Key Points
-
Cryptococcosis is a widespread opportunistic fungal infection of humans and other animals.
-
Cryptococcus species that infect humans likely evolved as accidental pathogens in response to environmental selective pressure.
-
Recent genomic analyses have highlighted the evolutionary history of Cryptococcus spp. and narrowed down the geographical origin of an unusual, hypervirulent outbreak.
-
Despite being accidental pathogens, cryptococci display a remarkable ability to manipulate the human immune response to facilitate disease establishment and spread.
-
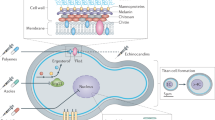
Detailed in vivo and in vitro characterization of Cryptococcus spp. has started to elucidate the details of multiple mechanisms of pathogenesis that probably have important roles in disease severity. These include changes in fungal morphology, interactions with host phagocytes and mechanisms that allow Cryptococcus spp. to disseminate from the lung to the central nervous system.
-
Renewed efforts to develop improved therapeutic approaches have highlighted potential new drugs and new uses for old drugs in the fight against cryptococcal disease.
Abstract
Cryptococcosis is a globally distributed invasive fungal infection that is caused by species within the genus Cryptococcus which presents substantial therapeutic challenges. Although natural human-to-human transmission has never been observed, recent work has identified multiple virulence mechanisms that enable cryptococci to infect, disseminate within and ultimately kill their human host. In this Review, we describe these recent discoveries that illustrate the intricacy of host–pathogen interactions and reveal new details about the host immune responses that either help to protect against disease or increase host susceptibility. In addition, we discuss how this improved understanding of both the host and the pathogen informs potential new avenues for therapeutic development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Kwon-Chung, J. K., Boekhout, T., Fell, J. W. & Diaz, M. Proposal to conserve the name Cryptococcus gattii against C. hondurianus and C. basillisporus (Basidiomycota, Hymenomycetes, Tremellomycetidae). Taxon 51, 804–806 (2002).
Hagen, F. et al. Recognition of seven species in the Cryptococcus gattii/Cryptococcus neoformans species complex. Fungal Genet. Biol. 78, 16–48 (2015).
Springer, D. J. et al. Cryptococcus gattii VGIII isolates causing infections in HIV/AIDS patients in Southern California: identification of the local environmental source as arboreal. PLoS Pathog. 10, e1004285 (2014).
Chowdhary, A., Rhandhawa, H. S., Prakash, A. & Meis, J. F. Environmental prevalence of Cryptococcus neoformans and Cryptococcus gattii in India: an update. Crit. Rev. Microbiol. 38, 1–16 (2012).
Litvintseva, A. P. et al. Evidence that the human pathogenic fungus Cryptococcus neoformans var. grubii may have evolved in Africa. PLoS ONE 6, e19688 (2011).
Baddley, J. W. et al. Transmission of Cryptococcus neoformans by organ transplantation. Clin. Infect. Dis. 52, e94–e98 (2011).
Lagrou, K. et al. Zoonotic transmission of Cryptococcus neoformans from a magpie to an immunocompetent patient. J. Intern. Med. 257, 385–388 (2005).
Goldman, D. L. et al. Serologic evidence for Cryptococcus neoformans infection in early childhood. Pediatrics 107, E66 (2001).
Giles, S. S., Dagenais, T. R., Botts, M. R., Keller, N. P. & Hull, C. M. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect. Immun. 77, 3491–3500 (2009).
Springer, D. J., Saini, D., Byrnes, E. J., Heitman, J. & Frothingham, R. Development of an aerosol model of Cryptococcus reveals humidity as an important factor affecting the viability of Cryptococcus during aerosolization. PLoS ONE 8, e69804 (2013).
Velagapudi, R., Hsueh, Y. P., Geunes-Boyer, S., Wright, J. R. & Heitman, J. Spores as infectious propagules of Cryptococcus neoformans. Infect. Immun. 77, 4345–4355 (2009).
Zaragoza, O. et al. The capsule of the fungal pathogen Cryptococcus neoformans. Adv. Appl. Microbiol. 68, 133–216 (2009).
