Key Points
-
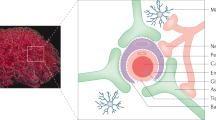
Microbial invasion and traversal of the blood–brain barrier is a prerequisite for meningitis and the associated blood–brain barrier dysfunction. Our current knowledge has been charted using Escherichia coli, with an emphasis on similarities and differences between different pathogens.
-
Transcellular penetration of the blood–brain barrier has been demonstrated for most meningitis-causing microorganisms, but the underlying mechanisms differ for different organisms.
-
E. coli invasion of the blood–brain barrier requires a high level of bacteraemia, as well as several bacterial and host factors. These microorganism–host interactions are unique to the blood–brain barrier and the relevant interactions have begun to be identified for E. coli and other meningitis-causing microorganisms.
-
Microbial binding to, and invasion of, the blood–brain barrier occurs through ligand–receptor interactions. Different microbial ligands interact with the same receptors, but the relevant interactions that are involved in traversal of the blood–brain barrier remain incompletely understood.
-
Microbial invasion and traversal of the blood–brain barrier requires rearrangement of the host cell cytoskeleton. Different microorganisms use different signal-transduction mechanisms to effect this rearrangement.
-
Central nervous system-infecting microorganisms induce blood–brain barrier dysfunction, but the underlying mechanisms differ from those that are involved in microbial binding to or invasion of the blood–brain barrier.
Abstract
Central nervous system (CNS) infections continue to be an important cause of morbidity and mortality. Microbial invasion and traversal of the blood–brain barrier is a prerequisite for CNS infections. Pathogens can cross the blood–brain barrier transcellularly, paracellularly and/or in infected phagocytes (the so-called Trojan-horse mechanism). Consequently, pathogens can cause blood–brain barrier dysfunction, including increased permeability, pleocytosis and encephalopathy. A more complete understanding of the microbial–host interactions that are involved in microbial traversal of the blood–brain barrier and the associated barrier dysfunction should help to develop new strategies to prevent CNS infections.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
World Health Organization. World health report: changing history (WHO, Geneva, 2004).
Klinger, G., Chin, C.-N., Beyene, J. & Perlman, M. Predicting the outcome of neonatal bacterial meningitis. Pediatrics 106, 477–482 (2000).
Stevens, J. P. et al. Long-term outcome of neonatal meningitis. Arch. Dis. Child. Fetal Neonatal Ed. 88, F179–F184 (2003).
Chang, C.-J. et al. Bacterial meningitis in infants: the epidemiology, clinical features, and prognostic factors. Brain Dev. 26, 168–175 (2004).
van de Beek, D. et al. Clinical features and prognostic factors in adults with bacterial meningitis. N. Engl. J. Med. 351, 1849–1859 (2004).
Kim, K. S. Pathogenesis of bacterial meningitis: from bacteraemia to neuronal injury. Nature Rev. Neurosci. 4, 376–385 (2003). A comprehensive review on the pathogenesis of meningitis and the associated neuronal injury.
Kim, K. S. Microbial translocation of the blood–brain barrier. Int. J. Parasitol. 36, 607–614 (2006).
Kim, Y. V., Di Cello, F., Hillaire, C. S. & Kim, K. S. Differential Ca2+ signaling by thrombin and protease-activated receptor-1-activating peptide in human brain microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 286, C31–C42 (2004).
Ruffer, C., Strey, A., Janning, A., Kim, K. S. & Gerke, V. Cell–cell junctions of dermal microvascular endothelial cells contain tight and adherens junction proteins in spatial proximity. Biochemistry 43, 5360–5369 (2004).
Stins, M. F., Badger, J. L. & Kim, K. S. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb. Pathog. 30, 19–28 (2001).
Rubin, L. L. & Staddon, J. M. The cell biology of the blood–brain barrier. Annu. Rev. Neurosci. 22, 11–28 (1999).
Mucke, L. & Eddleston, M. Astrocytes in infectious and immune-mediated diseases of the central nervous system. FASEB J. 7, 1226–1232 (1993).
Daubener, W. et al. Restriction of Toxoplasma gondii growth in human brain microvascular endothelial cells by activation of indoleamine 2,3-dioxygenase. Infect. Immun. 69, 6527–6531 (2001).
