Key Points
-
Leptospira spp. belong to the bacterial phylum Spirochaetes, an evolutionarily and structurally unique group of bacteria. The genus Leptospira is composed of saprophytic and pathogenic species that are all fastidious and slow-growing bacteria.
-
Leptospirosis is a zoonotic disease with a worldwide distribution. More than half a million cases are reported annually. Acute disease and chronic colonization can be reproduced in experimental animal models.
-
Determination and comparison of the genome sequences of saprophytic and pathogenic strains could shed light on the virulence determinants involved in disease.
-
Compared to other bacterial species, work to gather genetic data for leptospires and to elucidate the molecular basis of the pathogenesis of these organisms is in its infancy.
-
Further development of genetic tools should allow a better understanding of the virulence and survival mechanisms that are used by these bacteria to ensure their persistence in different ecological niches.
Abstract
Leptospirosis is a zoonotic disease that has emerged as an important cause of morbidity and mortality among impoverished populations. One hundred years after the discovery of the causative spirochaetal agent, little is understood about Leptospira spp. pathogenesis, which in turn has hampered the development of new intervention strategies to address this neglected disease. However, the recent availability of complete genome sequences for Leptospira spp. and the discovery of genetic tools for their transformation have led to important insights into the biology of these pathogens and their pathogenesis. We discuss the life cycle of the bacterium, the recent advances in our understanding and the implications for the future prevention of leptospirosis.
Similar content being viewed by others
Main
Descriptions of leptospirosis-like syndromes were reported in the scripts of ancient civilizations1, but the first modern clinical description of leptospirosis was published by Weil in 1886 (Ref. 2). In a landmark study in 1916, Inada et al. isolated leptospires, identified the organism as the causal agent of leptospirosis and determined that rats are a reservoir for transmission to humans3. Leptospires were subsequently isolated from a wide range of animal reservoir species and classified into serogroups and serovars as a function of their antigenic determinants (Box 1).
Leptospirosis, a zoonotic disease with a worldwide distribution, is now recognized as an emerging infectious disease4. Over the past decade, outbreaks during sporting events, adventure tourism and disasters have underscored its ability to become a public health problem in non-traditional settings4,5,6. However, leptospirosis is a neglected disease that places its greatest burden on impoverished populations from developing countries and tropical regions6. In addition to being an endemic disease of subsistence farmers1,4,5, leptospirosis has emerged as a widespread problem in urban slums, where inadequate sanitation has produced the conditions for rat-borne transmission7,8,9. More than 500,000 cases of severe leptospirosis are reported each year, with case fatality rates exceeding 10%10. This Review focuses on the pathogenesis of leptospirosis and then highlights the recent advances in the field with respect to the genetic approaches that have been recently developed and the virulence factors that have been discovered.
The question mark-shaped bacterium
The genus Leptospira belongs to the phylum Spirochaetes and comprises saprophytic and pathogenic species11(Box 1). Saprophytic leptospires, such as Leptospira biflexa , are free-living organisms found in water and soil and, unlike pathogenic Leptospira spp., do not infect animal hosts1. Leptospires are thin, highly motile, slow-growing obligate aerobes with an optimal growth temperature of 30 °C and can be distinguished from other spirochaetes on the basis of their unique hook or question mark-shaped ends12 (Fig. 1a).
a | Leptospires are thin, helical bacteria with a diameter of 0.15 μm and a length ranging from 10 to 20 μm. The motility of leptospires is dependent on the presence of two endoflagella (or periplasmic flagella), one arising at each end of the spirochete, that extend along the cell body without overlapping in the central part of the cell. b | A scanning electron micrograph of a Leptospira interrogans biofilm on a glass surface. c | A scanning electron micrograph of L. interrogans adhered to polarized Mardin-Darby canine kidney cell monolayers. d | A photomicrograph of a Warthin-Starry-stained section of kidney tissue from a captured sewer rat ( Rattus norvegicus ). Leptospires are seen as silver-impregnated, filamentous structures in the proximal renal tubule lumen (×400 magnification).
In addition to L. biflexa, the genomes of two pathogenic species, Leptospira interrogans and Leptospira borgpetersenii , have been sequenced13,14,15,16. Most (77–81%) of the genes in leptospiral genomes do not have orthologues in the genomes of other spirochaetes, indicating the large degree by which leptospires have diverged from other members of the phylum11. Furthermore, comparative analysis of the genomes of the pathogenic and saprophytic species16,17 has provided insights into the genetic determinants that may be involved in pathogenesis (Box 2).
The transmission cycle
Transmission of leptospirosis requires continuous enzootic circulation of the pathogen among animal reservoirs or, as they are commonly called, maintenance hosts (Fig. 2). Leptospira serovars demonstrate specific, although not entirely exclusive, host preferences; for example, rats serve as reservoirs for the Icterohaemorragiae serogroup, whereas house mice ( Mus musculus ) are the reservoir for the Ballum serogroup4,5,18. Furthermore, serovars often do not cause serious disease in reservoir hosts to which they are highly adapted.
Mammalian species excrete leptospiral pathogens in their urine and serve as reservoirs for their transmission. The pathogens are maintained in sylvatic and domestic environments by transmission among rodent species. In these reservoirs, infection produces chronic, asymptomatic carriage. Leptospires can then infect livestock and domestic and wild animals and cause a range of disease manifestations and carrier states. Maintenance of leptospirosis in these populations is due to their continued exposure to rodent reservoirs or to transmission within animal herds. Leptospirosis is transmitted to humans by direct contact with reservoir animals or by exposure to environmental surface water or soil that is contaminated with their urine. Leptospires penetrate abraded skin or mucous membranes, enter the bloodstream and disseminate throughout the body tissue. Infection causes an acute febrile illness during the early 'leptospiraemic' phase and progresses during the late 'immune' phase to cause severe multisystem manifestations such as hepatic dysfunction and jaundice, acute renal failure, pulmonary haemorrhage syndrome, myocarditis and meningoencephalitis. Although the immune response eventually eliminates the pathogens, leptospires may persist for prolonged periods in immunoprivileged sites, such as the renal tubules and the anterior chamber and vitreous humor of the eye, where they can produce, respectively, urinary shedding weeks after resolution of the illness and uveitis months after exposure. Humans are an accidental host and do not shed sufficient numbers of leptospires to serve as reservoirs for transmission.
Leptospires colonize and are shed from the renal tubules of a wide range of animals (see Supplementary information S1 (box)). The bacteria survive for weeks or even months in moist soil and water after excretion in the urine19. Cell aggregation19 and biofilm formation20 (Fig. 1b) may contribute to the survival of leptospires outside their hosts.
Disease pathogenesis
Pathogenic Leptospira spp. produce a systemic infection after environmental exposure, establish persistent renal carriage and urinary shedding in reservoir animals and cause tissue damage in multiple organs of susceptible hosts. Acute disease and chronic colonization represent the opposite poles of a wide range of disease presentations (see Supplementary information S1 (box)). Humans are incidental hosts: pathogenic Leptospira spp. cause acute disease manifestations but do not induce a carrier state that is required for their transmission.
Dissemination in the host. Leptospires penetrate abraded skin and mucous membranes and quickly establish a systemic infection by crossing tissue barriers and by haematogenous dissemination1. It was thought that leptospires, like other spirochaetes, spread through intercellular junctions21. However, they have been shown to efficiently enter host cells in vitro22,23 and to rapidly translocate across polarized cell monolayers without altering the trans-epithelial electrical resistance24,25. Leptospires are not facultative intracellular organisms; they are rarely observed within host cells but instead seem to reside only transiently within these cells as they cross cell monolayers in vitro25. The process by which leptospires enter host cells is not understood. Internalized leptospires have been observed in cytoplasmic24,25 and phagosomal compartments23 of normally non-phagocytic host cells. These findings suggest that leptospires use host cell entry and rapid translocation as mechanisms to spread to target organs and evade immune killing.
Infection causes prolonged leptospiraemia until the host mounts an effective acquired immune response, which occurs one to two weeks after exposure26(Fig. 3a). Leptospires can be isolated from the bloodstream within minutes after inoculation1 and are detected in multiple organs by the third day after infection26,27,28,29; they may reach 106–107 organisms per ml or per g in the blood and tissues of patients30,31 and infected animals29. Leptospires evade the host innate immune response during the initial stages of infection. They are resistant to the alternative pathway of complement activation32,33 and acquire complement factor H and related fluid-phase regulators34,35 through ligands such as the leptospiral endostatin-like (Len) proteins36,37. The host complement fragment C4b-binding protein alpha chain (C4BPA) binds to the surface of leptospires38, suggesting that a similar process may confer some protection against the classical pathway of complement activation.
a | The kinetics of leptospiral infection and disease. Infection produces leptospiraemia in the first few days after exposure, which is followed by migration of leptospires to the tissues of multiple organs by the third day of infection. In humans, a fever develops, with the appearance of agglutinating antibodies 5–14 days after exposure. Leptospires are cleared from the bloodstream and organs as the titres of serum agglutinating antibodies increase. Although early-phase illness is mild and resolves in most infected individuals, a subset of patients develop severe late-phase manifestations 4–6 days after the onset of illness, during the period of immune-mediated destruction and clearance of leptospires. b | Survival curves for hamsters during experimental leptospirosis. Inoculation with increasing amounts of Leptospira interrogans strain Fiocruz L1-130 is associated with a shortening of the incubation period and decreased survival among Golden Syrian hamsters.
