Key Points
-
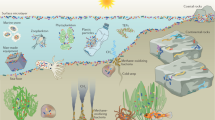
Biofilms dominate microbial life in stream ecosystems. These matrix-enclosed and surface-attached microbial communities are ubiquitous, prolific and highly active at the interfaces of the streambed. The biofilm mode of life is advantageous in streams with a fast flow of water and continuous export of nutrients and organic matter.
-
Biofilms in streams can be considered a 'microbial skin', regulating the processing and export of nutrients and organic matter from catchments and influencing the dispersal of microorganisms and their biodiversity dynamics at the scale of entire stream networks.
-
Interactions between the growth of biofilms, streamwater flow and substratum chemistry produce emergent environmental complexity in the streambed.
-
Proteobacteria and Bacteroidetes often dominate the communities of stream biofilms. Flavobacteriia and Sphingobacteriia seem to be especially important members of these communities. Archaea are found within niche microenvironments established by the metabolic activity of other microorganisms.
-
High biodiversity in stream biofilms is supported by continuous input of microorganisms from upstream catchments, environmental sorting induced by habitat heterogeneity (ranging from the scale of the biofilm to large stream networks) and episodic disturbance from streamwater flow.
-
New interdisciplinary approaches are needed to link structure and function of biofilms to their environment and, ultimately, to ecosystem processes and biogeochemical fluxes in streams. This is crucial to understand and predict implications of global ecosystem change and climate change on the microbial ecology and functioning of stream ecosystems.
Abstract
Streams and rivers form dense networks, shape the Earth's surface and, in their sediments, provide an immensely large surface area for microbial growth. Biofilms dominate microbial life in streams and rivers, drive crucial ecosystem processes and contribute substantially to global biogeochemical fluxes. In turn, water flow and related deliveries of nutrients and organic matter to biofilms constitute major constraints on microbial life. In this Review, we describe the ecology and biogeochemistry of stream biofilms and highlight the influence of physical and ecological processes on their structure and function. Recent advances in the study of biofilm ecology may pave the way towards a mechanistic understanding of the effects of climate and environmental change on stream biofilms and the biogeochemistry of stream ecosystems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Geesey, G. G., Mutch, R., Costerton, J. W. & Green, R. B. Sessile bacteria: an important component of the microbial population in small mountain stream. Limnol. Oceanogr. 23, 1214–1223 (1978). This study represented the first evaluation of the abundance and biomass of bacteria in the streamwater in benthic biofilms. It also presents the first microphotographs to depict the spatial organization of benthic biofilms.
Costerton, J. W., Geesey, G. G. & Cheng, K. J. How bacteria stick. Sci. Am. 238, 86–95 (1978).
Lock, M. A., Wallace, R. R., Costerton, J. W., Ventullo, R. M. & Charlton, S. E. River epilithon: toward a structural–functional model. Oikos 42, 10 (1984). This is the first study to propose a conceptual model of stream biofilm structure and function, including the interactions of biofilms with DOM.
Haack, T. K. & McFeters, G. A. Nutritional relationships among microorganisms in an epilithic biofilm community. Microb. Ecol. 8, 115–126 (1982). This study reported the first quantification of carbon fluxes between algae and microbial heterotrophs in stream biofilms.
Findlay, S. Stream microbial ecology. J. N. Am. Benth. Soc. 29, 170–181 (2010).
Battin, T. J., Kaplan, L. A., Denis Newbold, J. & Hansen, C. M. E. Contributions of microbial biofilms to ecosystem processes in stream mesocosms. Nature 426, 439–442 (2003).
Romaní, A. M. et al. Relevance of polymeric matrix enzymes during biofilm formation. Microb. Ecol. 56, 427–436 (2008).
Boano, F. et al. Hyporheic flow and transport processes: mechanisms, models, and biogeochemical implications. Rev. Geophys. 52, 603–679 (2014).
Battin, T. J. et al. Biophysical controls on organic carbon fluxes in fluvial networks. Nat. Geosci. 1, 95–100 (2008).
Raymond, P. A. et al. Global carbon dioxide emissions from inland waters. Nature 503, 355–359 (2014).
