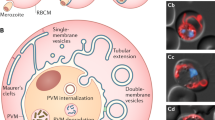
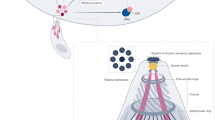
Key Points
-
The capacity of Plasmodium parasites to extensively remodel the erythrocytes in which they reside is fundamental to their ability to survive within these enucleated, metabolically inactive cells and to evade the immune response of the host.
-
Plasmodium falciparum exports ∼10% of its proteome across the parasite membrane and the surrounding vacuolar membrane into a host erythrocyte.
-
Many proteins that are exported by Plasmodium parasites contain a distinct trafficking motif, the Plasmodium export element (PEXEL), that requires processing in the endoplasmic reticulum by an aspartyl protease to direct their export.
-
A unique translocon known as the Plasmodium translocon of exported proteins (PTEX) is present at the vacuolar membrane and functions as a selective gateway for proteins to access host erythrocytes.
-
As erythrocytes lack their own trafficking machinery, Plasmodium parasites establish an exomembrane trafficking system in the host erythrocyte. This system functions as a sorting depot for exported proteins and has a role in the delivery of proteins to the erythrocyte membrane.
-
The export pathway of Plasmodium parasites contains novel constituents that are not found in higher eukaryotes and hence provides potential new drug targets for combating malaria.
Abstract
Plasmodium parasites, the causative agents of malaria, have developed elaborate strategies that they use to survive and thrive within different intracellular environments. During the blood stage of infection, the parasite is a master renovator of its erythrocyte host cell, and the changes in cell morphology and function that are induced by the parasite promote survival and contribute to the pathogenesis of severe malaria. In this Review, we discuss how Plasmodium parasites use the protein trafficking motif Plasmodium export element (PEXEL), protease-mediated polypeptide processing, a novel translocon termed the Plasmodium translocon of exported proteins (PTEX) and exomembranous structures to export hundreds of proteins to discrete subcellular locations in the host erythrocytes, which enables the parasite to gain access to vital nutrients and to evade the immune defence mechanisms of the host.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Sutherland, C. J. et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 201, 1544–1550 (2010).
Keeling, P. J. & Rayner, J. C. The origins of malaria: there are more things in heaven and earth. Parasitology 142 (Suppl. 1), 16–25 (2015).
Prudencio, M., Mota, M. M. & Mendes, A. M. A toolbox to study liver stage malaria. Trends Parasitol. 27, 565–574 (2011).
Cowman, A. F. & Crabb, B. S. Invasion of red blood cells by malaria parasites. Cell 124, 755–766 (2006).
Miller, L. H., Baruch, D. I., Marsh, K. & Doumbo, O. K. The pathogenic basis of malaria. Nature 415, 673–679 (2002).
Guttery, D. S., Roques, M., Holder, A. A. & Tewari, R. Commit and transmit: molecular players in Plasmodium sexual development and zygote differentiation. Trends Parasitol. 31, 676–685 (2015).
Sinden, R. E. The cell biology of malaria infection of mosquito: advances and opportunities. Cell. Microbiol. 17, 451–466 (2015).
Haase, S. & de Koning-Ward, T. F. New insights into protein export in malaria parasites. Cell. Microbiol. 12, 580–587 (2010).
Sargeant, T. J. et al. Lineage-specific expansion of proteins exported to erythrocytes in malaria parasites. Genome Biol. 7, R12 (2006).
Maier, A. G. et al. Exported proteins required for virulence and rigidity of Plasmodium falciparum-infected human erythrocytes. Cell 134, 48–61 (2008). This is the first large-scale gene-knockout study to be carried out in P. falciparum , which when combined with functional screens, is used to identify exported proteins that have a role in host cell remodelling and parasite virulence.
Decherf, G., Egee, S., Staines, H. M., Ellory, J. C. & Thomas, S. L. Anionic channels in malaria-infected human red blood cells. Blood Cells Mol. Dis. 32, 366–371 (2004).
Desai, S. A. Why do malaria parasites increase host erythrocyte permeability? Trends Parasitol. 30, 151–159 (2014).
Su, X.-Z. et al. The large diverse gene family var encodes proteins involved in cytoadherence and antigenic variation of Plasmodium falciparum-infected erythrocytes. Cell 82, 89–100 (1995).
Deitsch, K. W. & Wellems, T. E. Membrane modifications in erythrocytes parasitized by Plasmodium falciparum. Mol. Biochem. Parasitol. 76, 1–10 (1996).
