Key Points
-
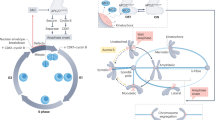
The spindle checkpoint monitors the interactions between chromosomes and microtubules.
-
The Mad and Bub proteins are core components of this cell-cycle control.
-
The checkpoint can monitor kinetochore–microtubule attachment and/or the tension exerted at kinetochores upon bipolar attachment.
-
Kinetochores are a key site for monitoring defects and signalling, and for effecting the cell-cycle delay. They can act as the catalytic site for the production of the checkpoint signal ('wait anaphase'), which must then be transmitted globally.
-
Microtubule motors (CENP-E and dynein) and protein kinases (Aurora B) have roles in kinetochore binding and bipolar attachment, and could be involved in sensing tension.
-
Mad2 and other checkpoint proteins have a very dynamic association with kinetochores (half-life of ∼20 s).
-
These dynamics might also be involved in checkpoint silencing. Several checkpoint components (Mad2, BubR1, CENP-E and Rod) are continuously removed from kinetochores by a dynein-dependent mechanism.
-
Two related interactions of Mad2 (with Mad1 and Cdc20) are central to the checkpoint, and crystal structures of a Mad1–Mad2 complex have revealed a 'safety-belt' bind-and-release mechanism.
-
Mad2 and/or BubR1 complexes might stoichiometrically inhibit Cdc20 and thereby the activity of the anaphase-promoting complex.
Abstract
Chromosome segregation is a complex and astonishingly accurate process whose inner working is beginning to be understood at the molecular level. The spindle checkpoint plays a key role in ensuring the fidelity of this process. It monitors the interactions between chromosomes and microtubules, and delays mitotic progression to allow extra time to correct defects. Here, we review and integrate findings on the dynamics of checkpoint proteins at kinetochores with structural information about signalling complexes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Li, R. & Murray, A. W. Feedback control of mitosis in budding yeast. Cell 66, 519–531 (1991).
Hoyt, M. A., Totis, L. & Roberts, B. T. S. cerevisiae genes required for cell cycle arrest in response to loss of microtubule function. Cell 66, 507–517 (1991).Refs 1 and 2 describe genetic screens that led to the first molecular identification of spindle-checkpoint components.
Nasmyth, K. Disseminating the genome: joining, resolving, and separating sister chromatids during mitosis and meiosis. Annu. Rev. Genet. 35, 673–745 (2001).
Rieder, C. L. & Salmon, E. D. The vertebrate cell kinetochore and its roles during mitosis. Trends Cell Biol. 8, 310–318 (1998).
Pidoux, A. L. & Allshire, R. C. Centromeres: getting a grip of chromosomes. Curr. Opin. Cell Biol. 12, 308–319 (2000).
Hwang, L. H. et al. Budding yeast Cdc20: a target of the spindle checkpoint. Science 279, 1041–1044 (1998).Refs 6 and 7 show that CDC20 mutants that are resistant to the checkpoint cannot bind Mad2, establishing Cdc20 as the target of the spindle checkpoint. Furthermore, ref. 6 describes a set of mutual binding dependencies between budding-yeast checkpoint proteins, including an absolute requirement for Mad1 for Mad2–Cdc20-complex formation.
Kim, S. H. et al. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science 279, 1045–1047 (1998).
Peters, J. M. The anaphase-promoting complex: proteolysis in mitosis and beyond. Mol. Cell 9, 931–943 (2002).
Hoyt, M. A. A new view of the spindle checkpoint. J. Cell Biol. 154, 909–911 (2001).
Rieder, C. L., Cole, R. W., Khodjakov, A. & Sluder, G. The checkpoint delaying anaphase in response to chromosome monoorientation is mediated by an inhibitory signal produced by unattached kinetochores. J. Cell Biol. 130, 941–948 (1995).This paper identifies the kinetochores of unattached chromosomes as the site of production of the checkpoint signal. PtK1 cells are followed by video microscopy before and after specific chromosomal regions are destroyed by laser irradiation. The checkpoint is relieved after the centromere is destroyed on the last mono-oriented chromosome. Thus, the checkpoint mechanism monitors an inhibitor of anaphase produced by unattached kinetochores.