Mulholland, P. J. et al. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452, 202–205 (2008).
Beaulieu, J. J., Tank, J. L. & Hamilton, S. K. Nitrous oxide emission from denitrification in stream and river networks. Proc. Natl Acad. Sci. USA 108, 214–219 (2011).
Vorholt, J. A. Microbial life in the phyllosphere. Nat. Rev. Microbiol. 10, 828–840 (2012).
Daniel, R. The metagenomics of soil. Nat. Rev. Microbiol. 3, 470–478 (2005).
Baschien, C., Manz, W., Neu, T. R., Marvanova, L. & Szewzyk, U. In situ detection of freshwater fungi in an Alpine stream by new taxon-specific fluorescence in situ hybridization probes. Appl. Environ. Microbiol. 74, 6427–6436 (2008).
Bärlocher, F. & Murdoch, J. H. Hyporheic biofilms — a potential food source for interstitial animals. Hydrobiologia 184, 61–67 (1989).
Hakenkamp, C. C. & Morin, A. The importance of meiofauna to lotic ecosystem functioning. Freshw. Biol. 44, 165–175 (2000).
Dopheide, A., Lear, G., Stott, R. & Lewis, G. Molecular characterization of ciliate diversity in stream biofilms. Appl. Environ. Microbiol. 74, 1740–1747 (2008).
Lawrence, J. R., Scharf, B., Packroff, G. & Neu, T. R. Microscale evaluation of the effects of grazing by invertebrates with contrasting feeding modes on river biofilm architecture and composition. Microb. Ecol. 44, 199–207 (2002).
Böhme, A., Risse-Buhl, U. & Küsel, K. Protists with different feeding modes change biofilm morphology. FEMS Microbiol. Ecol. 69, 158–169 (2009).
Wey, J. K., Jürgens, K. & Weitere, M. Seasonal and successional influences on bacterial community composition exceed that of protozoan grazing in river biofilms. Appl. Environ. Microbiol. 78, 2013–2024 (2012).
Risse-Buhl, U. et al. Tracking the autochthonous carbon transfer in stream biofilm food webs. FEMS Microbiol. Ecol. 79, 118–131 (2012).
Jacquet, S., Miki, T., Noble, R., Peduzzi, P. & Wilhelm, S. Viruses in aquatic ecosystems: important advancements of the last 20 years and prospects for the future in the field of microbial oceanography and limnology. Adv. Oceanogr. Limnol. 1, 97–141 (2010).
Sutherland, I. W., Hughes, K. A., Skillman, L. C. & Tait, K. The interaction of phage and biofilms. FEMS Microbiol. Lett. 232, 1–6 (2004).
Zeglin, L. Stream microbial diversity responds to environmental changes: review and synthesis of existing research. Front. Microbiol. 6, 454 (2015).
Romaní, A. M. et al. Shifts in microbial community structure and function in light- and dark-grown biofilms driven by warming. Environ. Microbiol. 16, 2550–2567 (2014).
Timoner, X., Borrego, C. M., Acuña, V. & Sabater, S. The dynamics of biofilm bacterial communities is driven by flow wax and wane in a temporary stream. Limnol. Oceanogr. 59, 2057–2067 (2014).
Newton, R. J., Jones, S. E., Eiler, A., McMahon, K. D. & Bertilsson, S. A guide to the natural history of freshwater lake bacteria. Microbiol. Mol. Biol. Rev. 75, 14–49 (2011).
Kirchman, D. L. The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 39, 91–100 (2002).
Zhang, W., Sileika, T. & Packman, A. I. Effects of fluid flow conditions on interactions between species in biofilms. FEMS Microbiol. Ecol. 84, 344–354 (2013).
Widder, S. et al. Fluvial network organization imprints on microbial co-occurrence networks. Proc. Natl Acad. Sci. USA 111, 12799–12804 (2014).
Wilhelm, L., Singer, G. A., Fasching, C., Battin, T. J. & Besemer, K. Microbial biodiversity in glacier-fed streams. ISME J. 7, 1651–1660 (2013).
