S. K. Interleukin-8 and intercellular adhesion molecule 1 regulation in oral epithelial cells by selected periodontal bacteria: multiple effects of Porphyromonas gingivalis via antagonistic mechanisms. Infect. Immun. 69, 1364–1372 (2001).
Vankeerberghen, A. et al. Differential induction of human beta-defensin expression by periodontal commensals and pathogens in periodontal pocket epithelial cells. J. Periodontol. 76, 1293–1303 (2005).
Hasegawa, Y. et al. Role of Porphyromonas gingivalis SerB in gingival epithelial cell cytoskeletal remodeling and cytokine production. Infect. Immun. 76, 2420–2427 (2008).
Tribble, G. D., Mao, S., James, C. E. & Lamont, R. J. A Porphyromonas gingivalis haloacid dehalogenase family phosphatase interacts with human phosphoproteins and is important for invasion. Proc. Natl Acad. Sci. USA 103, 11027–11032 (2006). This article describes a novel protein that is secreted by P. gingivalis and that disrupts gingival epithelial cell function. The authors postulate that P. gingivalis has adapted a former metabolic enzyme to facilitate entry into host cells by modulating host cytoskeletal architecture.
Brissette, C. A., Pham, T. T., Coats, S. R., Darveau, R. P. & Lukehart, S. A. Treponema denticola does not induce production of common innate immune mediators from primary gingival epithelial cells. Oral Microbiol. Immunol. 23, 474–481 (2008).
Hajishengallis, G., Wang, M., Liang, S., Triantafilou, M. & Triantafilou, K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc. Natl Acad. Sci. USA 105, 13532–13537 (2008).
Coats, S. R., Pham, T. T., Bainbridge, B. W., Reife, R. A. & Darveau, R. P. MD-2 mediates the ability of tetra-acylated and penta-acylated lipopolysaccharides to antagonize Escherichia coli lipopolysaccharide at the TLR4 signaling complex. J. Immunol. 175, 4490–4498 (2005).
Coats, S. R., Do, C. T., Karimi-Naser, L. M., Braham, P. H. & Darveau, R. P. Antagonistic lipopolysaccharides block E. coli lipopolysaccharide function at human TLR4 via interaction with the human MD-2 lipopolysaccharide binding site. Cell. Microbiol. 9, 1191–1202 (2007).
Silipo, A., Lanzetta, R., Amoresano, A., Parrilli, M. & Molinaro, A. Ammonium hydroxide hydrolysis: a valuable support in the MALDI-TOF mass spectrometry analysis of lipid A fatty acid distribution. J. Lipid Res. 43, 2188–2195 (2002).
Rund, S., Lindner, B., Brade, H. & Holst, O. Structural analysis of the lipopolysaccharide from Chlamydia trachomatis serotype L2. J. Biol. Chem. 274, 16819–16824 (1999).
Que, N. L., Lin, S., Cotter, R. J. & Raetz, C. R. Purification and mass spectrometry of six lipid A species from the bacterial endosymbiont Rhizobium etli. Demonstration of a conserved distal unit and a variable proximal portion. J. Biol. Chem. 275, 28006–28016 (2000).
Aussel, L., Brisson, J. R., Perry, M. B. & Caroff, M. Structure of the lipid A of Bordetella hinzii ATCC 51730. Rapid Commun. Mass Spectrom. 14, 595–599 (2000).
Therisod, H., Monteiro, M. A., Perry, M. B. & Caroff, M. Helicobacter mustelae lipid A structure differs from that of Helicobacter pylori. FEBS Lett. 499, 1–5 (2001).
Bainbridge, B. W., Coats, S. R. & Darveau, R. P. Porphyromonas gingivalis lipopolysaccharide displays functionally diverse interactions with the innate host defense system. Ann. Periodontol. 7, 1–9 (2002).
Kumada, H., Haishima, Y., Umemoto, T. & Tanamoto, K.-I. Structural study on the free lipid A isolated from lipopolysaccharide of Porphyromonas gingivalis. J. Bacteriol. 177, 2098–2106 (1995).
Yi, E. C. & Hackett, M. Rapid isolation method for lipopolysaccharide and lipid A from Gram-negative bacteria. Analyst 125, 651–656 (2000).
Reife, R. A. et al. Porphyromonas gingivalis lipopolysaccharide lipid A heterogeneity: differential activities of tetra- and penta-acylated lipid A structures on E-selectin expression and TLR4 recognition. Cell. Microbiol. 8, 857–868 (2006).
Fujiwara, T., Ogawa, T., Sobue, S. & Hamada, S. Chemical, immunobiological and antigenic characterizations of lipopolysaccharides from Bacteroides gingivalis strains. J. Gen. Microbiol. 136, 319–326 (1990).
Zhang, Y., Gaekwad, J., Wolfert, M. A. & Boons, G. J. Synthetic tetra-acylated derivatives of lipid A from Porphyromonas gingivalis are antagonists of human TLR4. Org. Biomol. Chem. 6, 3371–3381 (2008).