Li, X. & Nicklas, R. B. Mitotic forces control a cell-cycle checkpoint. Nature 373, 630–632 (1995).The classic demonstration that lack of tension at kinetochores can activate the spindle checkpoint. In 10% of praying-mantis spermatocytes, a free X chromosome delays anaphase by 5–6 h. Applying tension to this X chromosome with a micromanipulation needle is enough to relieve the checkpoint delay and to induce anaphase.
Chen, R. H., Waters, J. C., Salmon, E. D. & Murray, A. W. Association of spindle assembly checkpoint component XMAD2 with unattached kinetochores. Science 274, 242–246 (1996).
Li, Y. & Benezra, R. Identification of a human mitotic checkpoint gene: hsMAD2. Science 274, 246–248 (1996).Refs 12 and 13 provided the first demonstration of the kinetochore localization of a defined checkpoint protein.
Maney, T., Ginkel, L. M., Hunter, A. W. & Wordeman, L. The kinetochore of higher eucaryotes: a molecular view. Int. Rev. Cytol. 194, 67–131 (2000).
Brunet, S. & Vernos, I. Chromosome motors on the move. From motion to spindle checkpoint activity. EMBO Rep. 2, 669–673 (2001).
Kapoor, T. M. & Compton, D. A. Searching for the middle ground. J. Cell Biol. 157, 551–556 (2002).
Chan, G. K. T., Schaar, B. T. & Yen, T. J. Characterization of the kinetochore binding domain of CENP-E reveals interactions with the kinetochore proteins CENP-F and hBUBR1. J. Cell Biol. 143, 49–63 (1998).
Chan, G. K. et al. Human BUBR1 is a mitotic checkpoint kinase that monitors CENP-E functions at kinetochores and binds the cyclosome/APC. J. Cell Biol. 146, 941–954 (1999).
Yao, X. et al. CENP-E forms a link between attachment of spindle microtubules to kinetochores and the mitotic checkpoint. Nature Cell Biol. 2, 484–491 (2000).
Abrieu, A., Kahana, J. A., Wood, K. W. & Cleveland, D. W. CENP-E as an essential component of the mitotic checkpoint in vitro. Cell 102, 817–826 (2000).
McEwen, B. F. et al. CENP-E is essential for reliable bioriented spindle attachment, but chromosome alignment can be achieved via redundant mechanisms in mammalian cells. Mol. Biol. Cell 12, 2776–2789 (2001).
Banks, J. D. & Heald, R. Chromosome movement: dynein-out at the kinetochore. Curr. Biol. 11, R128–R131 (2001).
Starr, D. A., Williams, B. C., Hays, T. S. & Goldberg, M. L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 142, 763–774 (1998).
Savoian, M. S., Goldberg, M. L. & Rieder, C. L. The rate of poleward chromosome motion is attenuated in Drosophila zw10 and rod mutants. Nature Cell Biol. 2, 948–952 (2000).
Sharp, D. J., Rogers, G. C. & Scholey, J. M. Cytoplasmic dynein is required for poleward chromosome movement during mitosis in Drosophila embryos. Nature Cell Biol. 2, 922–930 (2000).
Chan, G. K. et al. Human Zw10 and ROD are mitotic checkpoint proteins that bind to kinetochores. Nature Cell Biol. 2, 944–947 (2000).
Basto, R., Gomes, R. & Karess, R. E. Rough deal and Zw10 are required for the metaphase checkpoint in Drosophila. Nature Cell Biol. 2, 939–943 (2000).
Yu, H. G., Muszynski, M. G. & Dawe, R. K. The maize homologue of the cell cycle checkpoint protein MAD2 reveals kinetochore substructure and contrasting mitotic and meiotic localization patterns. J. Cell Biol. 145, 425–435 (1999).
Waters, J. C., Chen, R. H., Murray, A. W. & Salmon, E. D. Localization of Mad2 to kinetochores depends on microtubule attachment, not tension. J. Cell Biol. 141, 1181–1191 (1998).