Persistent colonization. The essential component of the life cycle of pathogenic Leptospira spp. is their ability to give rise to persistent renal carriage in reservoir animals. In rats, leptospires cause a systemic infection but are subsequently cleared from all organs except the renal tubules28,39. Colonized tubules are densely populated with leptospires, which aggregate into an amorphous, biofilm-like structure (Fig. 1d). Rats have been shown to excrete leptospires in high concentrations (107 organisms per ml (Ref. 39)) for 9 months after experimental infection40.
Leptospires isolated from chronically infected rat kidneys have substantially higher amounts of lipopolysaccharide (LPS) O antigen than those isolated from the livers of hamsters with acute disease, suggesting that the expression of O antigen facilitates the induction of carriage39. The renal tubule is an immunoprivileged site, a feature that may contribute to high-grade persistence of the pathogen. Moreover, those leptospires that are shed in the urine downregulate the expression of proteins that are recognized by the humoral immune response in rats41.
Disease manifestations and determinants. The incubation period for leptospirosis is 5–14 days on average, with a range of 2–30 days1(Fig. 3a). In humans, leptospirosis causes a febrile illness that, in its early phase, often cannot be differentiated from other acute fevers. In most patients, illness resolves after the first week of symptoms. However, a subset (5–15%) of patients develop severe late-phase manifestations6. Unlike bacterial infections such as Gram-negative sepsis, leptospirosis does not cause fulminating disease shortly after the onset of illness, which may relate to the low endotoxic potency of leptospiral LPS1. Severe late-phase manifestations usually occur 4 to 6 days after the onset of illness (Fig. 3a) but can vary depending on the infecting inoculum dose and other disease determinants. Weil's disease is the classic presentation of severe leptospirosis and is characterized by jaundice, acute renal failure and bleeding. In addition, there is increasing awareness of an emerging severe disease form, leptospirosis-associated pulmonary haemorrhage syndrome (LPHS) (Box 3), for which the case fatality rate is more than 50%6.
The development of leptospirosis and disease progression are influenced by host susceptibility factors, the dose of the infecting inoculum and the virulence characteristics of the infecting strain. Certain Leptospira species and serovars are more frequently found to cause severe disease in humans than others42,43. A single circulating clone caused a large and sustained nationwide epidemic in Thailand44. Clonal transmission of strains has been described in other outbreaks and in settings of endemic transmission45,46 and may reflect localized transmission clusters45. However, the magnitude and duration of the epidemic in Thailand suggest that predominant clones may indeed possess specific factors that contribute to their overall biological success. The advent of high-throughput, whole-genome sequencing provides an opportunity to determine whether such factors exist by screening isolate genomes for genetic polymorphisms that are associated with clinical and transmission-related phenotypes.
Our understanding of the acquired and innate host factors that influence infection and disease progression remains limited. An investigation of a triathlon-related outbreak identified the human leukocyte antigen serotype HLA-DQ6 as the first and to date only genetic susceptibility factor for leptospirosis47. The authors found a synergistic risk interaction between HLA-DQ6 and swallowing water while swimming during the triathlon event. This environmental exposure was a likely proxy for an inoculum size effect. It is well known that increasing the inoculum size shortens the incubation period and decreases survival in a dose-dependent manner in experimental animals26,48 (Fig. 3b). The synergism between HLA-DQ6 and environmental exposure found during the triathlon outbreak constitutes the first gene–environment interaction to be identified for an infectious disease.
Tissue damage. The onset of disease correlates with the appearance of agglutinating antibodies and the clearance of leptospires by antibody-mediated opsonization and lysis1 (Fig. 3a). Vascular endothelial damage is a hallmark feature of severe leptospirosis49,50 and causes capillary leakage, haemorrhage and, in a subset of cases, vasculitis. Leptospirosis activates the coagulation cascade51,52 and has been reported to cause disseminated intravascular coagulation in up to 50% of patients with severe disease manifestations51.
Leptospiral components that are released after immune killing stimulate the production of pro-inflammatory cytokines53,54,55,56 and mediate inflammation and damage of end-organ tissues. The Jarisch-Herxheimer reaction, which is caused by the sudden release of these cytokines, is a complication of antimicrobial therapy for leptospirosis. Moreover, tumour necrosis factor may play a key part in disease progression, as levels of this cytokine are a predictor of poor clinical outcomes57.
Leptospiral LPS has been shown to activate Toll-like receptor 2 (TLR2) in human cells rather than the TLR4 pathway58, an unusual finding that may relate to a 1-methylphosphate moiety that is not found in the lipid Aof other bacteria59. In addition, leptospirallipoproteins induce innate immune responses by activating the TLR2 pathway58,60. However, leptospiral LPS activates both TLR2 and TLR4 pathways in mouse cells, indicating that there are species-specific differences with respect to TLR activation61. Leptospires stimulate the expansion ofγδ T cell populations in naive peripheral blood mononuclear cells, and patients with leptospirosis have increased numbers of these specific T cells54; this suggests that acquired cell-mediated responses may promote inflammation, in addition to the stimulation of inflammation by the innate and acquired humoral responses.
Leptospires can induce a peculiar hypokalaemic, non-oliguric form of acute renal failure that is characterized by impaired tubular Na+ reabsorption62. Although non-esterified unsaturated fatty acids derived from leptospires have been found to inhibit the kidney Na+–K+ ATPase63, it seems more plausible that the renal manifestations of infection are the direct result of a focal tubulo-interstitial nephritis. Leptospiral outer membrane proteins, such as LipL32, activate TLR-dependent pathways, which lead to the activation of nuclear factor-κB, mitogen-activated kinases and cytokines and, subsequently, to tubular damage60. Furthermore, activation of these pathways may provide an explanation for the dysregulation of Na+ transporters in infected kidneys, a finding that has been shown to be associated with impaired Na+ reabsorption64,65.
Leptospires can induce apoptosis in macrophages and hepatocytes22,66,67, but the overall contribution of apoptosis to disease pathogenesis has not been clarified. Leptospirosis elicits the production of autoantibodies, such as cardiolipin-specific antibodies68 and several reports suggest that autoimmune mechanisms may play a part in the development of uveitis37 and LPHS (Box 3)69 during infection.
Genetic tools for Leptospira spp.
The virulence mechanisms and, more generally, the biology of the causative agents of leptospirosis remain largely unknown. Before 2000, the lack of genetic tools available for use in pathogenic or saprophytic leptospires precluded the full characterization of genes of interest. In the first genetic studies, carried out in the 1990s, several Leptospira spp. genes were isolated by the functional complementation of Escherichia coli mutants. This method led to the identification of the L. biflexa recombinase A (reca) gene70, the L. interrogans rfb genes71 and a number of amino acid biosynthesis genes, such as aspartate semi-aldehyde dehydrogenase (asd) and the tryptophan biosynthesis gene trpE72,73.
The origin of replication from the LE1 temperate leptophage74, a 74 kb extrachromosomal element of L. biflexa16 and a genomic island that can excise from the L. interrogans chromosome75 were used to generate a plasmid vector that can replicate autonomously in both L. biflexa and E. coli76. However, although DNA can be introduced into leptospires by electroporation76,77 and conjugation78, there is currently no replicative plasmid vector available for pathogenic leptospires.
Deletion of chromosomal genes (including trpE, recA, the haeme synthetase gene hemH, the flagellar filament core proteingene flaB and the methionine biosynthesis genes metY, metX and metW) was achieved by targeted mutagenesis in the saprophyte L. biflexa using a suicide plasmid79. Recently the first gene to be successfully targeted, ligB, was disrupted in the pathogenic L. interrogans80 by site-directed homologous recombination.
A system for random mutagenesis using the Himar1 mariner transposon has been developed for various Leptospira spp. strains77,81,82. In L. biflexa an extensive library of mutants can be generated and screened for phenotypes that affect diverse aspects of metabolism and physiology, such as amino acid biosynthesis and iron acquisition systems82,83. However, pathogenic leptospires remain much less easily transformable with Himar1 (Ref. 77). Transformation experiments with L. interrogans that were performed in two different laboratories resulted in approximately 1,000 random mutations, of which 721 affected the protein-coding regions of 551 different genes81 (Table 1). Further improvements to the methods and the identification of more readily transformable L. interrogans strains may allow the generation of a mutant library for high-throughput screening for specific processes that are known to be involved in pathogenesis.