Anfuso, C. D. et al. Endothelial cell–pericyte cocultures induce PLA2 protein expression through activation of PKCα and MAPK/ERK cascade. J. Lipid Res. 48, 782–793 (2007).
Stins, M. F., Gilles, F. & Kim, K. S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J. Neuroimmunol. 76, 81–90 (1997).
Das, A. et al. Differential role of cytosolic phospholipase A2 in the invasion of brain microvascular endothelial cells by Escherichia coli and Listeria monocytogenes. J. Infect. Dis. 184, 732–737 (2001). Described the contribution of cPLA2 to E. coli invasion of brain microvascular endothelial cells.
Kim, K. S. et al. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Invest. 90, 897–905 (1992).
Tan, T. Q., Smith, C. W., Hawkins, E. P., Mason, E. O. Jr & Kaplan, S. L. Hematogenous bacterial meningitis in an intercellular adhesion molecule-1-deficient infant mouse model. J. Infect. Dis. 171, 342 (1995).
Munoz, F. M., Hawkins, E. P., Bullard, D. C., Beauchet, A. L. & Kaplan, S. L. Host defense against systemic infection with Streptococcus pneumoniae is impaired in E-, P-, and E-/P-selectin-deficient mice. J. Clin. Invest. 100, 2099–2106 (1997).
Nizet, V. et al. Invasion of brain microvascular endothelial cells by group B streptococci. Infect. Immun. 65, 5074–5081 (1997). The first study to show transcellular penetration of HBMECs by S. agalactiae.
Ring, A., Weiser, J. N. & Tuomanen, E. I. Pneumococcal trafficking across the blood–brain barrier. Molecular analysis of a novel bi-directional pathway. J. Clin. Invest. 102, 347–360 (1998).
Unkmeir, A. et al. Fibronectin mediates Opc-dependent internalization of Neisseria meningitidis in human brain microvascular endothelial cells. Mol. Microbiol. 46, 933–946 (2002).
Jong, A. Y., Stins, M. F., Huang, S. H. & Kim, K. S. Traversal of Candida albicans across human blood–brain barrier in vitro. Infect. Immun. 69, 4536–4544 (2001).
Chang, Y. C. et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood–brain barrier. Infect. Immun. 72, 4985–4995 (2004). The first study to show transcellular penetration of the blood–brain barrier by C. neoformans.
Masocha, W., Rottenberg, M. E. & Kristensson, K. Migration of African trypanosomes across the blood–brain barrier. Physiol. Behav. 92, 110–114 (2007).
Grab, D. J. et al. African trypanosome interactions with an in vitro model of the human blood–brain barrier. J. Parasitol. 90, 970–979 (2004).
Grab, D. et al. Borrelia burgdorferi, host-derived proteases, and the blood–brain barrier. Infect. Immun. 73, 1014–1022 (2005).
Sethi, N., Sondey, M., Bai, Y., Kim, K. S. & Cadavid, D. Interaction of a neurotropic strain of Borrelia turicatae with the cerebral microcirculation system. Infect. Immun. 74, 6408–6418 (2006).
Drevets, D. A., Leenen P. J. & Greenfield, R. A. Invasion of the central nervous system by intracellular bacteria. Clin. Microbiol. Rev. 17, 323–347 (2004).
Greiffenberg, L., Goebel, W., Kim, K. S. & Kuhn, M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect. Immun. 66, 5260–5267 (1998).
Jain, S. K., Paul-Satyaseela, M., Lamichhane, G., Kim, K. S. & Bishai, W. R. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood–brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J. Infect. Dis. 193, 1287–1295 (2006). The first paper to show transcellular penetration of HBMECs by M. tuberculosis.
Be, N. A. et al. Bacterial genetic determinants for Mycobacterium tuberculosis invasion and survival in the central nervous system. J. Infect. Dis. (in the press).
Ferrieri, P., Burke, B. & Nelson, J. Production of bacteremia and meningitis in infant rats with group B streptococcal serotypes. Infect. Immun. 27, 1023–1032 (1980).