Besemer, K. et al. Unraveling assembly of stream biofilm communities. ISME J. 6, 1459–1468 (2012).
Battin, T. J., Wille, A., Sattler, B. & Psenner, R. Phylogenetic and functional heterogeneity of sediment biofilms along environmental gradients in a glacial stream. Appl. Environ. Microbiol. 67, 799–807 (2001).
Olapade, O. A. & Leff, L. G. Seasonal response of stream biofilm communities to dissolved organic matter and nutrient enrichments. Appl. Environ. Microbiol. 71, 2278–2287 (2005).
Merbt, S. N., Auguet, J.-C., Casamayor, E. O. & Marti, E. Biofilm recovery in a wastewater treatment plant-influenced stream and spatial segregation of ammonia-oxidizing microbial populations. Limnol. Oceanogr. 56, 1054–1064 (2011).
Buriánková, I. et al. Identification of methanogenic archaea in the hyporheic sediment of Sitka stream. PLoS ONE 8, e80804 (2013).
Leibold, M. A. et al. The metacommunity concept: a framework for multi-scale community ecology. Ecol. Lett. 7, 601–613 (2004).
Battin, T. J., Kaplan, L. A., Newbold, J. D., Cheng, X. & Hansen, C. Effects of current velocity on the nascent architecture of stream microbial biofilms. Appl. Environ. Microbiol. 69, 5443–5452 (2003).
Besemer, K., Hödl, I., Singer, G. & Battin, T. J. Architectural differentiation reflects bacterial community structure in stream biofilms. ISME J. 3, 1318–1324 (2009).
Besemer, K., Singer, G., Hödl, I. & Battin, T. J. Bacterial community composition of stream biofilms in spatially variable-flow environments. Appl. Environ. Microbiol. 75, 7189–7195 (2009).
Woodcock, S., Besemer, K., Battin, T. J., Curtis, T. P. & Sloan, W. T. Modelling the effects of dispersal mechanisms and hydrodynamic regimes upon the structure of microbial communities within fluvial biofilms. Environ. Microbiol. 15, 1216–1225 (2013).
Wang, J. et al. Phylogenetic beta diversity in bacterial assemblages across ecosystems: deterministic versus stochastic processes. ISME J. 7, 1310–1321 (2013).
Besemer, K. et al. Headwaters are critical reservoirs of microbial diversity for fluvial networks. Proc. Biol. Sci. 280, 20131760 (2013). This is the first study that shows how α- and β-diversity of biofilm bacteria change across a stream network; the study discusses metacommunity dynamics and hydrology as potential drivers of biodiversity dynamics.
Crump, B. C., Amaral-Zettler, L. A. & Kling, G. W. Microbial diversity in arctic freshwaters is structured by inoculation of microbes from soils. ISME J. 6, 1629–1639 (2012).
Ruiz-González, C., Niño-García, J. P. & del Giorgio, P. A. Terrestrial origin of bacterial communities in complex boreal freshwater networks. Ecol. Lett. 18, 1198–1206 (2015).
Lear, G. et al. The biogeography of stream bacteria. Glob. Ecol. Biogeogr. 22, 544–554 (2013).
Fierer, N., Morse, J. L., Berthrong, S. T., Bernhardt, E. S. & Jackson, R. B. Environmental controls on the landscape-scale biogeography of stream bacterial communities. Ecology 88, 2162–2173 (2007).
Costerton, J. W., Lewandowski, Z., Caldwell, D. E., Korber, D. R. & Lappin-Scott, H. M. Microbial biofilms. Annu. Rev. Microbiol. 49, 711–745 (1995).
Parsek, M. R. & Tolker-Nielsen, T. Pattern formation in Pseudomonas aeruginosa biofilms. Curr. Opin. Microbiol. 11, 560–566 (2008).
Celler, K., Hodl, I., Simone, A., Battin, T. J. & Picioreanu, C. A mass-spring model unveils the morphogenesis of phototrophic Diatoma biofilms. Sci. Rep. 4, 3649 (2014).