Skoufias, D. A. et al. Mammalian Mad2 and Bub1/BubR1 recognize distinct spindle-attachment and kinetochore-tension checkpoints. Proc. Natl Acad. Sci. USA 98, 4492–4497 (2001).
Zhou, J. et al. Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J. Biol. Chem. 277, 17200–17208 (2002).
Gorbsky, G. J. & Ricketts, W. A. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J. Cell Biol. 122, 1311–1321 (1993).
Taylor, S. S. et al. Kinetochore localisation and phosphorylation of the mitotic checkpoint components Bub1 and BubR1 are differentially regulated by spindle events in human cells. J. Cell Sci. 114, 4385–4395 (2001).
Kapoor, T. M., Mayer, T. U., Coughlin, M. L. & Mitchison, T. J. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150, 975–988 (2000).
Shonn, M. A., McCarroll, R. & Murray, A. W. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science 289, 300–303 (2000).
Stern, B. M. & Murray, A. W. Lack of tension at kinetochores activates the spindle checkpoint in budding yeast. Curr. Biol. 11, 1462–1467 (2001).
Hoffman, D. B. et al. Microtubule-dependent changes in assembly of microtubule motor proteins and mitotic spindle checkpoint proteins at PtK1 kinetochores. Mol. Biol. Cell 12, 1995–2009 (2001).
Taylor, S. S. & McKeon, F. Kinetochore localization of murine Bub1 is required for normal mitotic timing and checkpoint response to spindle damage. Cell 89, 727–735 (1997).
Taylor, S. S., Ha, E. & McKeon, F. The human homologue of Bub3 is required for kinetochore localization of Bub1 and a Mad3/Bub1-related protein kinase. J. Cell Biol. 142, 1–11 (1998).
Jablonski, S. A. et al. The hBUB1 and hBUBR1 kinases sequentially assemble onto kinetochores during prophase with hBUBR1 concentrating at the kinetochore plates in mitosis. Chromosoma 107, 386–396 (1998).
Basu, J. et al. Localization of the Drosophila checkpoint control protein Bub3 to the kinetochore requires Bub1 but not Zw10 or Rod. Chromosoma 107, 376–385 (1998).
Martinez-Exposito, M. J., Kaplan, K. B., Copeland, J. & Sorger, P. K. Retention of the BUB3 checkpoint protein on lagging chromosomes. Proc. Natl Acad. Sci. USA 96, 8493–8498 (1999).
Adams, R. R., Carmena, M. & Earnshaw, W. E. Chromosomal passengers and the (aurora) ABCs of mitosis. Trends Cell Biol. 11, 49–54 (2001).
Tanaka, T. U. et al. Evidence that the Ipl1–Sli15 (Aurora kinase–INCENP) complex promotes chromosome bi-orientation by altering kinetochore–spindle pole connections. Cell 108, 317–329 (2002).
Shimoda, S. L. & Solomon, F. Integrating functions at the kinetochore. Cell 109, 9–12 (2002).
Biggins, S. & Murray, A. W. The budding yeast protein kinase Ipl1/Aurora allows the absence of tension to activate the spindle checkpoint. Genes Dev. 15, 3118–3129 (2001).
Piatti, S., Lengauer, C. & Nasmyth, K. Cdc6 is an unstable protein whose de novo synthesis in G1 is important for the onset of S phase and for preventing a 'reductional' anaphase in the budding yeast Saccharomyces cerevisiae. EMBO J. 14, 3788–3799 (1995).
Kitagawa, K. & Hieter, P. Evolutionary conservation between budding yeast and human kinetochores. Nature Rev. Mol. Cell Biol. 2, 678–687 (2001).
Murata-Hori, M. & Wang, Y. The kinase activity of Aurora B is required for kinetochore–microtubule interactions in mitosis. Curr. Biol. 12, 894–899 (2002).
Kallio, M. J., McCleland, M. L., Stukenberg, P. T. & Gorbsky, G. J. Inhibition of Aurora B kinase blocks chromosome segregation, overrides the spindle checkpoint, and perturbs microtubule dynamics in mitosis. Curr. Biol. 12, 900–905 (2002).