McDonald, T., Wiesner, D. L. & Nielsen, K. Cryptococcus. Curr. Biol. 22, R554–R555 (2012).
Zaragoza, O. & Nielsen, K. Titan cells in Cryptococcus neoformans: cells with a giant impact. Curr. Opin. Microbiol. 16, 409–413 (2013).
Okagaki, L. H. et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog. 6, e1000953 (2010).
Zaragoza, O. et al. Fungal cell gigantism during mammalian infection. PLoS Pathog. 6, e1000945 (2010). References 15 and 16 simultaneously reported the identification of titan cells, which are likely to play a key role in cryptococcal pathogenesis.
Wiesner, D. L. et al. Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal Infection. PLoS Pathog. 11, e1004701 (2015).
Gerstein, A. C. et al. Polyploid titan cells produce haploid and aneuploid progeny to promote stress adaptation. mBio 6, e01340–01415 (2015).
Alanio, A., Vernel-Pauillac, F., Sturny-Leclère, A. & Dromer, F. Cryptococcus neoformans host adaptation: toward biological evidence of dormancy. mBio 6, e02580–e02614 (2015).
Feldmesser, M., Kress, Y. & Casadevall, A. Dynamic changes in the morphology of Cryptococcus neoformans during murine pulmonary infection. Microbiology 147, 2355–2365 (2001).
Neilson, J. B., Fromtling, R. A. & Bulmer, G. S. Pseudohyphal forms of Cryptococcus neoformans: decreased survival in vivo. Mycopathologia 73, 57–59 (1981).
Wang, L., Zhai, B. & Lin, X. The link between morphotype transition and virulence in Cryptococcus neoformans. PLoS Pathog. 8, e1002765 (2012).
Magditch, D. A., Liu, T. B., Xue, C. & Idnurm, A. DNA mutations mediate microevolution between host-adapted forms of the pathogenic fungus Cryptococcus neoformans. PLoS Pathog. 8, e1002936 (2012).
Lin, J., Idnurm, A. & Lin, X. Morphology and its underlying genetic regulation impact the interaction between Cryptococcus neoformans and its hosts. Med. Mycol. 53, 493–504 (2015).
Bouklas, T. & Fries, B. C. Aging as an emergent factor that contributes to phenotypic variation in Cryptococcus neoformans. Fungal Genet. Biol. 78, 59–64 (2014).
Bouklas, T. et al. Old Cryptococcus neoformans cells contribute to virulence in chronic cryptococcosis. mBio 4, e00455–00413 (2013).
Jain, N. et al. Isolation and characterization of senescent C. neoformans and its implications for phenotypic switching and the pathogenesis of chronic cryptococcosis. Eukaryot. Cell 8, 858–866 (2009).
Lee, H., Chang, Y. C., Nardone, G. & Kwon-Chung, K. J. TUP1 disruption in Cryptococcus neoformans uncovers a peptide-mediated density-dependent growth phenomenon that mimics quorum sensing. Mol. Microbiol. 64, 591–601 (2007).
Albuquerque, P. et al. Quorum sensing-mediated, cell density-dependent regulation of growth and virulence in Cryptococcus neoformans. mBio 5, e00986–e00913 (2014).
Wang, L., Tian, X., Gyawali, R. & Lin, X. Fungal adhesion protein guides community behaviors and autoinduction in a paracrine manner. Proc. Natl Acad. Sci. USA 110, 11571–11576 (2013).
Albuquerque, P. C. et al. Cryptococcus neoformans glucuronoxylomannan fractions of different molecular masses are functionally distinct. Future Microbiol. 9, 147–161 (2014).
Idnurm, A. & Heitman, J. Light controls growth and development via a conserved pathway in the fungal kingdom. PLoS Biol. 3, e95 (2005).
Schoffelen, T. et al. Cryptococcus gattii induces a cytokine pattern that is distinct from other cryptococcal species. PLoS ONE 8, e55579 (2013).
Piccioni, M. et al. A purified capsular polysaccharide markedly inhibits inflammatory response during endotoxic shock. Infect. Immun. 81, 90–98 (2013).