Hödl, I. et al. Biophysical controls on cluster dynamics and architectural differentiation of microbial biofilms in contrasting flow environments. Environ. Microbiol. 16, 802–812 (2014).
Stewart, P. S. & Franklin, M. J. Physiological heterogeneity in biofilms. Nat. Rev. Microbiol. 6, 199–210 (2008).
Wimpenny, J. & Colasanti, R. A unifying hypothesis for the structure of microbial biofilms based on cellular automaton models. FEMS Microbiol. Ecol. 22, 1–16 (1997).
Neu, T. Development and structure of microbial biofilms in river water studied by confocal laser scanning microscopy. FEMS Microbiol. Ecol. 24, 11–25 (1997). This is one of the first studies to use confocal laser scanning microscopy to comprehensively describe the physical structure of river biofilms.
Besemer, K. et al. Biophysical controls on community succession in stream biofilms. Appl. Environ. Microbiol. 73, 4966–4974 (2007).
Taherzadeh, D., Picioreanu, C. & Horn, H. Mass transfer enhancement in moving biofilm structures. Biophys. J. 102, 1483–1492 (2012).
Nepf, H. M. Flow and transport in regions with aquatic vegetation. Annu. Rev. Fluid Mech. 44, 123–142 (2012).
Vignaga, E. et al. Erosion of biofilm-bound fluvial sediments. Nat. Geosci. 6, 1–5 (2013).
Singer, G., Besemer, K., Hochedlinger, G., Chlup, A. K. & Battin, T. J. Monomeric carbohydrate uptake and structure–function coupling in stream biofilms. Aquat. Microb. Ecol. 62, 71–83 (2011).
Lawrence, J. R., Swerhone, G. D. W., Kuhlicke, U. & Neu, T. R. In situ evidence for microdomains in the polymer matrix of bacterial microcolonies. Can. J. Microbiol. 53, 450–458 (2007).
Arnon, S., Gray, K. A. & Packman, A. I. Biophysicochemical process coupling controls nitrogen use by benthic biofilms. Limnol. Oceanogr. 52, 1665–1671 (2007).
Rusconi, R., Lecuyer, S., Guglielmini, L. & Stone, H. A. Laminar flow around corners triggers the formation of biofilm streamers. J. R. Soc. Interface 7, 1293–1299 (2010).
Drescher, K., Shen, Y. & Bassler, B. L. Biofilm streamers cause catastrophic disruption of flow with consequences for environmental and medical systems. Proc. Natl Acad. Sci. USA 110, 4345–4350 (2013).
Pintelon, T. R. R., Picioreanu, C., van Loosdrecht, M. C. M. & Johns, M. L. The effect of biofilm permeability on bio-clogging of porous media. Biotechnol. Bioeng. 109, 1031–1042 (2012).
Chen, C., Packman, A. I. & Gaillard, J.-F. Pore-scale analysis of permeability reduction resulting from colloid deposition. Geophys. Res. Lett. 35, L07404 (2008).
Naeem, S., Duffy, J. E. & Zavaleta, E. The functions of biological diversity in an age of extinction. Science 336, 1401–1406 (2012).
Loreau, M. et al. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294, 804–808 (2001).
Ylla, I., Borrego, C., Romaní, A. M. & Sabater, S. Availability of glucose and light modulates the structure and function of a microbial biofilm. FEMS Microbiol. Ecol. 69, 27–42 (2009).
Romaní, A. M. et al. Biofilm structure and function and possible implications for riverine DOC dynamics. Microb. Ecol. 47, 316–328 (2004).
Guenet, B., Danger, M., Abbadie, L. & Lacroix, G. Priming effect: bridging the gap between terrestrial and aquatic ecology. Ecology 91, 2850–2861 (2010).
Danger, M. et al. Benthic algae stimulate leaf litter decomposition in detritus-based headwater streams: a case of aquatic priming effect? Ecology 94, 1604–1613 (2013).
Kuehn, K. A., Francoeur, S. N., Findlay, R. H. & Neely, R. K. Priming in the microbial landscape: periphytic algal stimulation of litter-associated microbial decomposers. Ecology 95, 749–762 (2014).