Nicklas, R. B. & Ward, S. C. Elements of error correction in mitosis: microtubule capture, release, and tension. J. Cell Biol. 126, 1241–1253 (1994).
Nicklas, R. B., Waters, J. C., Salmon, E. D. & Ward, S. C. Checkpoint signals in grasshopper meiosis are sensitive to microtubule attachment, but tension is still essential. J. Cell Sci. 114, 4173–4183 (2001).
Chen, R. H., Shevchenko, A., Mann, M. & Murray, A. W. Spindle checkpoint protein Xmad1 recruits Xmad2 to unattached kinetochores. J. Cell Biol. 143, 283–295 (1998).
Millband, D. N. & Hardwick, K. G. Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localises to kinetochores in a Bub1p, Bub3p and Mph1p dependent manner. Mol. Cell. Biol. 22, 2728–2742 (2002).
Sharp-Baker, H. & Chen, R. H. Spindle checkpoint protein Bub1 is required for kinetochore localization of Mad1, Mad2, Bub3, and CENP-E, independently of its kinase activity. J. Cell Biol. 153, 1239–1250 (2001).
Abrieu, A. et al. Mps1 is a kinetochore-associated kinase essential for the vertebrate mitotic checkpoint. Cell 106, 83–93 (2001).
Chen, R. H. BubR1 is required for kinetochore localization of other spindle checkpoint proteins and its phosphorylation requires Mad1. J. Cell Biol. 158, 487–496 (2002).
Brady, D. M. & Hardwick, K. G. Complex formation between Mad1p, Bub1p and Bub3p is crucial for spindle checkpoint function. Curr. Biol. 10, 675–678 (2000).
Chung, E. & Chen, R.-H. Spindle checkpoint requires Mad1-bound and Mad1-free Mad2. Mol. Biol. Cell 13, 1501–1511 (2002).
Howell, B. J. et al. Visualization of Mad2 dynamics at kinetochores, along spindle fibers, and at spindle poles in living cells. J. Cell Biol. 150, 1233–1250 (2000).A dynamic cycle of interaction of Mad2 with kinetochores, spindle fibres and spindle poles is revealed using an elegant fluorescence-recovery after photobleaching approach. The transient nature of the interaction of Mad2 with kinetochores is consistent with the catalytic model of kinetochore function in generating the 'wait anaphase' signal.
Howell, B. J., Farrar, E., Fang, G. & Salmon, E. D. Visualization of Cdc20 and BubRI dynamics in living cells. Mol. Biol. Cell 12(S), 315a (2001).
Sudakin, V., Chan, G. K. & Yen, T. J. Checkpoint inhibition of the APC/C in HeLa cells is mediated by a complex of BUBR1, BUB3, CDC20, and MAD2. J. Cell Biol. 154, 925–936 (2001).An APC/C inhibitory factor purified from HeLa cells was found to consist of BubR1, Bub3, Cdc20 and Mad2 checkpoint proteins in near equal stoichiometry. The APC inhibitory activity of this complex is 3,000 times than that of recombinant Mad2. This complex might not be generated from kinetochores because it is also present and active in interphase cells. Together with ref. 63 , this paper uncovers a direct role of BubR1 in Cdc20 binding and APC inhibition, but some of the conclusions do not coincide.
Tang, Z., Bharadwaj, R., Li, B. & Yu, H. Mad2-independent inhibition of APC–Cdc20 by the mitotic checkpoint protein BubR1. Dev. Cell 1, 227–237 (2001).A checkpoint complex containing BubR1 and Bub3 is purified from mitotic human cells and found to interact with Cdc20 in the absence of other proteins and to block the binding of Cdc20 to APC. Together with ref. 62 this paper establishes a direct role of BubR1 in Cdc20 binding and checkpoint activity.
Fraschini, R. et al. Bub3 interaction with Mad2, Mad3 and Cdc20 is mediated by WD40 repeats and does not require intact kinetochores. EMBO J. 20, 6648–6659 (2001).
Wojcik, E. et al. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nature Cell Biol. 3, 1001–1007 (2001).Together with ref. 66 , this paper shows that dynein might contribute to shutting off the metaphase checkpoint, allowing anaphase to ensue. A complementary set of kinetochore proteins is monitored relative to those described in ref. 66 , revealing a general mechanism of checkpoint inactivation.