Berman, P. H. & Banker, B. Q. Neonatal meningitis. A clinical and pathological study of 29 cases. Pediatrics 38, 6–24 (1966).
Kim, K. S. Effect of antimicrobial therapy for experimental infections due to group B streptococcus on mortality and clearance of bacteria. J. Infect. Dis. 155, 1233–1241 (1987).
Dietzman, D. E., Fischer, G. W. & Schoenknecht, F. D. Neonatal Escherichia coli septicemia — bacterial counts in blood. J. Pediatr. 85, 128–130 (1974).
Bell, L. M., Alpert, G., Campos, J. M. & Plotkin, S. A. Routine quantitative blood cultures in children with Haemophilus influenzae or Streptococcus pneumoniae bacteremia. Pediatrics 76, 901–904 (1985).
Sullivan, T. D., LaScolea, L. J. & Neter, E. Relationship between the magnitude of bacteremia in children and the clinical disease. Pediatrics 69, 699–702 (1982).
Marra, A. & Brigham, M. D. Streptococcus pneumoniae causes experimental meningitis following intranasal and otitis media infections via a nonhematogenous route. Infect. Immun. 69, 7318–7325 (2001).
van Ginkle, F. W. et al. Pneumococcal carriage results in ganglioside-mediated olfactory tissue infection. Proc. Natl Acad. Sci. USA 24, 14363–14367 (2003).
Cross, A. S., Kim, K. S., Wright, D. C., Sadoff, J. C. & Gemski, P. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J. Infect. Dis. 154, 497–503 (1986).
Xie, Y. et al. Identification and characterization of Escherichia coli RS218-derived islands in the pathogenesis of E. coli meningitis. J. Infect. Dis. 194, 358–364 (2006). The first comparative genomics study to demonstrate that E. coli K1-derived genomic islands contribute to meningitis.
Whitney, C. G. et al. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348, 1737–1746 (2003).
Tsai, C. J., Griffin, M. R., Pekka Nuorti, J. & Grijalva, C. G. Changing epidemiology of pneumococcal meningitis after the introduction of pneumococcal conjugate vaccine in the United States. Clin. Infect. Dis. 46, 1664–1672 (2008).
Moore, M. R. et al. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197, 1016–1027 (2008).
Borrow, R. & Miller, E. Long-term protection in children with meningococcal C conjugate vaccination: lessons learned. Expert Rev. Vaccines 5, 851–857 (2006).
Huang, S. H. et al. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect. Immun. 63, 4470–4475 (1995). First identification of the E. coli gene that contributes to the invasion of HBMECs and penetration into the brain.
Huang, S. H. et al. Identification and characterization of an Escherichia coli invasion gene locus, ibeB, required for penetration of brain microvascular endothelial cells. Infect. Immun. 67, 2103–2109 (1999).
Khan, N. A. et al. Cytotoxic necrotizing factor-1 contributes to Escherichia coli K1 invasion of the central nervous system. J. Biol. Chem. 277, 15607–15612 (2002).
Wang, Y., Huang, S. H., Wass, C. & Kim, K. S. The gene locus yijP contributes to Escherichia coli K1 invasion of brain microvascular endothelial cells. Infect. Immun. 67, 4751–4756 (1999).
Wang, Y. & Kim, K. S. Role of OmpA and IbeB in Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo. Pediatr. Res. 51, 559–563 (2002).
Prasadarao, N. V., Wass, C. A., Stins, M., Shimada, H. & Kim, K. S. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect. Immun. 67, 5775–5783 (1999).
Knodler, L. A., Celli, J. & Finlay, B. B. Pathogenic trickery: deception of host cell processes. Nature Rev. Mol. Cell Biol. 2, 578–588 (2001).
Cossart, P. & Sansonetti, P. J. Bacterial invasion: the paradigms of enteroinvasive pathogens. Science 304, 242–248 (2004).
Reddy, M. A., Wass, C. A., Kim, K. S., Schlaepfer, D. D. & Prasadarao, N. V. Involvement of focal adhesion kinase in Escherichia coli invasion of human brain microvascular endothelial cells. Infect. Immun. 68, 6423–6430 (2000).