Angkasekwinai, P. et al. Cryptococcus gattii infection dampens Th1 and Th17 responses by attenuating dendritic cell function and pulmonary chemokine expression in the immunocompetent hosts. Infect. Immun. 82, 3880–3890 (2014).
Qiu, Y. et al. Immune modulation mediated by cryptococcal laccase promotes pulmonary growth and brain dissemination of virulent Cryptococcus neoformans in mice. PLoS ONE 7, e47853 (2012).
Davis, M. J. et al. Macrophage M1/M2 polarization dynamically adapts to changes in cytokine microenvironments in Cryptococcus neoformans infection. mBio 4, e00264–e00213 (2013).
Voelz, K., Lammas, D. A. & May, R. C. Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect. Immun. 77, 3450–3457 (2009).
Muller, U. et al. Abrogation of IL-4 receptor-α-dependent alternatively activated macrophages is sufficient to confer resistance against pulmonary cryptococcosis despite an ongoing Th2 response. Int. Immunol. 25, 459–470 (2013).
Hardison, S. E. et al. Protective immunity against pulmonary cryptococcosis is associated with STAT1-mediated classical macrophage activation. J. Immunol. 189, 4060–4068 (2012).
Flaczyk, A. et al. IL-33 signaling regulates innate and adaptive immunity to Cryptococcus neoformans. J. Immunol. 191, 2503–2513 (2013).
Chen, G. H. et al. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect. Immun. 76, 2379–2391 (2008).
Coelho, C., Bocca, A. L. & Casadevall, A. The intracellular life of Cryptococcus neoformans. Annu. Rev. Pathol. 9, 219–238 (2014).
Alvarez, M. & Casadevall, A. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol. 8, 16 (2007).
Ma, H., Croudace, J. E., Lammas, D. A. & May, R. C. Direct cell-to-cell spread of a pathogenic yeast. BMC Immunol. 8, 15 (2007).
Ma, H., Croudace, J. E., Lammas, D. A. & May, R. C. Expulsion of live pathogenic yeast by macrophages. Curr. Biol. 16, 2156–2160 (2006).
Alvarez, M. & Casadevall, A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr. Biol. 16, 2161–2165 (2006).
Nicola, A. M., Robertson, E. J., Albuquerque, P., Derengowski Lda, S. & Casadevall, A. Nonlytic exocytosis of Cryptococcus neoformans from macrophages occurs in vivo and is influenced by phagosomal pH. mBio 2, e00167–e00111 (2011).
Okagaki, L. H. & Nielsen, K. Titan cells confer protection from phagocytosis in Cryptococcus neoformans infections. Eukaryot. Cell 11, 820–826 (2012).
Smith, L. M., Dixon, E. F. & May, R. C. The fungal pathogen Cryptococcus neoformans manipulates macrophage phagosome maturation. Cell. Microbiol. (2014).
Davis, M. J. et al. Cryptococcus neoformans-induced macrophage lysosome damage crucially contributes to fungal virulence. J. Immunol. 194, 2219–2231 (2015).
Johnston, S. A. & May, R. C. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog. 6, e1001041 (2010).
Erb-Downward, J. R., Noggle, R. M., Williamson, P. R. & Huffnagle, G. B. The role of laccase in prostaglandin production by Cryptococcus neoformans. Mol. Microbiol. 68, 1428–1437 (2008).
Evans, R. J. et al. Cryptococcal phospholipase B1 is required for intracellular proliferation and control of titan cell morphology during macrophage infection. Infect. Immun. 83, 1296–1304 (2015).
Vu, K. et al. Invasion of the central nervous system by Cryptococcus neoformans requires a secreted fungal metalloprotease. mBio 5, e01101–e01114 (2014).
Shi, M. et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J. Clin. Invest. 120, 1683–1693 (2010). The first observation of cryptococcal invasion into the brain in vivo.
Olszewski, M. A. et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am. J. Pathol. 164, 1761–1771 (2004).
Chang, Y. C. et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect. Immun. 72, 4985–4995 (2004).