McMillan, P. J. et al. Spatial and temporal mapping of the PfEMP1 export pathway in Plasmodium falciparum. Cell. Microbiol. 15, 1401–1418 (2013).
Da Silva, E. et al. The Plasmodium falciparum protein RESA interacts with the erythrocyte cytoskeleton and modifies erythrocyte thermal stability. Mol. Biochem. Parasitol. 66, 59–69 (1994).
Knuepfer, E., Rug, M., Klonis, N., Tilley, L. & Cowman, A. F. Trafficking determinants for PfEMP3 export and assembly under the Plasmodium falciparum-infected red blood cell membrane. Mol. Microbiol. 58, 1039–1053 (2005).
Nolte, D., Hundt, E., Langsley, G. & Knapp, B. A. Plasmodium falciparum blood stage antigen highly homologous to the glycophorin binding protein GBP. Mol. Biochem. Parasitol. 49, 253–264 (1991).
Tilley, L., Sougrat, R., Lithgow, T. & Hanssen, E. The twists and turns of Maurer's cleft trafficking in P. falciparum-infected erythrocytes. Traffic 9, 187–197 (2008).
Hanssen, E. et al. Electron tomography of the Maurer's cleft organelles of Plasmodium falciparum-infected erythrocytes reveals novel structural features. Mol. Microbiol. 67, 703–718 (2008).
Wickham, M. E. et al. Trafficking and assembly of the cytoadherence complex in Plasmodium falciparum-infected human erythrocytes. EMBO J. 20, 5636–5649 (2001).
Marti, M., Good, R. T., Rug, M., Knuepfer, E. & Cowman, A. F. Targeting malaria virulence and remodeling proteins to the host erythrocyte. Science 306, 1930–1933 (2004). Together with reference 23 , this study reveals the identity of the sequence motifs that are required for protein export into the erythrocyte, thereby enabling the prediction of the size of the Plasmodium exportome.
Hiller, N. L. et al. A host-targeting signal in virulence proteins reveals a secretome in malarial infection. Science 306, 1934–1937 (2004).
Boddey, J. A. et al. Role of plasmepsin V in export of diverse protein families from the Plasmodium falciparum exportome. Traffic 14, 532–550 (2013).
Schulze, J. et al. The Plasmodium falciparum exportome contains non-canonical PEXEL/HT proteins. Mol. Microbiol. 97, 301–314 (2015).
van Ooij, C. et al. The malaria secretome: from algorithms to essential function in blood stage infection. PLoS Pathog. 4, e1000084 (2008).
Spielmann, T. et al. A cluster of ring stage-specific genes linked to a locus implicated in cytoadherence in Plasmodium falciparum codes for PEXEL-negative and PEXEL-positive proteins exported into the host cell. Mol. Biol. Cell 17, 3613–3624 (2006). This manuscript reveals that some of the proteins that are exported by P. falciparum lack a PEXEL motif, and such proteins are now referred to as PEXEL-negative exported proteins (PNEPs).
Spielmann, T. & Gilberger, T. W. Protein export in malaria parasites: do multiple export motifs add up to multiple export pathways? Trends Parasitol. 26, 6–10 (2009).
Heiber, A. et al. Identification of new PNEPs indicates a substantial non-PEXEL exportome and underpins common features in Plasmodium falciparum protein export. PLoS Pathog. 9, e1003546 (2013).
Kulzer, S. et al. Plasmodium falciparum-encoded exported Hsp70/Hsp40 chaperone/co-chaperone complexes within the host erythrocyte. Cell. Microbiol. 14, 1784–1795 (2012).
Spielmann, T. & Gilberger, T. W. Critical steps in protein export of Plasmodium falciparum blood stages. Trends Parasitol. 31, 514–525 (2015).
Pasini, E. M. et al. Proteomic and genetic analyses demonstrate that Plasmodium berghei blood stages export a large and diverse repertoire of proteins. Mol. Cell. Proteomics 12, 426–448 (2013).
Haase, S., Hanssen, E., Matthews, K., Kalanon, M. & de Koning-Ward, T. F. The exported protein PbCP1 localises to cleft-like structures in the rodent malaria parasite Plasmodium berghei. PLoS ONE 8, e61482 (2013).
Siau, A. et al. Identification of a new export signal in Plasmodium yoelii: identification of a new exportome. Cell. Microbiol. 16, 673–686 (2014).