Kumada, H. et al. Biological properties of the native and synthetic lipid A of Porphyromonas gingivalis lipopolysaccharide. Oral Microbiol. Immunol. 23, 60–69 (2008).
Sawada, N., Ogawa, T., Asai, Y., Makimura, Y. & Sugiyama, A. Toll-like receptor 4-dependent recognition of structurally different forms of chemically synthesized lipid As of Porphyromonas gingivalis. Clin. Exp. Immunol. 148, 529–536 (2007).
Al-Qutub, M. N. et al. Hemin-dependent modulation of the lipid A structure of Porphyromonas gingivalis lipopolysaccharide. Infect. Immun. 74, 4474–4485 (2006).
Coats, S. R. et al. Human Toll-like receptor 4 responses to P. gingivalis are regulated by lipid A 1- and 4'-phosphatase activities. Cell. Microbiol 11, 1587–1599 (2009).
Lu, Q., Darveau, R., Samaranayke, L., Wang, C., Lijian, J. Differential modulation of human β-defensins expression in human gingival epithelia by Porphyromonas gingivalis lipopolysaccharide with tetra- and penta-acylated lipid A structures. Innate Immun. 15, 325–335 (2009).
Quinchia-Rios, B. H. et al. Down-regulation of epidermal growth factor receptor-dependent signaling by Porphyromonas gingivalis lipopolysaccharide in life-expanded human gingival fibroblasts. J. Periodont. Res. 43, 290–304 (2008).
Cardelli, P. et al. The modification of the extracellular matrix synthesized in vitro by human gingival fibroblasts in relation to aging. G. Chir. 13, 83–86 (1992) (in Italian).
Marsh, P. D. Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC Oral Health 6 (Suppl. 1), S14 (2006).
Kuramitsu, H. K., He, X., Lux, R., Anderson, M. H. & Shi, W. Interspecies interactions within oral microbial communities. Microbiol. Mol. Biol. Rev. 71, 653–670 (2007).
Costerton, W. et al. The application of biofilm science to the study and control of chronic bacterial infections. J. Clin. Invest. 112, 1466–1477 (2003).
Darveau, R. P. et al. Ability of bacteria associated with chronic inflammatory disease to stimulate E-selectin expression and promote neutrophil adhesion. Infect. Immun. 63, 1311–1317 (1995).
Grenier, D. & Mayrand, D. Functional characterization of extracellular vesicles produced by Bacteroides gingivalis. Infect. Immun. 55, 111–117 (1987).
Schwartz, J., Stinson, F. L. & Parker, R. B. The passage of tritiated bacterial endotoxin across intact gingival crevicular epithelium. J. Periodontol. 43, 270–276 (1972).
Potempa, J., Banbula, A. & Travis, J. Role of bacterial proteinases in matrix destruction and modulation of host responses. Periodontol. 2000 24, 153–192 (2000).
Tanner, A. C. & Izard, J. Tannerella forsythia, a periodontal pathogen entering the genomic era. Periodontol. 2000 42, 88–113 (2006).
Finlay, B. B. & Falkow, S. Common themes in microbial pathogenicity. Microbiol. Rev. 53, 210–230 (1989).
McNab, R. et al. LuxS-based signaling in Streptococcus gordonii: autoinducer 2 controls carbohydrate metabolism and biofilm formation with Porphyromonas gingivalis. J. Bacteriol. 185, 274–284 (2003).
Yuan, L., Hillman, J. D. & Progulske-Fox, A. Microarray analysis of quorum-sensing-regulated genes in Porphyromonas gingivalis. Infect. Immun. 73, 4146–4154 (2005).
Yuan, L., Rodrigues, P. H., Belanger, M., Dunn, W. A. Jr & Progulske-Fox, A. Porphyromonas gingivalis htrA is involved in cellular invasion and in vivo survival. Microbiology 154, 1161–1169 (2008).
Xie, H., Lin, X., Wang, B. Y., Wu, J. & Lamont, R. J. Identification of a signalling molecule involved in bacterial intergeneric communication. Microbiology 153, 3228–3234 (2007).
Chalmers, N. I., Palmer, R. J. Jr., Cisar, J. O. & Kolenbrander, P. E. Characterization of a Streptococcus sp-Veillonella sp. community micromanipulated from dental plaque. J. Bacteriol. 190, 8145–8154 (2008).
Ramsey, M. M. & Whiteley, M. Polymicrobial interactions stimulate resistance to host innate immunity through metabolite perception. Proc. Natl Acad. Sci. USA 106, 1578–1583 (2009).
Haffajee, A. D. & Socransky, S. S. Microbiology of periodontal diseases: introduction. Periodontol. 2000 38, 9–12 (2005).
Bonito, A. J., Lux, L. & Lohr, K. N. Impact of local adjuncts to scaling and root planing in periodontal disease therapy: a systematic review. J. Periodontol. 76, 1227–1236 (2005).