Bengtsson, M. M. et al. No evidence of aquatic priming effects in hyporheic zone microcosms. Sci. Rep. 4, 5187 (2014).
Cardinale, B. J. Biodiversity improves water quality through niche partitioning. Nature 472, 86–89 (2011).
Freimann, R., Bürgmann, H., Findlay, S. E. G. & Robinson, C. T. Bacterial structures and ecosystem functions in glaciated floodplains: contemporary states and potential future shifts. ISME J. 7, 2361–2373 (2013).
Frossard, A., Gerull, L., Mutz, M. & Gessner, M. O. Disconnect of microbial structure and function: enzyme activities and bacterial communities in nascent stream corridors. ISME J. 6, 680–691 (2012).
Wagner, K. et al. Functional and structural responses of hyporheic biofilms to varying sources of dissolved organic matter. Appl. Environ. Microbiol. 80, 6004–6012 (2014).
Hector, A. & Bagchi, R. Biodiversity and ecosystem multifunctionality. Nature 448, 188–190 (2007).
Peter, H. et al. Multifunctionality and diversity in bacterial biofilms. PLoS ONE 6, e23225 (2011).
Wilhelm, L. & Besemer, K. Altitudinal patterns of diversity and functional traits of metabolically active microorganisms in-stream biofilms. ISME J. 9, 2454–2464 (2015).
Dopheide, A., Lear, G., He, Z., Zhou, J. & Lewis, G. D. Functional gene composition, diversity and redundancy in microbial stream biofilm communities. PLoS ONE 10, e0123179 (2015).
Martiny, J. B. H., Jones, S. E., Lennon, J. T. & Martiny, A. C. Microbiomes in light of traits: a phylogenetic perspective. Science 350, aac9323 (2015).
Decho, A. W. et al. Sediment properties influencing upwelling spectral reflectance signatures: the 'biofilm gel effect'. Limnol. Oceanogr. 48, 431–443 (2003).
Rier, S. T., Shirvinski, J. M. & Kinek, K. C. In situ light and phosphorus manipulations reveal potential role of biofilm algae in enhancing enzyme-mediated decomposition of organic matter in streams. Freshw. Biol. 59, 1039–1051 (2014).
Van Horn, D. J., Sinsabaugh, R. L., Takacs-Vesbach, C. D., Mitchell, K. R. & Dahm, C. N. Response of heterotrophic stream biofilm communities to a gradient of resources. Aquat. Microb. Ecol. 64, 149–161 (2011).
Lyon, D. R. & Ziegler, S. E. Carbon cycling within epilithic biofilm communities across a nutrient gradient of headwater streams. Limnol. Oceanogr. 54, 439–449 (2009).
Ziegler, S. E., Lyon, D. R. & Townsend, S. L. Carbon release and cycling within epilithic biofilms in two contrasting headwater streams. Aquat. Microb. Ecol. 55, 285–300 (2009).
Findlay, S. E. G., Sinsabaugh, R. L., Sobczak, W. V. & Hoostal, M. Metabolic and structural response of hyporheic microbial communities to variations in supply of dissolved organic matter. Limnol. Oceanogr. 48, 1608–1617 (2003).
Sinsabaugh, R. L. & Follstad Shah, J. J. Ecoenzymatic stoichiometry and ecological theory. Annu. Rev. Ecol. Evol. Syst. 43, 313–343 (2012).
Singer, G., Besemer, K., Schmitt-Kopplin, P., Hödl, I. & Battin, T. J. Physical heterogeneity increases biofilm resource use and its molecular diversity in stream mesocosms. PLoS ONE 5, e9988–e9911 (2010).
Cardinale, B. J., Palmer, M. A., Swan, C. M., Brooks, S. & Poff, N. L. The influence of substrate heterogeneity on biofilm metabolism in a stream ecosystem. Ecology 83, 412 (2002).
Watrous, J. D. & Dorrestein, P. C. Imaging mass spectrometry in microbiology. Nat. Rev. Microbiol. 9, 683–694 (2011).
Liu, J. et al. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523, 550–554 (2015).