Chang, H. H. et al. N-terminal processing of proteins exported by malaria parasites. Mol. Biochem. Parasitol. 160, 107–115 (2008).
Boddey, J. A., Moritz, R. L., Simpson, R. J. & Cowman, A. F. Role of the Plasmodium export element in trafficking parasite proteins to the infected erythrocyte. Traffic 10, 285–299 (2009).
Boddey, J. A. et al. An aspartyl protease directs malaria effector proteins to the host cell. Nature 463, 627–631 (2010). Together with reference 38 , this study describes the identity of the aspartyl protease that is responsible for cleaving the PEXEL/HT motif.
Russo, I. et al. Plasmepsin V licenses Plasmodium proteins for export into the host erythrocyte. Nature 463, 632–636 (2010).
Gruring, C. et al. Uncovering common principles in protein export of malaria parasites. Cell Host Microbe 12, 717–729 (2012).
Tarr, S. J., Cryar, A., Thalassinos, K., Haldar, K. & Osborne, A. R. The C-terminal portion of the cleaved HT motif is necessary and sufficient to mediate export of proteins from the malaria parasite into its host cell. Mol. Microbiol. 87, 835–850 (2013).
Haase, S. et al. Sequence requirements for the export of the Plasmodium falciparum Maurer's clefts protein REX2. Mol. Microbiol. 71, 1003–1017 (2009).
Bhattacharjee, S., Stahelin, R. V., Speicher, K. D., Speicher, D. W. & Haldar, K. Endoplasmic reticulum PI(3)P lipid binding targets malaria proteins to the host cell. Cell 148, 201–212 (2012).
Bhattacharjee, S., Speicher, K. D., Stahelin, R. V., Speicher, D. W. & Haldar, K. PI(3)P-independent and -dependent pathways function together in a vacuolar translocation sequence to target malarial proteins to the host erythrocyte. Mol. Biochem. Parasitol. 185, 106–113 (2012).
Boddey, J. A. et al. Export of malaria proteins requires co-translational processing of the PEXEL motif independent of phosphatidylinositol-3-phosphate binding. Nat. Commun. 7, 10470 (2016).
Papakrivos, J., Newbold, C. I. & Lingelbach, K. A potential novel mechanism for the insertion of a membrane protein revealed by a biochemical analysis of the Plasmodium falciparum cytoadherence molecule PfEMP-1. Mol. Microbiol. 55, 1272–1284 (2005).
Desai, S. A. & Rosenberg, R. L. Pore size of the malaria parasite's nutrient channel. Proc. Natl Acad. Sci. USA 94, 2045–2049 (1997).
de Koning-Ward, T. F. et al. A newly discovered protein export machine in malaria parasites. Nature 459, 945–949 (2009). This paper reveals the identity of a protein complex at the PVM that is predicted to be involved in protein export.
Gehde, N. et al. Protein unfolding is an essential requirement for transport across the parasitophorous vacuolar membrane of Plasmodium falciparum. Mol. Microbiol. 71, 613–628 (2009). This manuscript provides evidence that implicates that a translocon rather than a vesicular mechanism is used to traffic exported proteins across the PVM.
Ansorge, I., Paprotka, K., Bhakdi, S. & Lingelbach, K. Permeabilization of the erythrocyte membrane with streptolysin O allows access to the vacuolar membrane of Plasmodium falciparum and a molecular analysis of membrane topology. Mol. Biochem. Parasitol. 84, 259–261 (1997).
Matthews, K. et al. The Plasmodium translocon of exported proteins (PTEX) component thioredoxin-2 is important for maintaining normal blood-stage growth. Mol. Microbiol. 89, 1167–1186 (2013).
Matz, J. M., Matuschewski, K. & Kooij, T. W. Two putative protein export regulators promote Plasmodium blood stage development in vivo. Mol. Biochem. Parasitol. 191, 44–52 (2013).
Beck, J. R., Muralidharan, V., Oksman, A. & Goldberg, D. E. HSP101/PTEX mediates export of diverse malaria effector proteins into the host erythrocyte. Nature 511, 592–595 (2014). Together with reference 53 , this study presents functional proof that PTEX provides a gateway for diverse types of cargo to access the host erythrocyte.
Elsworth, B. et al. PTEX is an essential nexus for protein export in malaria parasites. Nature 511, 587–591 (2014).