Jong, A. et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell. Microbiol. 10, 1313–1326 (2008).
Jong, A. et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells requires protein kinase C-α activation. Cell. Microbiol. 10, 1854–1865 (2008).
Liu, T. B. et al. Brain inositol is a novel stimulator for promoting Cryptococcus penetration of the blood-brain barrier. PLoS Pathog. 9, e1003247 (2013).
Kechichian, T. B., Shea, J. & Del Poeta, M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect. Immun. 75, 4792–4798 (2007).
Charlier, C. et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect. Immun. 77, 120–127 (2009).
Chen, Y. et al. The Cryptococcus neoformans transcriptome at the site of human meningitis. mBio 5, e01087–01013 (2014).
Robertson, E. J. et al. Cryptococcus neoformans ex vivo capsule size is associated with intracranial pressure and host immune response in HIV-associated cryptococcal meningitis. J. Infect. Dis. 209, 74–82 (2014).
Jarvis, J. N. et al. Cerebrospinal fluid cytokine profiles predict risk of early mortality and immune reconstitution inflammatory syndrome in HIV-associated cryptococcal meningitis. PLoS Pathog. 11, e1004754 (2015).
Datta, K. et al. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg. Infect. Dis. 15, 1185–1191 (2009).
Harris, J. R. et al. Cryptococcus gattii in the United States: clinical aspects of infection with an emerging pathogen. Clin. Infect. Dis. 53, 1188–1195 (2011).
Ma, H. et al. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc. Natl Acad. Sci. USA 106, 12980–12985 (2009).
Voelz, K. et al. 'Division of labour' in response to host oxidative burst drives a fatal Cryptococcus gattii outbreak. Nat. Commun. 5, 5194 (2014). Description of a new virulence mechanism that underpins the hypervirulent Pacific Northwest outbreak.
Brizendine, K. D., Baddley, J. W. & Pappas, P. G. Predictors of mortality and differences in clinical features among patients with cryptococcosis according to immune status. PLoS ONE 8, e60431 (2013).
Siddiqi, O. K. et al. Molecular diagnosis of central nervous system opportunistic infections in HIV-infected Zambian adults. Clin. Infect. Dis. 58, 1771–1777 (2014).
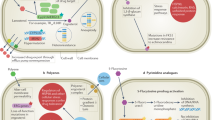
Anderson, T. M. et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 10, 400–406 (2014).
Belenky, P., Camacho, D. & Collins, J. J. Fungicidal drugs induce a common oxidative-damage cellular death pathway. Cell Rep. 3, 350–358 (2013).
Gray, K. C. et al. Amphotericin primarily kills yeast by simply binding ergosterol. Proc. Natl Acad. Sci. USA 109, 2234–2239 (2012).
Brouwer, A. E. et al. Combination antifungal therapies for HIV-associated cryptococcal meningitis: a randomised trial. Lancet 363, 1764–1767 (2004).
Day, J. N. et al. Combination antifungal therapy for cryptococcal meningitis. N. Engl. J. Med. 368, 1291–1302 (2013).
Perfect, J. R. et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases society of America. Clin. Infect. Dis. 50, 291–322 (2010).
Loyse, A. et al. Cryptococcal meningitis: improving access to essential antifungal medicines in resource-poor countries. Lancet Infect. Dis. 13, 629–637 (2013).
ISRCTN Registry. A phase III, randomised, controlled trial for the treatment of HIV-associated cryptococcal meningitis: oral fluconazole plus flucytosine or one week amphotericin B-based therapy vs two weeks amphotericin B-based therapy. ISRCTN registry[online], (2015).
Kelly, S. L. et al. Resistance to amphotericin B associated with defective sterol Δ8⊠7 isomerase in a Cryptococcus neoformans strain from an AIDS patient. FEMS Microbiol. Lett. 122, 39–42 (1994).
Bicanic, T., Harrison, T., Niepieklo, A., Dyakopu, N. & Meintjes, G. Symptomatic relapse of HIV-associated cryptococcal meningitis after initial fluconazole monotherapy: the role of fluconazole resistance and immune reconstitution. Clin. Infect. Dis. 43, 1069–1073 (2006).