Animal models of virulence
Guinea pigs and hamsters are the standard experimental models for acute leptospirosis1. Infection with low inocula (<100 leptospires) produces similar disease kinetics (Fig. 3b) and severity to those observed in humans48. Mice and gerbils have been used to study the genetics of the immune response to leptospirosis61,84,85 and as models for vaccine-mediated immunity86 (Table 2). However, mice are relatively resistant to infection and high inocula (up to 108 organisms) are required to produce disease, a situation that may not parallel natural exposure. Furthermore, when mice do develop disease it is more fulminant and they tend to die within markedly shorter intervals (5 days) than hamsters infected with low-inoculum lethal challenges (Fig. 3b). This finding raises concerns that this experimental animal model may not reproduce the disease dynamics and pathogenic processes that are observed in natural infections. Rats have been used as a model system to study persistent colonization but also require high inocula28,39. Similar to the situation in mice, it is not understood why this common reservoir in nature is relatively difficult to infect experimentally. Natural infection with leptospirosis occurs in non-human primates, which have been used as models to study the disease87 and, more recently, the development of pulmonary haemorrhage syndrome88.
Virulence factors
The virulence factors that have been identified to date are primarily surface proteins, which are thought to mediate the interaction between the bacterium and the host tissues. Although several proteins are secreted by Leptospira spp., including degradative enzymes, there is no evidence for a dedicated protein secretion pathway similar to the type III and type IV secretion machinery that is used by Gram-negative bacteria to inject proteins into host cells.
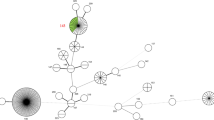
Virulent leptospires, but not culture-attenuated or saprophytic organisms, adhere to and enter mammalian host cells22,24,25,89(Fig. 1c). Proteins that are present on the surface of leptospires, including several that have been shown to bind in vitro to various components of the extracellular matrix36,90,91,92,93,94are thought to mediate the host cell–leptospire interaction (Fig. 4). Virulent leptospires have different protein and LPS profiles from those of culture-attenuated strains and have fewer protein particles on the outer membrane surface, as determined by freeze-fracture electron microscopy95.
Leptospires have an inner membrane (IM) and an outer membrane (OM). The peptidoglycan cell wall is associated with the IM100 and together these contain the FeoA–FeoB-type iron transporter (FeoAB)83, penicillin-binding proteins (PBPs) and the lipoprotein LipL31. The leptospiral OM contains lipopolysaccharide (LPS), the transmembrane porin outer membrane protein L1 (OmpL1) and the lipoproteins LipL32, LipL36 (on the inner surface of the OM), LipL41 and LigB. Several TonB-dependent receptors (TBDRs) were identified by genome analysis, of which three are involved in the transport of iron citrate, the siderophore desferrioxamine and hemin83,154. Transport requires energy transduction from the TonB–ExbB–ExbD complex in the inner membrane (for simplicity, only one TonB–ExbB–ExbD–TBDR system is shown). Leptospira spp. possess orthologues of the Escherichia coli export systems that transport OMPs and lipoproteins155, including the IM lipoprotein signal peptidase I (SPase I) and SPase II. Lipoproteins are first transported through the Sec system and then bind to the ABC transporter formed by LolC, LolD and LolE. OMPs are transported through the Sec translocon, bound by the periplasmic chaperone Skp and then bound by Omp85 before being integrated into the lipid bilayer. An incomplete set of type II secretion-like genes is also present in the Leptospira spp. genomes. Several cytoplasmic membrane ABC transporters are found in leptospires, including a metallic cation uptake family ABC transporter83. As in other spirochaetes, the endoflagella are located in the periplasm. The surface-exposed Loa22, leptospiral endostatin-like protein A (LenA), LenD, LigA and LigC proteins are not shown but are also known to be present at the surface of leptospires.
Like those of other spirochaetes, the genomes of Leptospira spp. encode more lipoproteins than non-spirochaete genomes. The L. interrogans genome contains approximately 145 genes that encode putative lipoproteins96 in addition to putative extracellular and outer membrane proteins97,98.
Consistent with the predicted ability of Leptospira spp. to migrate through host tissues, the genomes of these organisms encode a wide range of putative haemolysins and proteases that may facilitate this process. An analysis of the L. interrogans genome identified nine genes that encode putative haemolysins, including a pore-forming protein99 and a sphingomyelinase16 that are not found in the saprophyte L. biflexa. The L. interrogans genome also contains a microbial collagenase that is proposed to be involved in the destruction of host tissues.
Few proteins have been shown experimentally to be present on the leptospiral surface100. Approximately 12 outer membrane proteins, including OmpL1 (Ref. 101), LipL32 (Ref. 102), immunoglobulin-like repeat containing protein B (LigB)103, LenA36, LenD36 and Loa22 (Ref. 104) have been identified. Our knowledge of the outer surface of leptospires therefore remains limited, and further development of tools for the accurate localization of surface-associated determinants is required.
Loa22. The only gene found to date that fulfils Koch's molecular postulates for a virulence factor gene is loa22. In L. interrogans, disruption of loa22 by Himar1 insertion led to a complete loss of virulence in the guinea pig disease model104(Table 1). Loa22 is exposed on the bacterial surface104 and recognized by sera from human patients with leptospirosis105 and its expression is upregulated in an acute model of infection106. The in vitro binding between Loa22 and components of the extracellular matrix is weak107. The carboxyl terminus of Loa22 consists of an OmpA domain, which contains a predicted peptidoglycan-binding motif. Although the non-pathogenic L. biflexa genome contains an orthologue of loa22 (Ref. 16), differential expression of this gene in pathogenic and non-pathogenic leptospires or pathogen-specific sialylation of Loa22, which is dependent on pathogen-specific sialic acid modification pathways (J. Ricaldi and J. Vinetz, personal communication), may explain why this protein is a crucial determinant of L. interrogans virulence.
LipL32. LipL32 (Ref. 102) (also known as haemolysis-associated protein 1 (Hap1)108 is surface exposed102 and accounts for 75% of the outer membrane proteome109. This lipoprotein is highly conserved among pathogenic Leptospira spp.110, whereas there are no orthologues in the saprophytic L. biflexa16. LipL32 was long thought to be a putative virulence factor. Higher levels of LipL32 are expressed in leptospires during acute lethal infections than during in vitro culture106. In addition, the C terminus of LipL32 binds in vitro to laminin, collagen I, collagen IV, collagen V and plasma fibronectin92,93. Furthermore, the crystal structure of LipL32 was recently elucidated and shown to have structural homologies with proteins such as collagenase that bind to components of the extracellular matrix111. However, a LipL32-mutant strain, obtained by Himar1 insertion mutagenesis, was found to be as efficient as the wild-type strain in causing acute disease and chronic colonization in experimental animals112(Table 1). The role of this major outer membrane protein in pathogenesis remains unclear.
Leptospiral immunoglobulin-like proteins. Three high-molecular-mass Leptospira spp. proteins — LigA, LigB and LigC — are recently characterized members of the bacterial immunoglobulin-like protein superfamily86,103,113. Lig proteins are anchored to the outer membrane and have 12 to 13 tandem bacterial immunoglobulin-like repeat domains. Like lipL32, lig genes are exclusively present in pathogenic Leptospira spp. Recombinant Lig proteins bind in vitro to host extracellular matrix proteins, including fibronectin, fibrinogen, collagen and laminin94,114. Furthermore, the repeat domain portion of the LigB molecule binds Ca2+, which seems to enhance its ability to adhere to fibronectin115. The lig genes are upregulated under physiological osmolarity116 and encode surface-exposed proteins that are strongly recognized by sera from patients with leptospirosis103,117,118. Lig proteins are considered to be putative virulence factors103, as members of the bacterial immunoglobulin-like protein superfamily mediate pathogen–host cell interactions, such as invasion and host cell attachment, in other bacteria. However, a ligB mutation in L. interrogans, which also contains a ligA gene80, does not affect the ability of the bacterium to cause acute leptospirosis in hamsters or persistent renal colonization in rats. The presence of several other putative adhesins with potentially redundant functions, including LigA, may have obscured the detection of clear phenotypes for the ligB mutant.
Other potential virulence proteins. The motility of the bacteria may be of relevance to their basic biology and, despite also being common to saprophytes, may be considered a virulence factor. Freshly isolated pathogenic leptospires have higher translational and helical motility than strains passaged in vitro119. Their corkscrew motility allows these organisms to swim through gel-like media such as connective tissues12. However, it has not been determined whether loss of motility results in attenuation of virulence for pathogenic leptospires. L. biflexa flaB mutants cannot form functional endoflagella, but their cell bodies remain intact and helical120. The endoflagella are therefore not responsible for dictating the helical shape of the cell body in Leptospira spp., as they are in Borrelia burgdorferi 121. Proteins that are involved in the morphogenetic system of rod-shaped bacteria, such as MreB, MreC, MreD and penicillin-binding proteins, are encoded in the leptospiral genome. Leptospiral cell morphology may therefore be determined by the cytoskeleton and maintained by the rigid murein layer.