Bullen, H. E. et al. Biosynthesis, localisation and macromolecular arrangement of the Plasmodium falciparum translocon of exported proteins; PTEX. J. Biol. Chem. 287, 7871–7884 (2012).
Elsworth, B. et al. Proteomic analysis reveals novel proteins associated with the Plasmodium protein exporter PTEX and a loss of complex stability upon truncation of the core PTEX component, PTEX150. Cell. Microbiol. http://dx.doi.org/10.1111/cmi.12596 (2016).
Riglar, D. T. et al. Spatial association with PTEX complexes defines regions for effector export into Plasmodium falciparum-infected erythrocytes. Nat. Commun. 4, 1415 (2013). This paper provides evidence for the compartmentalization of the PTEX protein trafficking machinery in the parasitophorous vacuole.
Atkinson, C. T. & Aikawa, M. Ultrastructure of malaria-infected erythrocytes. Blood Cells 16, 351–368 (1990).
Elford, B. C., Cowan, G. M. & Ferguson, D. J. Parasite-regulated membrane transport processes and metabolic control in malaria-infected erythrocytes. Biochem. J. 308, 361–374 (1995).
Lauer, S. A., Rathod, P. K., Ghori, N. & Haldar, K. A membrane network for nutrient import in red cells infected with the malaria parasite. Science 276, 1122–1125 (1997).
Matz, J. M. et al. The Plasmodium berghei translocon of exported proteins reveals spatiotemporal dynamics of tubular extensions. Sci. Rep. 5, 12532 (2015).
Adisa, A. et al. The signal sequence of exported protein-1 directs the green fluorescent protein to the parasitophorous vacuole of transfected malaria parasites. J. Biol. Chem. 278, 6532–6542 (2003).
Meibalan, E. et al. Host erythrocyte environment influences the localization of exported protein 2, an essential component of the Plasmodium translocon. Eukaryot. Cell 14, 371–384 (2015).
Ito, K. The major pathways of protein translocation across membranes. Genes Cells 1, 337–346 (1996).
Johnson, D. et al. Characterization of membrane proteins exported from Plasmodium falciparum into the host erythrocyte. Parasitology 109, 1–9 (1994).
Mesén-Ramírez, P. et al. Stable translocation intermediates jam global protein export in Plasmodium falciparum parasites and link the PTEX component EXP2 with translocation activity. PLoS Pathog. 12, e1005618 (2016).
Gold, D. A. et al. The Toxoplasma dense granule proteins GRA17 and GRA23 mediate the movement of small molecules between the host and the parasitophorous vacuole. Cell Host Microbe 17, 642–652 (2015).
Kalanon, M. et al. The Plasmodium translocon of exported proteins component EXP2 is critical for establishing a patent malaria infection in mice. Cell. Microbiol. 18, 399–412 (2015).
Denks, K. et al. The Sec translocon mediated protein transport in prokaryotes and eukaryotes. Mol. Membr. Biol. 31, 58–84 (2014).
Schleiff, E. & Becker, T. Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 12, 48–59 (2011).
Flores-Perez, U. & Jarvis, P. Molecular chaperone involvement in chloroplast protein import. Biochim. Biophys. Acta 1833, 332–340 (2013).
Banumathy, G., Singh, V. & Tatu, U. Host chaperones are recruited in membrane-bound complexes by Plasmodium falciparum. J. Biol. Chem. 277, 3902–3912 (2002).
Matz, J. M. et al. In vivo function of PTEX88 in malaria parasite sequestration and virulence. Eukaryot. Cell 14, 528–534 (2015).
Chisholm, S. A. et al. Contrasting inducible knockdown of the auxiliary PTEX component PTEX88 in P. falciparum and P. berghei unmasks a role in parasite virulence. PLoS ONE 11, e0149296 (2016).
Knuepfer, E., Rug, M., Klonis, N., Tilley, L. & Cowman, A. F. Trafficking of the major virulence factor to the surface of transfected P. falciparum-infected erythrocytes. Blood 105, 4078–4087 (2005).
Dixon, M. W. et al. Targeting of the ring exported protein 1 to the Maurer's clefts is mediated by a two-phase process. Traffic 9, 1316–1326 (2008).
Lanzer, M., Wickert, H., Krohne, G., Vincensini, L. & Braun Breton, C. Maurer's clefts: a novel multi-functional organelle in the cytoplasm of Plasmodium falciparum-infected erythrocytes. Int. J. Parasitol. 36, 23–36 (2006).