Reddy, M. A., Nemani, P. V., Wass, C. A. & Kim, K. S. Phosphatidylinositol 3-kinase activation and interaction with focal adhesion kinase in Escherichia coli K1 invasion of human brain microvascular endothelial cells. J. Biol. Chem. 275, 36769–36774 (2000). Showed for the first time that activation of FAK and phosphatidylinositol 3-kinase is required for E. coli K1 invasion of HBMECs.
Kim, K. J., Elliott, S. A., DiCello, F., Stins, M. F. & Kim, K. S. The K1 capsule modulates trafficking of E. coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell. Microbiol. 5, 245–252 (2003). First demonstration of the role of the K1 capsule in the modulation of E. coli movement in HBMECs.
Nikulin, J., Panzner, U., Frosch, M. & Schubert-Unkmeir, A. Intracellular survival and replication of Neisseria meningitidis in human brain microvascular endothelial cells. Int. J. Med. Microbiol. 296, 553–558 (2006).
Siddharthan, V., Kim, Y. V., Liu, S. & Kim, K. S. Human astrocytes/astrocyte conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells. Brain Res. 1147, 39–50 (2007).
Mairey, E. et al. Cerebral microcirculation shear stress levels determine Neisseria meningitidis attachment sites along the blood–brain barrier. J. Exp. Med. 203, 1939–1950 (2006).
Stins, M. F. et al. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am. J. Pathol. 145, 1228–1236 (1994).
Prasadarao, N. V., Wass, C. A. & Kim, K. S. Endothelial cell GlcNAcβ1–4 GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood–brain barrier. Infect. Immun. 64, 154–160 (1996).
Charland, N. et al. Streptococcus suis serotype 2 interactions with human brain microvascular endothelial cells. Infect. Immun. 68, 637–643 (2000).
Hoffmann, I., Eugene, E., Nassif, X., Couraud, P. & Bourdoulous, S. Activation of ErbB2 receptor tyrosine kinase supports invasion of endothelial cells by Neisseria meningitidis. J. Cell Biol. 155, 133–145 (2001).
Teng, C. H. et al. Escherichia coli K1 RS218 interacts with human brain microvascular endothelial cells via type 1 fimbria bacteria in the fimbriated state. Infect. Immun. 73, 2923–2931 (2005). Used DNA microarrays to identify type 1 fimbriae in E. coli that bind to HBMECs.
Khan, N. A. et al. Outer membrane protein A and cytotoxic necrotizing factor-1 use diverse signaling mechanisms for Escherichia coli K1 invasion of human brain microvascular endothelial cells. Microb. Pathog. 35, 35–42 (2003).
Khan, N. A., Kim, Y., Shin, S. & Kim, K. S. FimH-mediated Escherichia coli K1 invasion of human brain microvascular endothelial cells. Cell. Microbiol. 9, 169–178 (2007).
Shin, S., Lu, G., Cai, M. & Kim, K. S. Escherichia coli outer membrane protein A adheres to human brain microvascular endothelial cells. Biochem. Biophys. Res. Commun. 330, 1199–1204 (2005).
Prasadarao, N. V. et al. Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect. Immun. 71, 1680–1688 (2003).
Cabanes, D. et al. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J. 24, 2827–2838 (2005).
Teng, C.-H. et al. Effects of ompA deletion on expression of type 1 fimbriae in Escherichia coli K1 strain RS218 and on the association of E. coli with human brain microvascular endothelial cells. Infect. Immun. 74, 5609–5616 (2006).
Badger, J., Wass, C., Weissman, S. & Kim, K. S. Application of signature-tagged mutagenesis for identification of Escherichia coli K1 genes that contribute to invasion of human brain microvascular endothelial cells. Infect. Immun. 68, 5056–5061 (2000).
Huang, S.-H., Wan, Z.-S., Chen, Y.-H., Jong, A. Y. & Kim, K. S. Further characterization of Escherichia coli brain microvascular endothelial cell invasion gene ibeA by deletion, complementation, and protein expression. J. Infect. Dis. 183, 1071–1078 (2001).
Nemani, P. V., Wass, C. A., Huang, S.-H. & Kim, K. S. Identification and characterization of a novel Ibe10 binding protein that contributes to Escherichia coli invasion of brain microvascular endothelial cells. Infect. Immun. 67, 1131–1138 (1999).