Multiple methyl-accepting chemotaxis proteins have been identified in Leptospira spp., suggesting that chemotactic responses to various chemoattractants and repellents occurs. Unlike saprophytic strains, L. interrogans displays positive chemotaxis towards haemoglobin122.
Iron acquisition is important for virulence in many bacterial pathogens, and Leptospira spp. contain several iron-uptake systems, including TonB-dependent outer membrane receptors83. Leptospira spp. possess a haem oxygenase, encoded by hemO, that degrades the tetrapyrrole ring of the haem molecule, releasing ferrous iron. Disruption of the hemO gene in L. interrogans decreases virulence in the hamster model of leptospirosis123(Table 1), suggesting that Leptospira spp. use haem as their principal source of iron during infection.
Two attenuated L. interrogans mutants have disruptions in genes that encode hypothetical proteins that may be virulence factors81, but these findings need to be confirmed with complementation studies.
Previous microarray studies have shown that exposing L. interrogans to the osmolarity conditions found in host tissues induces a profound shift in its global transcription profile. Therefore, osmolarity and temperature116,124 are important factors for regulating the expression of proteins that mediate the infection of mammalian hosts. Nineteen of the twenty-five most strongly salt-induced L. interrogans genes encode hypothetical proteins116. These proteins may be response regulators and environment-sensing proteins that are involved in survival or persistence in the environment or in the infected host. In addition, sphingomyelinase C is upregulated by increases in osmolarity to the levels that are found in mammalian host tissues116.
Immunity
The humoral response is thought to be the primary mechanism of immunity to leptospirosis125. LPS seems to be the main target for the protective antibody response: passive transfer of immunity correlates with levels of agglutinating LPS-specific antibodies in transferred sera126 and LPS-specific monoclonal antibodies passively protect naive animals from leptospirosis127. However, it is not known whether antibody responses against leptospiral antigens other than LPS also confer protection.
Recent work has confirmed that immunity to leptospirosis is not limited to the humoral response. Mice require intact TLR2 (Ref. 128) and TLR4 (Ref. 85) activation pathways to control a lethal infection. In contrast to immunity in hosts that are susceptible to acute leptospirosis, protective immunity against L. borgpetersenii serovar Hardjo in bovine reservoirs is cell mediated. Immunization trials in cattle found that protection against this serovar, conferred by whole-leptospire-based vaccines, correlated with T helper 1 cell responses and not with agglutinating antibody titres129,130,131
Vaccines
In 1916, Ido et al. provided the first demonstration that immunization with killed leptospires protects against experimental infection132. Since then, whole-leptospire-based vaccines have been routinely administered to livestock and domestic animals and used for immunization of human populations6. However, there are serious concerns about their use133. Whole-leptospire-based vaccines are associated with high rates of adverse reactions and confer only short-term, serovar-specific immunity1. Polyvalent vaccines are used to provide coverage for circulating serovar agents and must be reformulated at substantial cost when new serovars emerge134. Furthermore, whole-leptospire-based vaccines are not universally effective in preventing carriage, which limits their use as a transmission-blocking intervention.
Owing to these limitations, efforts have focused on developing subunit vaccine candidates (Table 2) — more specifically, on identifying surface-associated proteins that are conserved among serovars, and targets for bactericidal immune responses. The first evidence forthe feasibility of this approach was the demonstration that immunization with E. coli outer membrane vesicles containing recombinant LipL41 and OmpL1 partially protected against an otherwise lethal challenge of leptospires in hamsters135. Subsequently, LipL32 has been shown to elicit immunoprotection when administered in naked DNA136, Mycobacterium bovis bacille Calmette–Guérin (BCG)137 and adenoviral138 delivery systems. However, the overall efficacy of these candidate vaccines is low (40–75%) in experimental animals. The most promising subunit vaccine candidates are the Lig proteins, which have been shown to confer high-level protection (Table 2) approaching 100% in mice86 and hamsters139,140,141. The ability of Lig proteins to elicit cross-protective immunity to a range of serovar agents must be determined, as amino acid sequence identities for these proteins are 70–100% among Leptospira spp.142.
The availability of multiple genome sequences provides an opportunity to use high-throughput strategies to identify new vaccine candidates105. The ultimate goal for vaccine development is to identify a candidate that protects against multiple Leptospira species. The L. interrogans and L. borgpetersenii genomes share 2,708 open reading frames, of which 656 are not present in the L. biflexa genome15,16 (Box 2). Strategies to refine the number of target candidates include the sequencing of a wider representation of pathogenic Leptospira spp. genomes and the bioinformatic analysis and selection of open reading frames that are highly conserved among these genomes and that encode outer membrane proteins98. The main barrier to pursuing this strategy is the lack of in vitro correlates for immunity against leptospirosis. High-throughput screening in experimental animals may not be feasible given the expected number of candidate antigens. A priority for vaccine development will be to determine whether infection with leptospirosis protects against subsequent reinfection in high-risk populations and to identify the mechanisms of immunity that are involved. Until epidemiologically validated immune correlates are identified, the discovery of vaccine candidates will probably continue to rely on the search for new virulence factors and outer membrane proteins.
Conclusions and future directions
There has been impressive recent progress in our knowledge of the basic aspects of the biology and pathogenesis of Leptospira spp., although modern molecular genetics was not applied to pathogenic leptospires until 2005, with the generation of the first mutants in L. interrogans77. Further studies are required to explain why it is so difficult to introduce DNA into pathogenic leptospires by methods that are commonly used for other bacteria. More efficient methods are needed to test the roles of putative virulence factors. The presence of prophage-like loci in the genomes of pathogenic Leptospira spp.75,143 suggests that transduction may occur and that phages could be used as tools for gene transfer. Despite the large evolutionary distance between the pathogenic and non-pathogenic species, Leptospira spp. share a core of approximately 2,000 genes16 L. biflexa could therefore be used as a model bacterium to identify the functions of these common genes and to gain an insight into the general biology of Leptospira spp.
The development of genetic tools to transform leptospires has circumvented a substantial barrier to the elucidation of pathogen-related determinants of virulence and has led to the identification of Loa22 as the first virulence factor in Leptospira spp.104. LipL32 and Lig proteins were long thought to be virulence factors, but mutagenesis of the corresponding genes did not result in attenuation of virulence. This suggests that there may be a high degree of redundancy in function among virulence factors and that classical knockout approaches may not be useful in identifying such factors. There is therefore a real need to use convergent genomic, proteomic and metabolomic approaches to systematically identify molecular phenotypes and link these phenotypes with the pathogen's ability to cause disease in humans and animals. Our next hurdle is to learn more about leptospiral gene regulation and the interactions among leptospiral proteins. Microarrays are a valuable tool to identify regulatory networks or analyse the pleiotropic effects of a mutation. The use of genetically distinct (or engineered) laboratory rodents together with microarrays or proteomic studies should permit researchers to better delineate the mechanisms leading to chronic renal shedding. Ecological and metagenomic studies of soils will possibly provide information on the environmental persistence of leptospires, which remains poorly understood.
Both host and microbiological factors probably contribute to the severity of leptospiral infection. Further studies will allow us to determine whether severe disease manifestations, such as LPHS, are due to strain-specific factors that enhance the pathogen's virulence or to innate or acquired host immune responses and susceptibility factors. Elucidation of the molecular mechanisms of pathogenesis will contribute to the development of novel strategies for the treatment and prevention of leptospirosis. Such advances are urgently needed to address the large disease burden that is attributable to this emerging infectious disease in impoverished populations.
References
Faine, S., Adler, B., Bolin, C. & Perolat, P. Leptospira and Leptospirosis (MedScience, Melbourne, 1999).
Weil, A. Ueber eine eigenthümliche, mit Milztumor, Icterus und Nephritis einhergehende acute Infectionskrankheit. Dtsch. Arch. Klin. Med. 39, 209–232 (1886) (in German).
Inada, R., Ido, Y., Hoki, R., Kakeno, R. & Ito, H. The etiology, mode of infection and specific therapy of Weil's disease (Spirochaetosis icterohaemorrhagica). J. Exp. Med. 23, 377–403 (1916). A landmark paper that describes the discovery of Leptospira spp. as the aetiological agents of leptospirosis.
Levett, P. N. Leptospirosis. Clin. Microbiol. Rev. 14, 296–326 (2001). This comprehensive review provides wide coverage of our knowledge of leptospirosis.