Bhattacharjee, S., van Ooij, C., Balu, B., Adams, J. H. & Haldar, K. Maurer's clefts of Plasmodium falciparum are secretory organelles that concentrate virulence protein reporters for delivery to the host erythrocyte. Blood 111, 2418–2426 (2008).
Gruring, C. et al. Development and host cell modifications of Plasmodium falciparum blood stages in four dimensions. Nat. Commun. 2, 165 (2011).
McHugh, E. et al. A repeat sequence domain of the ring-exported protein-1 of Plasmodium falciparum controls export machinery architecture and virulence protein trafficking. Mol. Microbiol. 98, 1101–1114 (2015).
Morlot, S. et al. Membrane shape at the edge of the dynamin helix sets location and duration of the fission reaction. Cell 151, 619–629 (2012).
Low, H. H., Sachse, C., Amos, L. A. & Lowe, J. Structure of a bacterial dynamin-like protein lipid tube provides a mechanism for assembly and membrane curving. Cell 139, 1342–1352 (2009).
Rug, M. et al. Export of virulence proteins by malaria-infected erythrocytes involves remodeling of host actin cytoskeleton. Blood 124, 3459–3468 (2014).
Kriek, N. et al. Characterization of the pathway for transport of the cytoadherence-mediating protein, PfEMP1, to the host cell surface in malaria parasite-infected erythrocytes. Mol. Microbiol. 50, 1215–1227 (2003).
Wickert, H. et al. Evidence for trafficking of PfEMP1 to the surface of P. falciparum-infected erythrocytes via a complex membrane network. Eur. J. Cell Biol. 82, 271–284 (2003).
Hanssen, E. et al. Whole cell imaging reveals novel modular features of the exomembrane system of the malaria parasite. Plasmodium falciparum. Int. J. Parasitol. 40, 123–134 (2010).
Regev-Rudzki, N. et al. Cell–cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell 153, 1120–1133 (2013).
Trelka, D. P., Schneider, T. G., Reeder, J. C. & Taraschi, T. F. Evidence for vesicle-mediated trafficking of parasite proteins to the host cell cytosol and erythrocyte surface membrane in Plasmodium falciparum infected erythrocytes. Mol. Biochem. Parasitol. 106, 131–145 (2000).
Pachlatko, E. et al. MAHRP2, an exported protein of Plasmodium falciparum, is an essential component of Maurer's cleft tethers. Mol. Microbiol. 77, 1136–1152 (2010).
Watermeyer, J. M. et al. A spiral scaffold underlies cytoadherent knobs in Plasmodium falciparum-infected erythrocytes. Blood 127, 343–351 (2015).
Crabb, B. S. et al. Targeted gene disruption shows that knobs enable malaria-infected red cells to cytoadhere under physiological shear stress. Cell 89, 287–296 (1997).
Glenister, F. K., Coppel, R. L., Cowman, A. F., Mohandas, N. & Cooke, B. M. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood 99, 1060–1063 (2002).
Shi, H. et al. Life cycle-dependent cytoskeletal modifications in Plasmodium falciparum-infected erythrocytes. PLoS ONE 8, e61170 (2013).
Millholland, M. G. et al. The malaria parasite progressively dismantles the host erythrocyte cytoskeleton for efficient egress. Mol. Cell. Proteomics 10, M111.010678 (2011).
Cyrklaff, M. et al. Hemoglobins S and C interfere with actin remodeling in Plasmodium falciparum-infected erythrocytes. Science 334, 1283–1286 (2011). Together with references 95 and 96 , this study describes and measures the parasite-induced modifications that underpin shifts in erythrocyte deformability and calculates the roles that these changes have on parasite adhesion under flow conditions.
Zhang, Y. et al. Multiple stiffening effects of nanoscale knobs on human red blood cells infected with Plasmodium falciparum malaria parasite. Proc. Natl Acad. Sci. USA 112, 6068–6073 (2015).
Chishti, A. H. et al. Phosphorylation of protein 4.1 in Plasmodium falciparum-infected human red blood cells. Blood 83, 3339–3345 (1994).
Kats, L. M. et al. An exported kinase (FIKK4.2) that mediates virulence-associated changes in Plasmodium falciparum-infected red blood cells. Int. J. Parasitol. 44, 319–328 (2014).
de Koning-Ward, T. F., Gilson, P. R. & Crabb, B. S. Advances in molecular genetic systems in malaria. Nat. Rev. Microbiol. 13, 373–387 (2015).