Sionov, E., Chang, Y. C., Garraffo, H. M. & Kwon-Chung, K. J. Heteroresistance to fluconazole in Cryptococcus neoformans is intrinsic and associated with virulence. Antimicrob. Agents Chemother. 53, 2804–2815 (2009).
Sionov, E., Lee, H., Chang, Y. C. & Kwon-Chung, K. J. Cryptococcus neoformans overcomes stress of azole drugs by formation of disomy in specific multiple chromosomes. PLoS Pathog. 6, e1000848 (2010). An elegant example of the mechanism driving antifungal resistance in cryptococci.
Sionov, E., Chang, Y. C. & Kwon-Chung, K. J. Azole heteroresistance in Cryptococcus neoformans: emergence of resistant clones with chromosomal disomy in the mouse brain during fluconazole treatment. Antimicrob. Agents Chemother. 57, 5127–5130 (2013).
Posteraro, B. et al. Identification and characterization of a Cryptococcus neoformans ATP binding cassette (ABC) transporter-encoding gene, CnAFR1, involved in the resistance to fluconazole. Mol. Microbiol. 47, 357–371 (2003).
Miyazaki, M. et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 55, 4652–4658 (2011).
Shibata, T. et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob. Agents Chemother. 56, 5892–5897 (2012).
Brown, J. C. et al. Unraveling the biology of a fungal meningitis pathogen using chemical genetics. Cell 159, 1168–1187 (2014). A powerful approach for identifying new antifungals and identifying their mode of action.
Dehdashti, S. J. et al. A high-throughput screening assay for assessing the viability of Cryptococcus neoformans under nutrient starvation conditions. Anal. Bioanal Chem. 405, 6823–6829 (2013).
Butts, A. et al. A repurposing approach identifies off-patent drugs with fungicidal cryptococcal activity, a common structural chemotype, and pharmacological properties relevant to the treatment of cryptococcosis. Eukaryot. Cell 12, 278–287 (2013).
Butts, A. et al. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio 5, e00765–e00713 (2014).
Zhai, B., Wu, C., Wang, L., Sachs, M. S. & Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 56, 3758–3766 (2012).
US National Library of Medicine. ClinicalTrials.gov [online], (2015).
Saha, D. C. et al. Serologic evidence for reactivation of cryptococcosis in solid-organ transplant recipients. Clin. Vaccine Immunol. 14, 1550–1554 (2007).
Fang, W., Fa, Z. & Liao, W. Epidemiology of Cryptococcus and cryptococcosis in China. Fungal Genet. Biol. 78, 7–15 (2014).
Beale, M. A. et al. Genotypic diversity is associated with clinical outcome and phenotype in cryptococcal meningitis across Southern Africa. PLoS Negl. Trop. Dis. 9, e0003847 (2015). A recent wide-ranging analysis that, for the first time, demonstrates an important role for cryptococcal genotype in human pathogenesis.
Litvintseva, A. P. & Mitchell, T. G. Most environmental isolates of Cryptococcus neoformans var. grubii (serotype A) are not lethal for mice. Infect. Immun. 77, 3188–3195 (2009).
Wiesner, D. L. et al. Cryptococcal genotype influences immunologic response and human clinical outcome after meningitis. mBio 3, e00196–e00112 (2012).
Khayhan, K. et al. Geographically structured populations of Cryptococcus neoformans variety grubii in Asia correlate with HIV status and show a clonal population structure. PLoS ONE 8, e72222 (2013).
Ou, X. T. et al. Genotypes coding for mannose-binding lectin deficiency correlated with cryptococcal meningitis in HIV-uninfected Chinese patients. J. Infect. Dis. 203, 1686–1691 (2011).
Hu, X. P. et al. Association of Fcγ receptor IIB polymorphism with cryptococcal meningitis in HIV-uninfected Chinese patients. PLoS ONE 7, e42439 (2012).