Bharti, A. R. et al. Leptospirosis: a zoonotic disease of global importance. Lancet Infect. Dis. 3, 757–771 (2003). This review provides important complementary information to reference 4 and discusses the main issues facing the field of leptospirosis.
McBride, A. J., Athanazio, D. A., Reis, M. G. & Ko, A. I. Leptospirosis. Curr. Opin. Infect. Dis. 18, 376–386 (2005).
Ko, A. I., et al. Urban epidemic of severe leptospirosis in Brazil. Lancet 354, 820–825 (1999).
Riley, L. W., Ko, A. I., Unger, A. & Reis, M. G. Slum health: diseases of neglected populations. BMC Int. Health Hum. Rights 7, 2 (2007).
Reis, R. B. et al. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl. Trop. Dis. 2, e228 (2008).
WHO. Leptospirosis worldwide, 1999. Wkly Epidemiol. Rec. 74, 237–242 (1999).
Paster, B. J. et al. Phylogenetic analysis of the spirochetes. J. Bacteriol. 173, 6101–6109 (1991).
Li, C., Motaleb, M. A., Sal, M., Goldstein, S. F. & Charon, N. Gyrations, rotations, periplasmic flagella: the biology of spirochete motility. J. Mol. Microbiol. Biotechnol. 2, 345–354 (2000).
Ren, S. et al. Unique and physiological pathogenic features of Leptospira interrogans revealed by whole genome sequencing. Nature 422, 888–893 (2003). This article describes the first complete genome sequence of a leptospiralspecies.
Nascimento, A. L. et al. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. J. Bacteriol. 186, 2164–2172 (2004).
Bulach, D. M. et al. Genome reduction in Leptospira borgpetersenii reflects limited transmission potential. Proc. Natl Acad. Sci. USA 103, 14560–14565 (2006). This work showed that the L. borgpetersenii genome is undergoing a process of reduction that seems to have impaired its ability to survive in the external environment and has caused it to rely on direct contact as its mode of transmission.
Picardeau, M. et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE 3, e1607 (2008). This paper describes the genome of the model bacterium L. biflexa and its comparison to the pathogenic L. interrogans and L. borgpetersenii.
Xue, F., Yan, J. & Picardeau, M. Evolution and pathogenesis of Leptospira spp.: lessons learned from the genomes. Microbes Infect. 11, 328–333 (2008).
Thiermann, A. B. The Norway rat as a selective chronic carrier of Leptospira icterohaemorrhagiae. J. Wildl. Dis. 17, 39–43 (1981).
Trueba, G., Zapata, S., Madrid, K., Cullen, P. & Haake, D. Cell aggregation: a mechanism of pathogenic Leptospira to survive in fresh water. Int. Microbiol. 7, 35–40 (2004).
Ristow, P. et al. Biofilm formation by saprophytic and pathogenic leptospires. Microbiology 154, 1309–1317 (2008). References 19 and 20 describe key findings about the survival mechanisms of pathogenic Leptospira spp. outside the host.
Haake, D. A. & Lovett, M. A. Interjunctional invasion of endothelial cell monolayers. Methods Enzymol. 236, 447–463 (1994).
Merien, F., Baranton, G. & Perolat, P. Invasion of Vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect. Immun. 65, 729–738 (1997).
Liu, Y., Zheng, W., Li, L., Mao, Y. & Yan, J. Pathogenesis of leptospirosis: interaction of Leptospira interrogans with in vitro cultured mammalian cells. Med. Microbiol. Immunol. 196, 233–239 (2007).
Thomas, D. D. & Higbie, L. M. In vitro association of leptospires with host cells. Infect. Immun. 58, 581–585 (1990).
Barocchi, M. A., Ko, A. I., Reis, M. G., McDonald, K. L. & Riley, L. W. Rapid translocation of polarized MDCK cell monolayers by Leptospira interrogans, an invasive but nonintracellular pathogen. Infect. Immun. 70, 6926–6932 (2002). This study found that leptospires rapidly translocate across polarized cell monolayers by invading host cells and transiently residing in the cytoplasmic compartment.
Faine, S. Virulence in Leptospira. I. Reactions of guinea-pigs to experimental infections with Leptospira Icterohaemorrhagiae. Br. J. Exp. Pathol. 38, 1–7 (1957).
Faine, S. Virulence in Leptospira. II. The growth in vivo of virulent Leptospira icterohaemorrhagiae. Br. J. Exp. Pathol. 38, 8–14 (1957).
Athanazio, D. A. et al. Rattus norvegicus as a model for persistent renal colonization by pathogenic Leptospira interrogans. Acta Trop. 105, 176–180 (2008).
Lourdault, K., Aviat, F. & Picardeau, M. The use of quantitative real-time PCR to study the dissemination of Leptospira interrogans in the guinea pig infection model of leptospirosis. J. Med. Microbiol. 58, 648–655 (2009).
Segura, E. R. et al. Clinical spectrum of pulmonary involvement in leptospirosis in a region of endemicity, with quantification of leptospiral burden. Clin. Infect. Dis. 40, 343–351 (2005).
Truccolo, J., Serais, O., Merien, F. & Perolat, P. Following the course of human leptospirosis: evidence of a critical threshold for the vital prognosis using a quantitative PCR assay. FEMS Microbiol. Lett. 204, 17–321 (2001).
Cinco, M. & Banfi, E. Activation of complement by leptospires and its bactericidal activity. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 254, 261–265 (1983).
Johnson, R. C. & Muschel, L. H. Antileptospiral activity of serum. I. Normal and immune serum. J. Bacteriol. 91, 1403–1409 (1966).
Meri, T., Murgia, R., Stefanel, P., Meri, S. & Cinco, M. Regulation of complement activation at the C3-level by serum resistant leptospires. Microb. Pathog. 39, 139–147 (2005).
Verma, A. et al. LfhA, a novel factor H-binding protein of Leptospira interrogans. Infect. Immun. 74, 2659–2666 (2006). This study is the first report of a leptospiral complement factor H-binding protein that potentially contributes to both Leptospira spp.resistance to complement-mediated killing and the evasion of the immune response as the pathogen disseminates in the bloodstream.
Stevenson, B. et al. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS ONE 2, e1188 (2007).
Verma, A. et al. LruA and LruB antibodies in sera of humans with leptospiral uveitis. Clin. Vaccine Immunol. 15, 1019–1023 (2008).
Barbosa, A. S. et al. Immune evasion of Leptospira species by acquisition of human complement regulator C4BP. Infect. Immun. 77, 1137–1143 (2009).
Nally, J. E., Chow, E., Fishbein, M. C., Blanco, D. R. & Lovett, M. A. Changes in lipopolysaccharide O antigen distinguish acute versus chronic Leptospira interrogans infections. Infect. Immun. 73, 3251–3260 (2005). This interesting study suggests that differential expression of LPS O antigen influences the ability of the pathogen to cause an acute lethal infection versus chronic renal carriage.
Sterling, C. R. & Thiermann, A. B. Urban rats as chronic carriers of leptospirosis: an ultrastructural investigation. Vet. Pathol. 18, 628–637 (1981).
Monahan, A. M., Callanan, J. J. & Nally, J. E. Proteomic analysis of Leptospira interrogans shed in urine of chronically infected hosts. Infect. Immun. 76, 4952–4958 (2008).
Ganoza, C. A. et al. Determining risk for severe leptospirosis by molecular analysis of environmental surface waters for pathogenic Leptospira. PLoS Med. 3, e308 (2006). This investigation found that an outbreak of severe leptospirosis was associated with exposure to high concentrations of pathogenic leptospires in environmental surface waters. The work was pioneering in that it developed new molecular tools to quantify leptospires in the environment.
Matthias, M. A. et al. Human leptospirosis caused by a new, antigenically unique Leptospira associated with a Rattus species reservoir in the peruvian Amazon. PLoS Negl. Trop. Dis. 2, e213 (2008).
Thaipadungpanit, J. et al. A dominant clone of Leptospira interrogans associated with an outbreak of human leptospirosis in Thailand. PLoS Negl. Trop. Dis. 1, e56 (2007).
Slack, A., Symonds, M., Dohnt, M. & Smythe, L. An improved multiple-locus variable number of tandem repeats analysis for Leptospira interrogans serovar Australis: a comparison with fluorescent amplified fragment length polymorphism analysis and its use to redefine the molecular epidemiology of this serovar in Queensland, Australia. J. Med. Microbiol. 55, 1549–1557 (2006).
Zuerner, R. L. & Alt, D. P. Variable nucleotide tandem repeat analysis reveals a unique group of Leptospira interrogans serovar Pomona isolates associated with California sea lions. J. Clin. Microbiol. 47, 1202–1205 (2009).
Lingappa, J. et al. HLA-DQ6 and ingestion of contaminated water: possible gene-environment interaction in an outbreak of Leptospirosis. Genes Immun. 5, 311–314 (2004). This is the first report of a host genetic susceptibility factor for leptospirosis and also of a gene–environment interaction for an infectious disease.