Sleebs, B. E. et al. Transition state mimetics of the Plasmodium export element are potent inhibitors of plasmepsin V from P. falciparum and P. vivax. J. Med. Chem. 57, 7644–7662 (2014).
Hsiao, C. H., Luisa Hiller, N., Haldar, K. & Knoll, L. J. A HT/PEXEL motif in Toxoplasma dense granule proteins is a signal for protein cleavage but not export into the host cell. Traffic 14, 519–531 (2013).
Pelle, K. G. et al. Shared elements of host-targeting pathways among apicomplexan parasites of differing lifestyles. Cell. Microbiol. 17, 1618–1639 (2015).
Curt-Varesano, A., Braun, L., Ranquet, C., Hakimi, M. A. & Bougdour, A. The aspartyl protease TgASP5 mediates the export of the Toxoplasma GRA16 and GRA24 effectors into host cells. Cell. Microbiol. 18, 151–167 (2015).
Hammoudi, P. M. et al. Fundamental roles of the golgi-associated Toxoplasma aspartyl protease, ASP5, at the host–parasite interface. PLoS Pathog. 11, e1005211 (2015).
Coffey, M. J. et al. An aspartyl protease defines a novel pathway for export of Toxoplasma proteins into the host cell. eLife 4, e10809 (2015).
Bougdour, A. et al. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe 13, 489–500 (2013).
Moore, R. B. et al. A photosynthetic alveolate closely related to apicomplexan parasites. Nature 451, 959–963 (2008).
Woo, Y. H. et al. Chromerid genomes reveal the evolutionary path from photosynthetic algae to obligate intracellular parasites. eLife 4, e06974 (2015).
Nguitragool, W. et al. Malaria parasite clag3 genes determine channel-mediated nutrient uptake by infected red blood cells. Cell 145, 665–677 (2011).
Trenholme, K. R. et al. clag9: A cytoadherence gene in Plasmodium falciparum essential for binding of parasitized erythrocytes to CD36. Proc. Natl Acad. Sci. USA 97, 4029–4033 (2000).
Kaneko, O. et al. Apical expression of three RhopH1/Clag proteins as components of the Plasmodium falciparum RhopH complex. Mol. Biochem. Parasitol. 143, 20–28 (2005).
Hanssen, E. et al. Targeted mutagenesis of the ring-exported protein-1 of Plasmodium falciparum disrupts the architecture of Maurer's cleft organelles. Mol. Microbiol. 69, 938–953 (2008).
Dixon, M. W. et al. Genetic ablation of a Maurer's cleft protein prevents assembly of the Plasmodium falciparum virulence complex. Mol. Microbiol. 81, 982–993 (2011).
Hawthorne, P. L. et al. A novel Plasmodium falciparum ring stage protein, REX, is located in Maurer's clefts. Mol. Biochem. Parasitol. 136, 181–189 (2004).
Spycher, C. et al. MAHRP-1, a novel Plasmodium falciparum histidine-rich protein, binds ferriprotoporphyrin IX and localizes to the Maurer's clefts. J. Biol. Chem. 278, 35373–35383 (2003).
Spycher, C. et al. Genesis of and trafficking to the Maurer's clefts of Plasmodium falciparum-infected erythrocytes. Mol. Cell. Biol. 26, 4074–4085 (2006).
Spycher, C. et al. The Maurer's cleft protein MAHRP1 is essential for trafficking of PfEMP1 to the surface of Plasmodium falciparum-infected erythrocytes. Mol. Microbiol. 68, 1300–1314 (2008).
Blisnick, T. et al. Pfsbp1, a Maurer's cleft Plasmodium falciparum protein, is associated with the erythrocyte skeleton. Mol. Biochem. Parasitol. 111, 107–121 (2000).
Cooke, B. M. et al. A Maurer's cleft-associated protein is essential for expression of the major malaria virulence antigen on the surface of infected red blood cells. J. Cell Biol. 172, 899–908 (2006).
Kats, L. M., Cooke, B. M., Coppel, R. L. & Black, C. G. Protein trafficking to apical organelles of malaria parasites — building an invasion machine. Traffic 9, 176–186 (2008).
Maier, A. G. et al. Skeleton-binding protein 1 functions at the parasitophorous vacuole membrane to traffic PfEMP1 to the Plasmodium falciparum-infected erythrocyte surface. Blood 109, 1289–1297 (2007).