Piggott, J. J., Salis, R. K., Lear, G., Townsend, C. R. & Matthaei, C. D. Climate warming and agricultural stressors interact to determine stream periphyton community composition. Glob. Chang. Biol. 21, 206–222 (2015). This is the first experimental study to unravel the effects of global warming and eutrophication on algal and bacterial diversity in stream biofilms.
Downing, J. Global abundance and size distribution of streams and rivers. Inland Wat. 2, 229–236 (2012).
Drummond, J. D. et al. Retention and remobilization dynamics of fine particles and microorganisms in pastoral streams. Wat. Res. 66, 459–472 (2014).
Cadenasso, M. L. et al. An interdisciplinary and synthetic approach to ecological boundaries. BioScience 53, 717–722 (2003).
Strayer, D. L., Power, M. E., Fagan, W. F., Pickett, S. & Belnap, J. A classification of ecological boundaries. BioScience 53, 723–729 (2003).
Flemming, H.-C. & Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 8, 623–633 (2010).
Freeman, C. & Lock, M. A. The biofilm polysaccharide matrix - a buffer against changing organic substrate supply. Limnol. Oceanogr. 40, 273–278 (1995). This study shows that the biofilm matrix serves as a notable carbon reservoir for microbial heterotrophs during deprivation of carbon from external sources.
Battin, T. J. et al. Microbial landscapes: new paths to biofilm research. Nat. Rev. Microbiol. 5, 76–81 (2007).
Garcia-Pichel, F. & Wojciechowski, M. F. The evolution of a capacity to build supra-cellular ropes enabled filamentous cyanobacteria to colonize highly erodible substrates. PLoS ONE 4, e7801 (2009).
Logue, J. B. & Lindström, E. S. Biogeography of bacterioplankton in inland waters. Freshw. Rev. 1, 99–114 (2008).
Martiny, J. B. H. et al. Microbial biogeography: putting microorganisms on the map. Nat. Rev. Microbiol. 4, 102–112 (2006).
Araya, R., Tani, K., Takagi, T., Yamaguchi, N. & Nasu, M. Bacterial activity and community composition in stream water and biofilm from an urban river determined by fluorescent in situ hybridization and DGGE analysis. FEMS Microbiol. Ecol. 43, 111–119 (2003).
Rendueles, O. & Ghigo, J.-M. Mechanisms of competition in biofilm communities. Microbiol. Spectr. 3, MB-0009-2014 (2015).
Acknowledgements
The authors thank A. Ulseth and M. Bogard for critically reading an earlier version of the manuscript. Financial support came from the Austrian Science Foundation (P23420-B17, START Y420-B17) and the Swiss Science Foundation (205321_159958 / 1) to T.J.B., from the Austrian Science Foundation (J3542-B22) to K.B. and from the Spanish Ministry of Economy and Competitiveness (FLUMED-HOTSPOT) to A.R.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Periphyton
-
Traditionally considered to be a phototrophic biofilm that coats benthic substrates in stream ecosystems.
- Epilithon
-
Traditionally considered to be a biofilm that grows on stones in stream ecosystems.
- Meiobenthos
-
Invertebrates living in aquatic ecosystems that have a body size typically not exceeding one millimetre.
- Ecosystem respiration
-
The respiration by both heterotrophic and autotrophic organisms within an ecosystem, in which heterotrophic respiration generates carbon dioxide from the breakdown of organic compounds.
- Primary production
-
The generation of organic carbon from carbon dioxide by photosynthesis, which uses light as an energy source.
- Catchments
-
Drainage basin of streams or rivers delineated by the watershed and within which water from rain, snow or ice melt converges at the valley bottom to contribute to streamwater flow.
- Hyporheic zone
-
The zone in the streambed sediment in which streamwater interacts with groundwater, as driven by hydrodynamic exchange. Typically considered to be a habitat with high rates of biodiversity and biogeochemical reaction.
- Reflective characteristics
-
The ability of an interface, or ecological boundary, to partially or entirely return matter, energy or organisms.