Silva, E. F. et al. Characterization of virulence of Leptospira isolates in a hamster model. Vaccine 26, 3892–3896 (2008).
Nicodemo, A. C. et al. Lung lesions in human leptospirosis: microscopic, immunohistochemical, and ultrastructural features related to thrombocytopenia. Am. J. Trop. Med. Hyg. 56, 181–187 (1997).
De Brito, T., Böhm, G. M. & Yasuda, P. H. Vascular damage in acute experimental leptospirosis of the guinea-pig. J. Pathol. 128, 177–182 (1979).
Chierakul, W. et al. Activation of the coagulation cascade in patients with leptospirosis. Clin. Infect. Dis. 46, 254–260 (2008). This study found that activation of the coagulation cascade, including disseminated intravascular coagulation, is a prominent feature in leptospirosis.
Wagenaar, J. F. et al. What role do coagulation disorders play in the pathogenesis of leptospirosis? Trop. Med. Int. Health 12, 111–122 (2007).
Diament, D., Brunialti, M. K. C., Romero, E. C., Kallas, E. G. & Salomao, R. Peripheral blood mononuclear cell activation induced by Leptospira interrogans glycolipoprotein. Infect. Immun. 70, 1677–1683 (2002).
Klimpel, G. R., Matthias, M. A. & Vinetz, J. M. Leptospira interrogans activation of human peripheral blood mononuclear cells: preferential expansion of TCR γδ+ T cells vs TCR αβ+ T cells. J. Immunol. 171, 1447–1455 (2003).
Vernel-Pauillac, F. & Merien, F. Proinflammatory and immunomodulatory cytokine mRNA time course profiles in hamsters infected with a virulent variant of Leptospira interrogans. Infect. Immun. 74, 4172–4179 (2006).
de Fost, M., Hartskeerl, R. A., Groenendijk, M. R. & van der Poll, T. Interleukin 12 in part regulates gamma interferon release in human whole blood stimulated with Leptospira interrogans. Clin. Diagn. Lab. Immunol. 10, 332–335 (2003).
Tajiki, M. H. & Salomao, R. Association of plasma levels of tumor necrosis factor alpha with severity of disease and mortality among patients with leptospirosis. Clin. Infect. Dis. 23, 1177–1178 (1996).
Werts, C. et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nature Immunol. 2, 346–352 (2001).
Que-Gewirth, N. L. et al. A methylated phosphate group and four amide-linked acyl chains in Leptospira interrogans lipid A. The membrane anchor of an unusual lipopolysaccharide that activates TLR. J. Biol. Chem. 279, 25420–25429 (2004). References 58 and 59 show that Leptospira spp. possess an unusual LPS that uses TLR2 rather than TLR4 for signalling in human cells.
Yang, C. W. et al. Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney Int. 69, 815–822 (2006).
Nahori, M. A. et al. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. J. Immunol. 175, 6022–6031 (2005).
Seguro, A. C., Lomar, A. V. & Rocha, A. S. Acute renal failure of leptospirosis: nonoliguric and hypokalemic forms. Nephron 55, 146–151 (1990).
Burth, P., Younes-Ibrahim, M., Santos, M. C., Castro- Faria Neto, H. C. & de Castro Faria, M. V. Role of nonesterified unsaturated fatty acids in the pathophysiological processes of leptospiral infection. J. Infect. Dis. 191, 51–57 (2005).
Wu, M. S., Yang, C. W., Pan, M. J., Chang, C. T. & Chen, Y. C. Reduced renal Na+–K+–Cl− co-transporter activity and inhibited NKCC2 mRNA expression by Leptospira shermani: from bed-side to bench. Nephrol. Dial. Transplant. 19, 2472–2479 (2004).
Andrade, L., Rodrigues, A. C. Jr, Sanches, T. R., Souza, R. B. & Seguro, A. C. Leptospirosis leads to dysregulation of sodium transporters in the kidney and lung. Am. J. Physiol. Renal Physiol. 292, F586–F592 (2007).
Isogai, E. et al. Apoptosis of lymphocytes in mice administered lipopolysaccharide from Leptospira interrogans. Zentralbl. Veterinarmed. B 45, 529–537 (1998).
Merien, F., Truccolo, J., Rougier, Y., Baranton, G. & Perolat, P. In vivo apoptosis of hepatocytes in guinea pigs infected with Leptospira interrogans serovar Icterohaemorrhagiae. FEMS Microbiol. Lett. 169, 95–102 (1998).
Rugman, F. P., Pinn, G., Palmer, M. F., Waite, M. & Hay, C. R. Anticardiolipin antibodies in leptospirosis. J. Clin. Pathol. 44, 517–519 (1991).
Nally, J. E. et al. Alveolar septal deposition of immunoglobulin and complement parallels pulmonary hemorrhage in a guinea pig model of severe pulmonary leptospirosis. Am. J. Pathol. 164, 1115–1127 (2004). This study raises the potential for the involvement of an autoimmune process in the development of this severe disease form.
Stamm, L. V., Parrish, E. A. & Gherardini, F. C. Cloning of the recA gene from a free-living leptospire and distribution of RecA-like protein among spirochetes. Appl. Environ. Microbiol. 57, 183–189 (1991).
Mitchison, M. et al. Identification and characterization of the dTDP-rhamnose biosynthesis and transfer genes of the lipopolysaccharide-related rfb locus in Leptospira interrogans serovar Copenhageni. J. Bacteriol. 179, 1262–1267 (1997).
Yelton, D. B. & Cohen, R. A. Analysis of cloned DNA from Leptospira biflexa serovar patoc which complements a deletion of the Escherichia coli trpE gene. J. Bacteriol. 165, 41–46 (1986).
Baril, C., Richaud, C., Fournie, E., Baranton, G. & Saint Girons, I. Cloning of dapD, aroD and asd of Leptospira interrogans serovar Icterohaemorrhagiae, and nucleotide sequence of the asd gene. J. Gen. Microbiol. 138, 47–53 (1992).
Saint Girons, I., Margarita, D., Amouriaux, P. & Baranton, G. First isolation of bacteriophages for a spirochaete: potential genetic tools for Leptospira. Res. Microbiol. 141, 1131–1138 (1990).
Bourhy, P. et al. A genomic island of the pathogen Leptospira interrogans serovar Lai can excise from its chromosome. Infect. Immun. 75, 677–683 (2007).
Saint Girons, I. et al. The LE1 bacteriophage replicates as a plasmid within Leptospira biflexa: construction of an L. biflexa-Escherichia coli shuttle vector. J. Bacteriol. 182, 5700–5705 (2000). The first study to show the successful introduction of DNA into Leptospira spp.
Bourhy, P., Louvel, H., Saint Girons, I. & Picardeau, M. Random insertional mutagenesis of Leptospira interrogans, the agent of leptospirosis, using a mariner transposon. J. Bacteriol. 187, 3255–3258 (2005). This paper describes the first evidence of gene transfer in pathogenic Leptospira spp. and the generation of the first mutants in these pathogens.
Picardeau, M. Conjugative transfer between Escherichia coli and Leptospira spp. as a new genetic tool. Appl. Environ. Microbiol. 74, 319–322 (2008).
Louvel, H. & Picardeau, M. Genetic manipulation of Leptospira biflexa. Curr. Protoc. Microbiol. 12, 12E.4 (2007).
Croda, J. et al. Targeted mutagenesis in pathogenic Leptospira: disruption of the ligB gene does not affect virulence in animal models of leptospirosis. Infect. Immun. 76, 5826–5833 (2008). The first and so far only description of targeted inactivation in pathogenic Leptospira spp.
Murray, G. L. et al. Genome-wide transposon mutagenesis in pathogenic Leptospira spp. Infect. Immun. 77, 810–816 (2009).
Louvel, H., Saint Girons, I. & Picardeau, M. Isolation and characterization of FecA- and FeoB- mediated iron acquisition systems of the spirochete Leptospira biflexa by random insertional mutagenesis. J. Bacteriol. 187, 3249–3254 (2005).
Louvel, H. et al. Comparative and functional genomic analyses of iron transport and regulation in Leptospira spp. J. Bacteriol. 188, 7893–7904 (2006).
Pereira, M. M., Andrade, J., Marchevsky, R. S. & Ribeiro dos Santos, R. Morphological characterization of lung and kidney lesions in C3H/HeJ mice infected with Leptospira interrogans serovar icterohaemorrhagiae: defect of CD4+ and CD8+ T-cells are prognosticators of the disease progression. Exp. Toxicol. Pathol. 50, 191–198 (1998).