Rifkin, B. R., Vernillo, A. T. & Golub, L. M. Blocking periodontal disease progression by inhibiting tissue-destructive enzymes: a potential therapeutic role for tetracyclines and their chemically-modified analogs. J. Periodontol. 64, 819–827 (1993).
Kirkwood, K. L., Cirelli, J. A., Rogers, J. E. & Giannobile, W. V. Novel host response therapeutic approaches to treat periodontal diseases. Periodontol. 2000 43, 294–315 (2007).
Booth, V., Ashley, F. P. & Lehner, T. Passive immunization with monoclonal antibodies against Porphyromonas gingivalis in patients with periodontitis. Infect. Immun. 64, 422–427 (1996).
Page, R. C. et al. Immunization of Macaca fascicularis against experimental periodontitis using a vaccine containing cysteine proteases purified from Porphyromonas gingivalis. Oral Microbiol. Immunol. 22, 162–168 (2007).
O' Brien-Simpson, N. M. et al. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J. Immunol. 175, 3980–3989 (2005).
Katz, J., Black, K. P. & Michalek, S. M. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infect. Immun. 67, 4352–4359 (1999).
Momoi, F. et al. Nasal vaccination with the 40-kilodalton outer membrane protein of Porphyromonas gingivalis and a nontoxic chimeric enterotoxin adjuvant induces long-term protective immunity with reduced levels of immunoglobulin E antibodies. Infect. Immun. 76, 2777–2784 (2008).
Potempa, J., Sroka, A., Imamura, T. & Travis, J. Gingipains, the major cysteine proteinases and virulence factors of Porphyromonas gingivalis: structure, function and assembly of multidomain protein complexes. Curr. Protein Pept. Sci. 4, 397–407 (2003).
Miyachi, K., Ishihara, K., Kimizuka, R. & Okuda, K. Arg-gingipain A DNA vaccine prevents alveolar bone loss in mice. J. Dent. Res. 86, 446–450 (2007).
Van Dyke, T. E. Control of inflammation and periodontitis. Periodontol. 2000 45, 158–166 (2007). This article describes a new class of pro-resolving agents and points out that active resolution of an inflammatory response can restore host homeostasis in chronic inflammatory diseases; periodontitis is used as an example of this fact.
Serhan, C. N., Chiang, N. & Van Dyke, T. E. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Rev. Immunol. 8, 349–361 (2008).
Graves, D. et al. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J. Clin. Periodontol. 35, 89–105 (2008).
Acknowledgements
The author thanks M. Curtis for critical review of the manuscript, M. Thomashow and F. Roberts for fruitful discussions and C. Zenobia for help with the figures. He also thanks the past and present members of his lab and department for continued inspiring conversations. The editorial assistance of N. Balch is greatly appreciated. Work in the author's laboratory is supported by the National Institute of Dental and Craniofacial Research.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
Entrez Genome
Entrez Genome Project
Aggregatibacter actinomycetemcomitans
FURTHER INFORMATION
Glossary
- Periodontium
-
The epithelial, connective and bone tissues that both surround and support the teeth. The word comes from the Greek terms 'peri', meaning 'around', and -'odons', meaning 'tooth'.
- Dental plaque
-
A polymicrobial biofilm community that builds up on the surface of the tooth and tooth root. Plaque can also become mineralized and form a calculus.
- Microbial-shift disease
-
A disease caused by a decrease in the number of beneficial symbionts and/or an increase in the number of pathogens. This concept is also known as dysbiosis.
- Junctional epithelium
-
A specialized epithelium located at the interface between the gingival sulcus, which is populated with bacteria, and the periodontal soft and mineralized connective tissues. It connects the tooth surface to the host tissue.
- Germ-free mice
-
Mice that are completely devoid of bacteria. They are generated by sterile Caesarean section, raised aseptically in an isolator with sterile filtered air and housed using sterile food, water and bedding. Germ-free mice are distinct from specific-pathogen-free (SPF) mice, which are devoid of only known mouse pathogens and still contain intestinal bacteria.
Rights and permissions
About this article
Cite this article
Darveau, R. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8, 481–490 (2010). https://doi.org/10.1038/nrmicro2337
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2337
This article is cited by
-
Transcriptomic analysis identifies diagnostic genes in polycystic ovary syndrome and periodontitis
European Journal of Medical Research (2024)
-
Triptolide mitigates the inhibition of osteogenesis induced by TNF-α in human periodontal ligament stem cells via the p-IκBα/NF-κB signaling pathway: an in-vitro study
BMC Complementary Medicine and Therapies (2024)
-
The oral microbiome: diversity, biogeography and human health
Nature Reviews Microbiology (2024)
-
Porphyromonas gingivalis Lipopolysaccharide Damages Mucosal Barrier to Promote Gastritis-Associated Carcinogenesis
Digestive Diseases and Sciences (2024)
-
Screening of feature genes related to immune and inflammatory responses in periodontitis
BMC Oral Health (2023)