Flatau, G. et al. Toxin-induced activation of the G protein p21 Rho by deamidation of glutamine. Nature 387, 729–733 (1997).
Schmidt, G. et al. Gln 63 of Rho is deamidated by Escherichia coli cytotoxic necrotizing factor-I. Nature 387, 725–729 (1997).
Contamin, S. et al. The p21 Rho-activating toxin cytotoxic necrotizing factor 1 is endocytosed by a clathrin-independent mechanism and enters the cytosol by an acidic-dependent membrane translocation step. Mol. Biol. Cell 11, 1775–1787 (2000).
Chung, J. W. et al. 37-kDa laminin receptor precursor modulates cytotoxic necrotizing factor 1-mediated RhoA activation and bacterial uptake. J. Biol. Chem. 278, 16857–16862 (2003). The first study to identify 37 LRP as the receptor for CNF1.
Massia, S. P., Rao, S. S. & Hubbell, J. A. Covalently immobilized laminin peptide Tyr-Ile-Gly-Ser-Arg (YIGSR) supports cell spreading and co-localization of the 67-kilodalton laminin receptor with α-actinin and vinculin. J. Biol. Chem. 268, 8053–8059 (1993).
Kim, K. J., Chung, J. W. & Kim, K. S. 67-kDa laminin receptor promotes internalization of cytotoxic necrotizing factor 1-expressing Escherichia coli K1 into human brain microvascular endothelial cells. J. Biol. Chem. 280, 1360–1368 (2005).
Ludwig, G. V., Kondig, J. P. & Smith, J. F. A putative receptor for Venezuelan equine encephalitis virus from mosquito cells. J. Virol. 70, 5592–5599 (1996).
Gauczynski, S. et al. The 37-kDa/67-kDa laminin receptor acts as the cell-surface receptor for the cellular prion protein. EMBO J. 20, 5863–5875 (2001).
Thepparit, C. & Smith, D. R. Serotype-specific entry of dengue virus into liver cells: identification of the 37-kilodalton/67-kilodalton high-affinity laminin receptor as a dengue virus serotype 1 receptor. J. Virol. 78, 12647–12656 (2004).
Akache, B. et al. The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J. Virol. 80, 9831–9836 (2006).
Tenenbaum, T. et al. Streptococcus agalactiae invasion of human brain microvascular endothelial cells is promoted by the laminin-binding protein Lmb. Microbes Infect. 9, 714–720 (2007).
Tenenbaum, T., Bloier, C., Adam, R., Reinscheid, D. J. & Schroten, H. Adherence to and invasion of human brain microvascular endothelial cells are promoted by fibrinogen-binding protein FbsA of Streptococcus agalactiae. Infect. Immun. 73, 4404–4409 (2005).
Doran, K. S. et al. Blood–brain barrier invasion by group B Streptococcus depends upon proper cell-surface anchoring of lipoteichoic acid. J. Clin. Invest. 115, 2499–2507 (2005).
Maisey, H. C., Hensler, M., Nizet, V. & Doran, K. S. Group B streptococcal pilus proteins contribute to adherence to and invasion of brain microvascular endothelial cells. J. Bacteriol. 189, 1464–1467 (2007).
Braun, L., Ghebrehiwet, B. & Cossart P. gC1q-R/p32, a C1q-binding protein, is a receptor for the InlB invasion protein of Listeria monocytogenes. EMBO J. 19, 1458–1466 (2000).
Shen, Y., Naujokas, M., Park, M. & Ireton, K. InIB-dependent internalization of Listeria is mediated by the Met receptor tyrosine kinase. Cell 103, 501–510 (2000).
Niemann, H. H. et al. Structure of the human receptor tyrosine kinase Met in complex with the Listeria invasion protein InIB. Cell 130, 235–246 (2007).
Biswas, A. K. et al. Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping. PloS Pathog. 3, 1271–1280 (2007).
Cundell, D. R., Gerard, N. P., Gerard, C., Idanpaan-Heikkila, I. & Tuomanen, E. I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature 377, 435–438 (1995).