Rohatgi, S. et al. Fcγ receptor 3A polymorphism and risk for HIV-associated cryptococcal disease. mBio 4, e00573–e00513 (2013).
Sabiiti, W. et al. Efficient phagocytosis and laccase activity affect the outcome of HIV-associated cryptococcosis. J. Clin. Invest. 124, 2000–2008 (2014).
Jarvis, J. N. et al. Evaluation of a novel point-of-care cryptococcal antigen test on serum, plasma, and urine from patients with HIV-associated cryptococcal meningitis. Clin. Infect. Dis. 53, 1019–1023 (2011).
Mfinanga, S. et al. Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 385, 60164–60167 (2015).
Findley, K. et al. Phylogeny and phenotypic characterization of pathogenic Cryptococcus species and closely related saprobic taxa in the Tremellales. Eukaryot. Cell 8, 353–361 (2009).
Xu, J., Vilgalys, R. & Mitchell, T. G. Multiple gene genealogies reveal recent dispersion and hybridization in the human pathogenic fungus Cryptococcus neoformans. Mol. Ecol. 9, 1471–1481 (2000). Compelling genetic evidence of an African origin for C. neoformans.
Litvintseva, A. P. & Mitchell, T. G. Population genetic analyses reveal the African origin and strain variation of Cryptococcus neoformans var. grubii. PLoS Pathog. 8, e1002495 (2012).
Litvintseva, A. P., Lin, X., Templeton, I., Heitman, J. & Mitchell, T. G. Many globally isolated AD hybrid strains of Cryptococcus neoformans originated in Africa. PLoS Pathog. 3, e114 (2007).
Billmyre, R. B. et al. Highly recombinant VGII Cryptococcus gattii population develops clonal outbreak clusters through both sexual macroevolution and asexual microevolution. mBio 5, e01494–e01414 (2014). Detailed analysis of C. gattii population structure, providing important insights into the evolution and dispersal of lineages within this species.
Hagen, F. et al. Ancient dispersal of the human fungal pathogen Cryptococcus gattii from the Amazon Rainforest. PLoS ONE 8, e71148 (2013).
Engelthaler, D. M. et al. Cryptococcus gattii in North American Pacific Northwest: whole-population genome analysis provides insights into species evolution and dispersal. mBio 5, e01464–e01414 (2014).
Fraser, J. A. et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437, 1360–1364 (2005).
Idnurm, A. et al. Deciphering the model pathogenic fungus Cryptococcus neoformans. Nat. Rev. Microbiol. 3, 753–764 (2005). Although now 10 years old, this is still an excellent all-round introduction to the pathogen.
Lin, X., Hull, C. M. & Heitman, J. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434, 1017–1021 (2005).
Lin, X. et al. Diploids in the Cryptococcus neoformans serotype A population homozygous for the α mating type originate via unisexual mating. PLoS Pathog. 5, e1000283 (2009).
Ni, M. et al. Unisexual and heterosexual meiotic reproduction generate aneuploidy and phenotypic diversity de novo in the yeast Cryptococcus neoformans. PLoS Biol. 11, e1001653 (2013).
Lin, X. et al. αADα hybrids of Cryptococcus neoformans: evidence of same-sex mating in nature and hybrid fitness. PLoS Genet. 3, 1975–1990 (2007).
Bovers, M. et al. Unique hybrids between the fungal pathogens Cryptococcus neoformans and Cryptococcus gattii. FEMS Yeast Res. 6, 599–607 (2006).
Casadevall, A. Evolution of intracellular pathogens. Annu. Rev. Microbiol. 62, 19–33 (2008).
Wang, Y. & Casadevall, A. Decreased susceptibility of melanized Cryptococcus neoformans to UV light. Appl. Environ. Microbiol. 60, 3864–3866 (1994).
Warpeha, K. M., Park, Y. D. & Williamson, P. R. Susceptibility of intact germinating Arabidopsis thaliana to human fungal pathogens Cryptococcus neoformans and C. gattii. Appl. Environ. Microbiol. 79, 2979–2988 (2013).