Viriyakosol, S., Matthias, M. A., Swancutt, M. A., Kirkland, T. N. & Vinetz, J. M. Toll-like receptor 4 protects against lethal Leptospira interrogans serovar icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infect. Immun. 74, 887–895 (2006). This article highlights the importance of TLR pathway activation in innate immunity against leptospirosis.
Koizumi, N. & Watanabe, H. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine 22, 1545–1552 (2004).
Minette, H. P. & Shaffer, M. F. Experimental leptospirosis in monkeys. Am. J. Trop. Med. Hyg. 17, 202–212 (1968).
Pereira, M. M. et al. Experimental leptospirosis in marmoset monkeys (Callithrix jacchus): a new model for studies of severe pulmonary leptospirosis. Am. J. Trop. Med. Hyg. 72, 13–20 (2005).
Ito, T. & Yanagawa, R. Leptospiral attachment to extracellular matrix of mouse fibroblast (L929) cells. Vet. Microbiol. 15, 89–96 (1987).
Merien, F., Truccolo, J., Baranton, G. & Perolat, P. Identification of a 36-kDa fibronectin-binding protein expressed by a virulent variant of Leptospira interrogans serovar icterohaemorrhagiae. FEMS Microbiol. Lett. 185, 17–22 (2000).
Atzingen, M. V. et al. Lsa21, a novel leptospiral protein binding adhesive matrix molecules and present during human infection. BMC Microbiol. 8, 70 (2008).
Hauk, P. et al. In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infect. Immun. 76, 2642–2650 (2008).
Hoke, D. E., Egan, S., Cullen, P. A. & Adler, B. LipL32 is an extracellular matrix-interacting protein of Leptospira spp. and Pseudoalteromonas tunicata. Infect. Immun. 76, 2063–2069 (2008).
Choy, H. A. et al. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect. Immun. 75, 2441–2450 (2007). The first study to determine that Lig proteins bind to extracellular matrix moieties.
Haake, D. A. et al. Changes in the surface of Leptospira interrogans serovar grippotyphosa during in vitro cultivation. Infect. Immun. 59, 1131–1140 (1991).
Setubal, J. C., Reis, M. G., Matsunaga, J. & Haake, D. A. Lipoprotein computational prediction in spirochaetal genomes. Microbiology 152, 113–121 (2006).
Viratyosin, W., Ingsriswang, S., Pacharawongsakda, E. & Palittapongarnpim, P. Genome-wide subcellular localization of putative outer membrane and extracellular proteins in Leptospira interrogans serovar Lai genome using bioinformatics approaches. BMC Genomics 9, 181 (2008).
Yang, H. L. et al. In silico and microarray-based genomic approaches to identifying potential vaccine candidates against Leptospira interrogans. BMC Genomics 7, 293 (2006).
Lee, S. H., Kim, S., Park, S. C. & Kim, M. J. Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore-forming protein on mammalian cells. Infect. Immun. 70, 315–322 (2002).
Cullen, P. A., Haake, D. A. & Adler, B. Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol. Rev. 28, 291–318 (2004).
Haake, D. A. et al. Molecular cloning and sequence analysis of the gene encoding OmpL1, a transmembrane outer membrane protein of pathogenic Leptospira spp. J. Bacteriol. 175, 4225–4234 (1993).
Haake, D. A. et al. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infect. Immun. 68, 2276–2285 (2000).
Matsunaga, J. et al. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Mol. Microbiol. 49, 929–945 (2003).
Ristow, P. et al. The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathog. 3, e97 (2007). The first identification of a virulence determinant according to Koch's molecular postulates.
Gamberini, M. et al. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiol. Lett. 244, 305–313 (2005).
Nally, J. E., Whitelegge, J. P., Bassilian, S., Blanco, D. R. & Lovett, M. A. Characterization of the outer membrane proteome of Leptospira interrogans expressed during acute lethal infection. Infect. Immun. 75, 766–773 (2007).
Barbosa, A. S. et al. A newly identified leptospiral adhesin mediates attachment to laminin. Infect. Immun. 74, 6356–6364 (2006).
Lee, S. H. et al. Identification and partial characterization of a novel hemolysin from Leptospira interrogans serovar lai. Gene 254, 19–28 (2000). Together with reference 102, the first description of LipL32, the major surface-exposed protein of pathogens.
Cullen, P. A., Cordwell, S. J., Bulach, D. M., Haake, D. A. & Adler, B. Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect. Immun. 70, 2311–2318 (2002).
Haake, D. A. et al. Molecular evolution and mosaicism of leptospiral outer membrane proteins involves horizontal DNA transfer. J. Bacteriol. 186, 2818–2828 (2004).
Vivian, J. P. et al. Crystal structure of LipL32, the most abundant surface protein of pathogenic Leptospira spp. J. Mol. Biol. 387, 1229–1238 (2009).
Murray, G. L. et al. The major surface protein LipL32 is not required for either acute or chronic infection with Leptospira interrogans. Infect. Immun. 77, 952–958 (2009).
Palaniappan, R. U. et al. Cloning and molecular characterization of an immunogenic LigA protein of Leptospira interrogans. Infect. Immun. 70, 5924–5930 (2002). This study and that in reference 103 identified LigA, LigB and LigC as members of the bacterial immunoglobulin-like superfamily of proteins.
Lin, Y. P. & Chang, Y. F. A domain of the Leptospira LigB contributes to high affinity binding of fibronectin. Biochem. Biophys. Res. Commun. 362, 443–448 (2007).
Lin, Y. P., Raman, R., Sharma, Y. & Chang, Y. F. Calcium binds to leptospiral immunoglobulin-like protein, LigB, and modulates fibronectin binding. J. Biol. Chem. 283, 25140–24149 (2008).
Matsunaga, J. et al. Response of Leptospira interrogans to physiologic osmolarity: relevance in signaling the environment-to-host transition. Infect. Immun. 75, 2864–2874 (2007). References 94 and 116 both found that physiological osmolarity is a key environmental signal for the differential regulation of leptospiral genes.
Srimanote, P. et al. Recombinant ligA for leptospirosis diagnosis and ligA among the Leptospira spp. clinical isolates. J. Microbiol. Methods 72, 73–81 (2008).
Croda, J. et al. Leptospira immunoglobulin-like proteins as a serodiagnostic marker for acute leptospirosis. J. Clin. Microbiol. 45, 1528–1534 (2007).
Ellis, W. A., Hovind-Hougen, K., Möller, S. & Birch-Andresen, A. Morphological changes upon subculturing of freshly isolated strains of Leptospira interrogans serovar hardjo. Zentralbl. Bakteriol. Mikrobiol. Hyg. A 255, 323–335 (1983).
Picardeau, M., Brenot, A. & Saint Girons, I. First evidence for gene replacement in Leptospira spp. Inactivation of L. biflexa flaB results in non-motile mutants deficient in endoflagella. Mol. Microbiol. 40, 189–199 (2001). The first report of targeted gene inactivation in Leptospira spp.; unlike that of the spirochaete B. burgdorferi , the cell body of the leptospiral flaB mutant remains helical.
Motaleb, M. A. et al. Borrelia burgdorferi periplasmic flagella have both skeletal and motility functions. Proc. Natl Acad. Sci. USA 97, 10899–10904 (2000).
Yuri, K. et al. Chemotaxis of leptospires to hemoglobin in relation to virulence. Infect. Immun. 61, 2270–2272 (1993).
Murray, G. L. et al. Leptospira interrogans requires heme oxygenase for disease pathogenesis. Microbes Infect. 11, 311–314 (2009).
Lo, M. et al. Effects of temperature on gene expression patterns in Leptospira interrogans serovar Lai as assessed by whole-genome microarrays. Infect. Immun. 74, 848–859 (2006).
Adler, B. & Faine, S. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infect. Immun. 17, 67–72 (1977).
Adler, B. & Faine, S. The antibodies involved in the human immune response to leptospiral infection. J. Med. Microbiol. 11, 387–400 (1978).
Jost, B. H., Adler, B., Vinh, T. & Faine, S. A monoclonal antibody reacting with a determinant on leptospiral lipopolysaccharide protects guinea pigs against leptospirosis. J. Med. Microbiol. 22, 269–275 (1986).
Chassin, C. et al. TLR4- and TLR2-mediated B cell responses control the clearance of the bacterial pathogen, Leptospira interrogans. J. Immunol. 183, 2669–2677 (2009).
Naiman, B. M. et al. Evaluation of type 1 immune response in naive and vaccinated animals following challenge with Leptospira borgpetersenii serovar Hardjo: involvement of WC1+ γδ and CD4 T cells. Infect. Immun. 70, 6147–6157 (2002). This study raises the possibility that cell-mediated immune responses confer protection against leptospirosis in certain hosts, such as cows.
Brown, R. A. et al. Comparison of three different leptospiral vaccines for induction of a type 1 immune response to Leptospira borgpetersenii serovar Hardjo. Vaccine 21, 4448–4458 (2003).