- Phyllosphere
-
The microbial communities colonizing the above-ground surfaces that are provided by terrestrial plants.
- Benthic zone
-
The upper zone of the streambed; the benthic zone is notable for its direct interface with streamwater flow and its exposure to light.
- Humic substances
-
A complex and heterogeneous mixture of polydispersed materials formed by biochemical and chemical reactions during the decay of plant tissue. This mixture is a major contributor to dissolved organic matter in aquatic ecosystems.
- Co-occurrence networks
-
Graphical visualization of potential relationships, between species or other entities, that have been derived from correlation analyses.
- Operational taxonomic units
-
(OTUs). Classification of microorganisms on the basis of an operational definition for species distinction that applies a percentage similarity threshold to 16S rRNA sequences.
- Flow fields
-
Flow patterns that are generated by a moving liquid over and around solids.
- Bedforms
-
Geomorphological features that develop at the interface of fluid and a movable bed, such as dunes and ripples on the beds of streams and rivers. Bedforms affect near-bottom hydraulics and hydrodynamic exchange with porewater in the streambed.
- β-diversity
-
The compositional similarity of ecological communities and the species turnover therein.
- Neutral models
-
In the context of biodiversity, models that assume that individuals of all ecologically similar species are competitively equal and that the stochasticity of demographic processes, such as immigration, birth and death, drive local community assembly.
- α-diversity
-
Local species diversity in a habitat or ecosystem, often referred to as species richness or Shannon or Simpson diversity.
- Competitive exclusion
-
Ecological process whereby two (or more) species that use the same resources cannot stably coexist.
- Headwaters
-
The smallest streams in a fluvial network and where streamflow is generated.
- Laminar flow
-
The flow of water in parallel layers that are not disrupted. Laminar flow often fosters copious biofilm growth, as turbulence-induced erosion of microbial biomass is low.
- Drag force
-
A force that acts on any solid objects exposed to water flow; the drag comes from forces caused by pressure distributions over the surface of the object.
- Hydrodynamic exchange
-
The exchange of water masses driven by the pressure differences that occur over rough streambeds.
- Priming effect
-
Phenomenon in which labile dissolved organic matter (DOM) compounds facilitate the breakdown of apparently recalcitrant DOM compounds by microbial heterotrophs. The mechanism is unclear but may involve the provision of energy for the expression of extracellular enzymes that degrade recalcitrant DOM.
- Recalcitrant DOM
-
Dissolved organic matter (DOM) that is resistant to degradation by microbial heterotrophs.
- Functional plasticity
-
The capacity of an ecological community to accommodate environmental changes by adjusting the overall performance of dominant phylotypes.
- Functional redundancy
-
A concept that relates changes in ecosystems to species loss, in which species that perform similar roles in communities can substitute for one another with little effect on the functioning of the community and ecosystem.
- Functional gene arrays
-
DNA array technology for assessing functional gene diversity and distribution in microbial communities.
- Microautoradiography
-
Method that visualizes and quantifies the uptake of a radioactively labelled compound at the level of single cells.
- Eutrophication
-
The process in which increased nutrient inputs drive an increase in algal biomass in aquatic systems, which in turn causes anoxia as a result of the breakdown of these algae by microbial heterotrophs.
Rights and permissions
About this article
Cite this article
Battin, T., Besemer, K., Bengtsson, M. et al. The ecology and biogeochemistry of stream biofilms. Nat Rev Microbiol 14, 251–263 (2016). https://doi.org/10.1038/nrmicro.2016.15
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2016.15
This article is cited by
-
Global emergent responses of stream microbial metabolism to glacier shrinkage
Nature Geoscience (2024)
-
Pseudomonas aeruginosa’s greenish-blue pigment pyocyanin: its production and biological activities
Microbial Cell Factories (2023)
-
Evaporation-induced hydrodynamics control plasmid transfer during surface-associated microbial growth
npj Biofilms and Microbiomes (2023)
-
Cooperative microbial interactions drive spatial segregation in porous environments
Nature Communications (2023)
-
Local eukaryotic and bacterial stream community assembly is shaped by regional land use effects
ISME Communications (2023)