Radin, J. N. et al. β-arrestin 1 participates in platelet-activating factor receptor-mediated endocytosis of Streptococcus pneumoniae. Infect. Immun. 73, 7827–7835 (2005).
Weiser, J. N. et al. Phosphorylcholine on the lipopolysaccharide of Haemophilus influenzae contributes to persistence in the respiratory tract and sensitivity to serum killing mediated by C-reactive protein. J. Exp. Med. 187, 631–640 (1998).
Cabellos, C. et al. Differing roles of platelet-activating factor during inflammation of the lung and subarachnoid space. The special case of Streptococcus pneumoniae. J. Clin. Invest. 90, 612–618 (1992).
Nassif, X., Pujol, C., Morand, P. & Eugene, E. Interactions of pathogenic Neisseria with host cells. Is it possible to assemble the puzzle? Mol. Microbiol. 32, 1124–1132 (1999).
Johansson, L. et al. CD46 in meningococcal disease. Science 301, 373–375 (2003).
Capecchi, B. et al. Neisseria meningitis NadA is a new invasin which promotes bacterial adhesion to and penetration into human epithelial cells. Mol. Microbiol. 55, 687–698 (2005).
Plant, L. et al. Lipooligosaccharide structure contributes to multiple steps in the virulence of Neisseria meningitidis. Infect. Immun. 74, 1360–1367 (2006).
Manchester, M. et al. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74, 3967–3974 (2000).
Gaggar, A., Shayakhmetov, D. M. & Lieber, A. CD46 is a cellular receptor for group B adenoviruses. Nature Med. 9, 1408–1412 (2003).
Santoro, F. et al. Interaction of glycoprotein H of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 278, 25964–25969 (2003).
Rich, A. R. & McCordock, H. A. The pathogenesis of tuberculous meningitis. Bull. Johns Hopkins Hosp. 52, 5–37 (1933).
Thomas, D. D. et al. Treponema pallidum invades intercellular junctions of endothelial cell monolayers. Proc. Natl Acad. Sci. USA 85, 3608–3612 (1988).
Zhang, G. W., Khan, N. A., Kim, K. J., Stins, M. & Kim, K. S. Transforming growth factor-β increases Escherichia coli K1 adherence, invasion and transcytosis in human brain microvascular endothelial cells. Cell Tissue Res. 309, 281–286 (2002).
Galanakis, E., Di Cello, F., Paul-Satyaseela, M. & Kim, K. S. Escherichia coli K1 induces IL-8 expression in human brain microvascular endothelial cells. Eur. Cytokine Netw. 17, 260–265 (2006).
Sokolova, O. et al. Interaction of Neisseria meningitidis with human brain microvascular endothelial cells: role of MAP- and tyrosine kinases in invasion and inflammatory cytokine release. Cell. Microbiol. 6, 1153–1166 (2004).
Tuomanen, E., Saukkonen, K., Sande, S., Cioffe, C. & Wright, S. D. Reduction of inflammation, tissue damage and mortality in bacterial meningitis in rabbits treated with monoclonal antibodies against adhesion-promoting receptors of leukocytes. J. Exp. Med. 170, 959–968 (1989).
Tauber, M. G., Borschberg, U. & Sande, M. A. Influence of granulocytes on brain edema, intracranial pressure, and cerebrospinal fluid concentrations of lactate and protein in experimental meningitis. J. Infect. Dis. 157, 456–464 (1988).
Lesse, A. J., Moxon, E. R., Zwahten, A. & Scheid, W. M. Role of cerebrospinal fluid pleocytosis and Haemophilus influenzae type b capsule on blood brain barrier permeability during experimental meningitis in the rat. J. Clin. Invest. 82, 102–109 (1988).
Vadeboncoeur, N., Segura, M., Al-Numani, D., Vanier, G. & Gottschalk, M. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol. Med. Microbiol. 35, 49–58 (2003).
Frigerio, S. et al. Immunocompetence of human microvascular brain endothelial cells: cytokine regulation of IL-1β, MCP-1, IL-10, sICAM-1 and sVCAM-1. J. Neurol. 245, 727–730 (1998).