Steenbergen, J. N., Shuman, H. A. & Casadevall, A. Cryptococcus neoformans interactions with amoebae suggest an explanation for its virulence and intracellular pathogenic strategy in macrophages. Proc. Natl Acad. Sci. USA 98, 15245–15250 (2001). The first report of cryptococcal parasitism of amoebae, and the associated proposal of an 'accidental pathogen' model for the evolution of cryptococcal virulence.
Zaragoza, O. et al. Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell. Microbiol. 10, 2043–2057 (2008).
Garcia-Hermoso, D., Janbon, G. & Dromer, F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J. Clin. Microbiol. 37, 3204–3209 (1999).
Kronstad, J. W. et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat. Rev. Microbiol. 9, 193–203 (2011).
Steenbergen, J. N., Nosanchuk, J. D., Malliaris, S. D. & Casadevall, A. Interaction of Blastomyces dermatitidis, Sporothrix schenckii, and Histoplasma capsulatum with Acanthamoeba castellanii. Infect. Immun. 72, 3478–3488 (2004).
Bliska, J. B. & Casadevall, A. Intracellular pathogenic bacteria and fungi—a case of convergent evolution? Nat. Rev. Microbiol. 7, 165–171 (2009).
Jarvis, J. N. et al. Adjunctive interferon-γ immunotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 26, 1105–1113 (2012). An important clinical trial, both in improving patient outcomes and in demonstrating the direct effect of IFNγ on cryptococcal immunity.
Saijo, T. et al. Anti-granulocyte-macrophage colony-stimulating factor autoantibodies are a risk factor for central nervous system infection by Cryptococcus gattii in otherwise immunocompetent patients. mBio 5, e00912–e00914 (2014).
Grahnert, A. et al. IL-4 receptor-α-dependent control of Cryptococcus neoformans in the early phase of pulmonary infection. PLoS ONE 9, e87341 (2014).
Schulze, B. et al. CD4+ FoxP3+ regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur. J. Immunol. 44, 3596–3604 (2014).
Murdock, B. J., Huffnagle, G. B., Olszewski, M. A. & Osterholzer, J. J. Interleukin-17A enhances host defense against cryptococcal lung infection through effects mediated by leukocyte recruitment, activation, and γ interferon production. Infect. Immun. 82, 937–948 (2014).
Szymczak, W. A., Sellers, R. S. & Pirofski, L. A. IL-23 dampens the allergic response to Cryptococcus neoformans through IL-17-independent and -dependent mechanisms. Am. J. Pathol. 180, 1547–1559 (2012).
Boulware, D. R. et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N. Engl. J. Med. 370, 2487–2498 (2014).
Chang, C. C. et al. Cryptococcosis-IRIS is associated with lower cryptococcus-specific IFN-γ responses before antiretroviral therapy but not higher T-cell responses during therapy. J. Infect. Dis. 208, 898–906 (2013).
ISRCTN Registry. Adjunctive dexamethasone in HIV-infected adults with cryptococcal meningitis. ISRCTN registry[online], (2015).
Acknowledgements
The authors gratefully acknowledge the help of S. Kannambath in preparing Figure 3 and apologize to those colleagues in the field whose work could not be included in this Review owing to space constraints. R.C.M. is supported by funding from the European Research Council, Medical Research Council, Lister Institute and Royal Society. D.L.W. received support from the US National Institutes of Health (NIH) T32 training grant AI007313, a University of Minnesota Doctoral Dissertation Fellowship and a Dennis W. Watson Fellowship. K.N. is supported by funding from the NIH. T.B. is supported by funding from the Wellcome Trust and the Medical Research Council (UK). N.R.H.S. is supported by a Wellcome Trust Strategic Award in Medical Mycology and Fungal Immunology to the University of Aberdeen.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Pacific Northwest outbreak
-
An unusual cluster of cryptococcal disease in otherwise healthy (rather than immunocompromised) individuals. First identified on Vancouver Island, British Columbia, in 1999 (and hence originally called the Vancouver Island outbreak), both the causative organism and cases of human and animal disease have now expanded into mainland Canada and the northwestern USA, prompting a renaming of the outbreak.