Rug, M., Prescott, S. W., Fernandez, K. M., Cooke, B. M. & Cowman, A. F. The role of KAHRP domains in knob formation and cytoadherence of P. falciparum-infected human erythrocytes. Blood 108, 370–378 (2006).
Tshefu, K. & James, M. A. Relationship of antibodies to soluble Plasmodium falciparum antigen (Pf70) and protection against malaria in a human population living under intense transmission in Kinshasa, Zaire. Trop. Med. Parasitol. 46, 72–76 (1995).
Dietz, O. et al. Characterization of the small exported Plasmodium falciparum membrane protein SEMP1. PLoS ONE 9, e103272 (2014).
Oh, S. S. et al. Plasmodium falciparum erythrocyte membrane protein 1 is anchored to the actin–spectrin junction and knob-associated histidine-rich protein in the erythrocyte skeleton. Mol. Biochem. Parasitol. 108, 237–247 (2000).
Waller, K. L., Cooke, B. M., Nunomura, W., Mohandas, N. & Coppel, R. L. Mapping the binding domains involved in the interaction between the Plasmodium falciparum knob-associated histidine-rich protein (KAHRP) and the cytoadherence ligand P. falciparum erythrocyte membrane protein 1 (PfEMP1). J. Biol. Chem. 274, 23808–23813 (1999).
Mills, J. P. et al. Effect of plasmodial RESA protein on deformability of human red blood cells harboring Plasmodium falciparum. Proc. Natl Acad. Sci. USA 104, 9213–9217 (2007).
Pei, X. et al. The ring-infected erythrocyte surface antigen (RESA) of Plasmodium falciparum stabilizes spectrin tetramers and suppresses further invasion. Blood 110, 1036–1042 (2007).
Diez-Silva, M. et al. Pf155/RESA protein influences the dynamic microcirculatory behavior of ring-stage Plasmodium falciparum infected red blood cells. Sci. Rep. 2, 614 (2012).
Waller, K. L. et al. Mature parasite-infected erythrocyte surface antigen (MESA) of Plasmodium falciparum binds to the 30-kDa domain of protein 4.1 in malaria-infected red blood cells. Blood 102, 1911–1914 (2003).
Petersen, C. et al. The mature erythrocyte surface antigen of Plasmodium falciparum is not required for knobs or cytoadherence. Mol. Biochem. Parasitol. 36, 61–65 (1989).
Waterkeyn, J. G. et al. Targeted mutagenesis of Plasmodium falciparum erythrocyte membrane protein 3 (PfEMP3) disrupts cytoadherence of malaria-infected red blood cells. EMBO J. 19, 2813–2823 (2000).
Tarr, S. J., Moon, R. W., Hardege, I. & Osborne, A. R. A conserved domain targets exported PHISTb family proteins to the periphery of Plasmodium infected erythrocytes. Mol. Biochem. Parasitol. 196, 29–40 (2014).
Proellocks, N. I. et al. A lysine-rich membrane-associated PHISTb protein involved in alteration of the cytoadhesive properties of Plasmodium falciparum-infected red blood cells. FASEB J. 28, 3103–3113 (2014).
Oberli, A. et al. A Plasmodium falciparum PHIST protein binds the virulence factor PfEMP1 and comigrates to knobs on the host cell surface. FASEB J. 28, 4420–4433 (2014).
Brandt, G. S. & Bailey, S. Dematin, a human erythrocyte cytoskeletal protein, is a substrate for a recombinant FIKK kinase from Plasmodium falciparum. Mol. Biochem. Parasitol. 191, 20–23 (2013).
Alexandre, J. S., Yahata, K., Kawai, S., Torii, M. & Kaneko, O. PEXEL-independent trafficking of Plasmodium falciparum SURFIN4.2 to the parasite-infected red blood cell and Maurer's clefts. Parasitol. Int. 60, 313–320 (2011).
Niang, M. et al. STEVOR is a Plasmodium falciparum erythrocyte binding protein that mediates merozoite invasion and rosetting. Cell Host Microbe 16, 81–93 (2014).
Sanyal, S. et al. Plasmodium falciparum STEVOR proteins impact erythrocyte mechanical properties. Blood 119, e1–e8 (2012).
Hinterberg, K. et al. Plasmodium falciparum: the Pf332 antigen is secreted from the parasite by a brefeldin A-dependent pathway and is translocated to the erythrocyte membrane via the Maurer's clefts. Exp. Parasitol. 79, 279–291 (1994).