Medana, I. M. & Turner, G. D. Human cerebral malaria and the blood–brain barrier. Int. J. Parasitol. 36, 555–568 (2006).
van der Heyde, H. C., Nolan, J., Combes, V., Gramaglia, I. & Grau, G. E. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol. 22, 503–508 (2006).
Tripathi, A. K., Sullivan, D. J. & Stins, M. F. Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood–brain barrier endothelial cell monolayers. J. Infect. Dis. 195, 942–950 (2007).
Kugler, S. et al. Pertussis toxin transiently affects barrier integrity, organelle organization and transmigration of monocytes in a human brain microvascular endothelial cell barrier model. Cell. Microbiol. 9, 619–632 (2007).
Perfect, J. R. & Casadevall, A. Cryptococcus. Infect. Dis. Clin. North Am. 4, 837–874 (2002).
Chretien, F. et al. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J. Infect. Dis. 4, 522–530 (2002).
Olszewski, M. A. et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 164, 1761–1771 (2004).
Noverr, M. C., Williamson, P. R., Fajardo, R. S. & Huffnagle, G. B. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect. Immun. 72, 1693–1699 (2004).
Noveer, M. C., Cox, G. M., Perfect, J. R. & Huffnagle, G. B. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect. Immun. 71, 1538–1547 (2003).
Santangelo, R. et al. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect. Immun. 72, 2229–2239 (2004).
Shea, J. M., Kechichian, T. B., Luberto, C. & Del Poeta, M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect. Immun. 74, 5977–5988 (2006).
Acknowledgements
The information contained in this Review is derived from studies carried out by members of the author's laboratory and his collaborators' laboratories. Work in the laboratory of K.S.K. was supported by National Institutes of Health grants.
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Entrez Genome Project
Entrez Protein
Glossary
- Astrocyte
-
A star-shaped glial cell that supports the tissue of the central nervous system.
- Pericyte
-
A cell that is found around capillaries and is related to smooth muscle cells. Pericytes surround the endothelium as single cells. Association with pericytes reduces endothelial apoptosis and stabilizes the vasculature.
- Pinocytosis
-
The cellular uptake of extracellular fluid. Involves the formation of caveolae by the cell membrane that pinch off to form vesicles in the cytoplasm.
- Microglial cell
-
An immune cell of the central nervous system that is derived from mesodermal precursor cells and could be of haematopoietic lineage.
- Choroid plexus
-
A site of production of cerebrospinal fluid in the adult brain that is formed by the invagination of ependymal cells into the ventricles, which then become vascularized.
- AB-type toxin
-
A bacterial toxin that modifies target proteins within the cytosol of host cells and is composed of two domains: one that is responsible for the enzymatic activity (A) and one that is responsible for cell-receptor binding (B).
- Pleocytosis
-
The presence of a higher than normal number of cells in the cerebrospinal fluid.
- Fibrinolytic system
-
A broad spectrum of proteolytic enzymes that includes the plasminogen activator system and plasmin. Plasmin and plasmin activators proteolytically degrade the extracellular matrix.
- Encephalopathy
-
Brain dysfunction that is associated with alterations of the neural microenvironment and results from metabolic, toxic, vascular, infectious and/or inflammatory insults.
Rights and permissions
About this article
Cite this article
Kim, K. Mechanisms of microbial traversal of the blood–brain barrier. Nat Rev Microbiol 6, 625–634 (2008). https://doi.org/10.1038/nrmicro1952
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro1952
This article is cited by
-
Egr-1 is a key regulator of the blood-brain barrier damage induced by meningitic Escherichia coli
Cell Communication and Signaling (2024)
-
Signatures in in vitro infection of NSC-34 mouse neurons and their cell nucleus with Rickettsia helvetica
BMC Microbiology (2023)
-
Application of metagenomics for diagnosis of broilers displaying neurological symptoms
BMC Veterinary Research (2023)
-
Long non-coding RNA lncC11orf54-1 modulates neuroinflammatory responses by activating NF-κB signaling during meningitic Escherichia coli infection
Molecular Brain (2022)
-
Bacteria loaded with glucose polymer and photosensitive ICG silicon-nanoparticles for glioblastoma photothermal immunotherapy
Nature Communications (2022)