- Iatrogenic
-
Caused by medical treatment. For instance, infections due to contaminated surgical instruments.
- Zoonotic
-
A disease transmitted from animals to people.
- Polyploid
-
Having multiple (that is, more than two) sets of homologous chromosomes.
- Founder
-
The initial, small group of individuals that seeds a new population. For instance, the inoculum that starts an infection, or the first individuals to arrive on a new island habitat.
- Quorum sensing
-
The regulation of gene expression or behaviour in response to changes in the local population size.
- Paracrine
-
A signal that acts close to where it is produced, for instance on neighbouring cells.
- Filamentation
-
The growth of an organism by elongation without division.
- Major histocompatibility complex class II
-
(MHC class II). Molecules that are expressed on the surface of professional antigen-presenting cells (such as macrophages and dendritic cells) and present extracellular antigens to the immune system to coordinate an immune response.
- T helper 1 response
-
A response by one subtype of CD4+ helper T (TH) cell that is generally provoked by intracellular pathogens. TH2 responses, by contrast, are typically involved in the elimination of parasitic worms, harmful allergic responses and dampening of TH1-mediated inflammation. In the context of cryptococcal infection, TH1 responses are widely thought to be protective, and TH2 responses to be detrimental.
- Coalescence analyses
-
An evolutionary analysis method in which genetic drift is 'played backwards' to calculate common ancestry of individuals within a population and thereby estimate lineage branch points within an evolutionary phylogenetic tree.
- Bipolar mating
-
A system to control sexual reproduction that relies on a single genetic locus at which individual organisms can carry one of two alleles, effectively generating a species with two sexes.
- Diploid
-
Having two homologous sets of chromosomes, one from each parent.
- Aneuploid
-
Having an 'unbalanced' set of chromosomes; for instance, having only a single copy of one chromosome in an otherwise diploid genome.
- Melanization
-
The production of the dark, insoluble pigment melanin, which provides protection from high energy radiation and reactive oxygen molecules.
- Blood–brain barrier
-
A specialized endothelial barrier that prevents the entry of cells or large molecules into the central nervous system.
- Paracytosis
-
Transitioning between tissues by moving between, rather than through, adjacent cells.
- Transcytosis
-
Transitioning between tissues by moving directly through cells, rather than between adjacent cells.
- Hyaluronic acid
-
An abundant, high-molecular-weight polysaccharide that forms part of the extracellular matrix, particularly in neural tissue.
- Cerebrospinal fluid
-
(CSF). A clear fluid produced in the brain that bathes the central nervous tissue and is slowly turned over.
- Regulatory T cells
-
A type of T cell that functions to regulate the immune system, typically by suppressing the function of pro-inflammatory effector T cells.
- Fungicidal
-
An antimicrobial agent that kills fungi, rather than simply preventing growth.
- Fungistatic
-
An antimicrobial agent that prevents fungal growth, but does not kill the organism.
Rights and permissions
About this article
Cite this article
May, R., Stone, N., Wiesner, D. et al. Cryptococcus: from environmental saprophyte to global pathogen. Nat Rev Microbiol 14, 106–117 (2016). https://doi.org/10.1038/nrmicro.2015.6
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrmicro.2015.6
This article is cited by
-
Molecular characterization of clinical and environmental isolates from the Cryptococcus neoformans/C. Gattii species complexes of Maceió, Alagoas, Brazil
Brazilian Journal of Microbiology (2024)
-
Retrospective analysis of pulmonary cryptococcosis and extrapulmonary cryptococcosis in a chinese tertiary hospital
BMC Pulmonary Medicine (2023)
-
Persistent neurological symptoms and elevated intracranial pressures in a previously healthy host with cryptococcal meningitis
BMC Infectious Diseases (2023)
-
Differentiated extracts from freshwater and terrestrial mollusks inhibit virulence factor production in Cryptococcus neoformans
Scientific Reports (2023)
-
Cryptococcus neoformans adapts to the host environment through TOR-mediated remodeling of phospholipid asymmetry
Nature Communications (2023)