Blumerman, S. L., Herzig, C. T. & Baldwin, C. L. WC1+ γδ T cell memory population is induced by killed bacterial vaccine. Eur. J. Immunol. 37, 1204–1216 (2007).
Ido, Y., Hoki, R., Ito, H. & Wani, H. The prophylaxis of Weil's disease (Spirochaetosis Icterohaemorrhagica). J. Exp. Med. 24, 471–483 (1916).
Koizumi, N. & Watanabe, H. Leptospirosis vaccines: past, present, and future. J. Postgrad. Med. 51, 210–214 (2005).
Gonzalez, A. et al. Immunogenicity and protective capacity of leptospiral whole-cell monovalent serogroup Ballum vaccines in hamsters. Rev. Argent. Microbiol. 37, 169–175 (2005).
Haake, D. A. et al. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infect. Immun. 67, 6572–6582 (1999). The first evidence that immunization with a recombinant protein confers protection against experimental leptospirosis.
Branger, C. et al. Protection against Leptospira interrogans sensu lato challenge by DNA immunization with the gene encoding hemolysin-associated protein 1. Infect. Immun. 73, 4062–4069 (2005).
Seixas, F. K. et al. Recombinant Mycobacterium bovis BCG expressing the LipL32 antigen of Leptospira interrogans protects hamsters from challenge. Vaccine 26, 88–95 (2007).
Branger, C. et al. Identification of the hemolysis-associated protein 1 as a cross-protective immunogen of Leptospira interrogans by adenovirus-mediated vaccination. Infect. Immun. 69, 6831–6838 (2001).
Palaniappan, R. U. et al. Immunoprotection of recombinant leptospiral immunoglobulin-like protein A against Leptospira interrogans serovar Pomona infection. Infect. Immun. 74, 1745–1750 (2006). References 86 and 139 were the first to report that Lig proteins induce high-level immunoprotection against a lethal L. interrogans infection in experimental animals.
Silva, E. F. et al. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine 25, 6277–6286 (2007).
Yan, W. et al. Immunogenicity and protective efficacy of recombinant Leptospira immunoglobulin-like protein B (rLigB) in a hamster challenge model. Microbes Infect. 11, 230–237 (2009).
McBride, A. J. et al. Genetic diversity of the Leptospiral immunoglobulin-like (Lig) genes in pathogenic Leptospira spp. Infect. Genet. Evol. 9, 196–205 (2009).
Qin, J. H. et al. Identification of a novel prophage-like gene cluster actively expressed in both virulent and avirulent strains of Leptospira interrogans serovar Lai. Infect. Immun. 76, 2411–2419 (2008).
Salaün, L., Mérien, F., Gurianova, S., Baranton, G. & Picardeau, M. Application of multilocus variable-number tandem-repeat analysis for molecular typing of the agent of leptospirosis. J. Clin. Microbiol. 44, 3954–3962 (2006).
Ahmed, N. et al. Multilocus sequence typing method for identification and genotypic classification of pathogenic Leptospira species. Ann. Clin. Microbiol. Antimicrob. 5, 28 (2006).
Bourhy, P. et al. Complete nucleotide sequence of the LE1 prophage from the spirochete Leptospira biflexa and characterization of its replication and partition functions. J. Bacteriol. 187, 3931–3940 (2005).
Park, S. K. et al. Leptospirosis in Chonbuk Province of Korea in 1987: a study of 93 patients. Am. J. Trop. Med. Hyg. 41, 345–351 (1989).
Trevejo, R. T. et al. Epidemic leptospirosis associated with pulmonary hemorrhage — Nicaragua, 1995. J. Infect. Dis. 178, 1457–1463 (1998).
Panaphut, T., Domrongkitchaiporn, S. & Thinkamrop, B. Prognostic factors of death in leptospirosis: a prospective cohort study in Khon Kaen, Thailand. Int. J. Infect. Dis. 6, 52–59 (2002).
Marotto, P. C. F. et al. Acute lung injury in leptospirosis: clinical and laboratory features, outcome, and factors associated with mortality. Clin. Infect. Dis. 29, 1561–1563 (1999).
Gouveia, E. L. et al. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerg. Infect. Dis. 14, 505–508 (2008).
Yang, G. G. & Hsu, Y. H. Nitric oxide production and immunoglobulin deposition in leptospiral hemorrhagic respiratory failure. J. Formos. Med. Assoc. 104, 759–763 (2005).
Croda, J. et al. Leptospirosis pulmonary hemorrhage syndrome is associated with linear deposition of immunoglobulin and complement on the alveolar surface. Clin. Microbiol. Infect. (in the press).
Asuthkar, S., Velineni, S., Stadlmann, J., Altmann, F. & Sritharan, M. Expression and characterization of an iron-regulated hemin-binding protein, HbpA, from Leptospira interrogans serovar Lai. Infect. Immun. 75, 4582–4591 (2007).
Bos, M. P., Robert, V. & Tommassen, J. Biogenesis of the Gram-negative bacterial outer membrane. Annu. Rev. Microbiol. 61, 191–214 (2007).
Faisal, S. M. et al. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine 26, 277–287 (2008).
Faisal, S. M., Yan, W., McDonough, S. P. & Chang, Y. F. Leptospira immunoglobulin-like protein A variable region (LigAvar) incorporated in liposomes and PLGA microspheres produces a robust immune response correlating to protective immunity. Vaccine 27, 378–387 (2009).
Acknowledgements
We thank C. Figueira, E. Wunder, E. Couture, M.-C. Prevost and P. Ristow for providing the figures of leptospires and their pathology. We also thank L. Riley, G. Baranton, I. Saint Girons, M. Reis and G. Ribeiro for their critical advice during the preparation of this manuscript. Some of the work described was supported by a cooperative agreement between Institut Pasteur and the Oswaldo Cruz Foundation, Brazilian National Research Council (grants 01.06.0298.00, 3773/2005 and 554788/2006, Instituto Nacional de Ciência e Tecnologia de Vacinas), by the Research Support Foundation for the State of Bahia (54663), by the National Institutes of Health (grants 2R01 AI052473 and 2D43 TW00919), by the Institut Pasteur and by Agence Nationale de la Recherche (n°05-JCJC-0105–01).
Author information
Authors and Affiliations
Corresponding author
Related links
Supplementary information
Supplementary information S1 (box)
Leptospirosis in animals (PDF 145 kb)
Glossary
- Zoonotic disease
-
An infectious disease of animals that can be transmitted to humans, whatever its transmission mode (vector borne, directly or indirectly through a product derived from the host animal), and that causes a disease syndrome in susceptible individuals.
- Enzootic
-
Pertaining to an infection that can be maintained in nature through continuous transmission among animal populations.
- Haematogenous
-
Pertaining to anything transported by the blood.
- Alternative pathway
-
The complement activation pathway that is stimulated by the spontaneous hydrolysis of the complement component C3 or the presence of microbial surface structures.
- Classical pathway
-
The complement activation pathway that is stimulated by the recognition of antigen–antibody complexes on foreign-cell surfaces by the hexameric complement component C1q.
- Human leukocyte antigen
-
Also known as the major histocompatibility complex, this is a key part of the human immune system.
- Jarisch-Herxheimer reaction
-
An inflammatory reaction (characterized by the sudden onset of fever, chills and, in some cases, hypotension) that is induced in certain cases by antimicrobial therapy and believed to be due to the rapid release of bacterial antigens.
- Koch's molecular postulates
-
A series of conditions that must be met to establish that a gene is a virulence factor. For example, the gene must be present in strains that are associated with the disease but not in those which are not, the mutation of the gene must reduce or abolish bacterial virulence and complementation of the mutant must restore it.
- T helper 1 cell
-
A type of activated Thelper cell that promotes responses associated with the production of a particular set of cytokines, including tumour necrosis factor and interferon-γ, the main function of which is to stimulate phagocytosis-mediated defences against pathogens.
Rights and permissions
About this article
Cite this article
Ko, A., Goarant, C. & Picardeau, M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol 7, 736–747 (2009). https://doi.org/10.1038/nrmicro2208
Issue Date:
DOI: https://doi.org/10.1038/nrmicro2208
This article is cited by
-
Leptospirosis-associated meningitis in a patient with sjögren’s syndrome: a case report
BMC Infectious Diseases (2023)
-
Development and validation of a simple machine learning tool to predict mortality in leptospirosis
Scientific Reports (2023)
-
Identification of the most effective serovars to be included in the MAT antigen panel to optimize the serodiagnosis of Leptospira infection in Northern Italy
Veterinary Research Communications (2023)
-
Evaluation of a genus-specific rGroEL1-524 IgM-ELISA and commercial ELISA kits during the course of leptospirosis in Thailand
Scientific Reports (2021)
-
Human, animal, water source interactions and leptospirosis in Thailand
Scientific Reports (2021)