Glenister, F. K. et al. Functional alteration of red blood cells by a megadalton protein of Plasmodium falciparum. Blood 113, 919–928 (2009).
Waller, K. L. et al. Interaction of the exported malaria protein Pf332 with the red blood cell membrane skeleton. Biochim. Biophys. Acta 1798, 861–871 (2010).
Wu, Y. & Craig, A. Comparative proteomic analysis of metabolically labelled proteins from Plasmodium falciparum isolates with different adhesion properties. Malar J. 5, 67 (2006).
Lavazec, C., Sanyal, S. & Templeton, T. J. Expression switching in the stevor and Pfmc-2TM superfamilies in Plasmodium falciparum. Mol. Microbiol. 64, 1621–1634 (2007).
Acknowledgements
The authors apologize to those colleagues whose work could not be cited owing to length constraints. They acknowledge support from the National Health and Medical Research Council, Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
Glossary
- Parasitophorous vacuole
-
A compartment that is formed in host cells in which apicomplexan parasites reside and develop.
- New permeation pathways
-
(NPPs). These are induced in the membrane of infected erythrocytes to enable the passage of low-molecular-weight molecules.
- Rhoptry
-
A specialized secretory organelle that is located at the apical end of apicomplexan parasites.
- Parasitophorous vacuole membrane
-
(PVM). The membrane that surrounds the parasitophorous vacuole that, in the case of erythrocytes, is formed through the invagination of the membrane bilayer during parasite invasion.
- P. falciparum erythrocyte membrane protein 1
-
(PfEMP1). A major virulence factor of Plasmodium falciparum. It is a member of a family of approximately 60 proteins of which one allele is expressed in a particular parasite.
- Exomembrane system
-
A network of membranes that, in addition to the limiting membrane of the parasite, is present in the cytoplasm of the host cell.
- Exportomes
-
The full complement of exported proteins in malaria parasites.
- Translocon
-
A complex of proteins that includes a membrane-spanning channel component and provides a passage for polypeptides to cross membranes.
- Knobs
-
Electron-dense protrusions on the membrane of erythrocytes that are infected with Plasmodium falciparum and that increase the stiffness and adhesiveness of the erythrocyte.
- Plasmodium export element
-
(PEXEL). A pentameric amino acid motif in the amino-terminal region of proteins that guides their export.
- PEXEL-negative proteins
-
(PNEPs). Plasmodium export element (PEXEL)-negative exported proteins lack a PEXEL motif and contain a hydrophobic region that mediates entry into the secretory pathway.
- Plasmepsin V
-
A parasite aspartyl protease that cleaves the Plasmodium export element (PEXEL) motif.
- Plasmodium translocon of exported proteins
-
(PTEX). The Plasmodium translocon of exported proteins provides a selective gateway for parasite proteins to traverse the parasitophorous vacuole membrane and access the host erythrocyte.
- Dense granules
-
Small vesicular bodies that are located at the apical end of the cytoplasm in the invasive form (merozoite) of the malaria parasite.
- Patency
-
The time point during an infection when parasites are detectable.
- J-dots
-
Proteinaceous chaperone complexes that are present in the cytoplasm of erythrocytes that are infected with Plasmodium falciparum.
- Maurer's clefts
-
Flattened, single-membrane-bound structures that are constructed by Plasmodium falciparum in the cytoplasm of infected erythrocytes. These clefts receive exported protein cargo and deliver it to the surface of the erythrocyte.
- Lamellae
-
Thin, plate-like membranous structures.
- Cisterna
-
Flattened membrane disc-like structures.
- Dynamins
-
Large GTPases that are implicated in the budding and scission of nascent vesicles from their parent membranes.
Rights and permissions
About this article
Cite this article
de Koning-Ward, T., Dixon, M., Tilley, L. et al. Plasmodium species: master renovators of their host cells. Nat Rev Microbiol 14, 494–507 (2016). https://doi.org/10.1038/nrmicro.2016.79
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2016.79
This article is cited by
-
Toxoplasma protein export and effector function
Nature Microbiology (2024)
-
In vitro model of brain endothelial cell barrier reveals alterations induced by Plasmodium blood stage factors
Parasitology Research (2023)
-
A cell surface-exposed protein complex with an essential virulence function in Ustilago maydis
Nature Microbiology (2021)
-
An integrated platform for genome engineering and gene expression perturbation in Plasmodium falciparum
Scientific Reports (2021)