Howell, B. J. et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155, 1159–1172 (2001).Inhibition of dynein and dynactin activity in PtK1 cells blocks the transport to the spindle poles of many proteins located in the kinetochore outer domain, including Mad2, BubR1 and CENP-E. Furthermore, it prevents Mad2 depletion from kinetochores and induces a mitotic block at metaphase without blocking chromosome congression or anaphase. Thus, dynein and dynactin participate in a kinetochore-disassembly pathway that inactivates the spindle checkpoint.
Gorbsky, G. J., Chen, R. H. & Murray, A. W. Microinjection of antibody to Mad2 protein into mammalian cells in mitosis induces premature anaphase. J. Cell Biol. 141, 1193–1205 (1998).
Canman, J. C., Hoffman, D. B. & Salmon, E. D. The role of pre- and post-anaphase microtubules in the cytokinesis phase of the cell cycle. Curr. Biol. 10, 611–614 (2000).
Basu, J. et al. Mutations in the essential spindle checkpoint gene bub1 cause chromosome missegregation and fail to block apoptosis in Drosophila. J. Cell Biol. 146, 13–28 (1999).
Ikui, A. E., Furuya, K., Yanagida, M. & Matsumoto, T. Control of localization of a spindle checkpoint protein, Mad2, in fission yeast. J. Cell Sci. 115, 1603–1610 (2002).
Hardwick, K. G., Johnston, R. C., Smith, D. L. & Murray, A. W. MAD3 encodes a novel component of the spindle checkpoint which interacts with Bub3p, Cdc20p, and Mad2p. J. Cell Biol. 148, 871–882 (2000).
Luo, X., Tang, Z., Rizo, J. & Yu, H. The Mad2 spindle checkpoint protein undergoes similar major conformational changes upon binding to either Mad1 or Cdc20. Mol. Cell 9, 59–71 (2002).The structure of Mad2 bound to a synthetic peptide. The Mad2 carboxy-terminal tail undergoes a striking conformational change relative to the unbound form of Mad2. Together with ref. 73 , the paper also identifies a common Mad2-binding motif in Mad1 and Cdc20, and proposes that the carboxy-terminal tail of Mad2 undergoes the same conformational change when bound to these ligands.
Sironi, L. et al. Crystal structure of the tetrameric Mad1–Mad2 core complex: implications of a 'safety belt' binding mechanism for the spindle checkpoint. EMBO J. 21, 2496–2506 (2002).Reports the structure of the Mad1–Mad2 tetramer. An interaction mechanism defined as a 'safety belt' is identified and its implications for the interaction of Mad2 with Mad1 and Cdc20 are discussed. With refs 72 and 75 , this is the first report of structural work on checkpoint proteins.
Jin, D. Y., Spencer, F. & Jeang, K. T. Human T cell leukemia virus type 1 oncoprotein Tax targets the human mitotic checkpoint protein MAD1. Cell 93, 81–91 (1998).
Luo, X. et al. Structure of the Mad2 spindle assembly checkpoint protein and its interaction with Cdc20. Nature Struct. Biol. 7, 224–229 (2000).
Zhang, Y. & Lees, E. Identification of an overlapping binding domain on Cdc20 for Mad2 and anaphase-promoting complex: model for spindle checkpoint regulation. Mol. Cell. Biol. 21, 5190–5199 (2001).
Chen, R. H. et al. The spindle checkpoint of budding yeast depends on a tight complex between the Mad1 and Mad2 proteins. Mol. Biol. Cell 10, 2607–2618 (1999).
Sironi, L. et al. Mad2 binding to Mad1 and Cdc20, rather than oligomerization, is required for the spindle checkpoint. EMBO J. 20, 6371–6382 (2001).
Pines, J. Cell cycle trials in Salamanca: workshop on G2/M progression and associated checkpoints. EMBO Rep. 3, 17–21 (2002).
Hardwick, K. G. et al. Activation of the budding yeast spindle assembly checkpoint without mitotic spindle disruption. Science 273, 953–956 (1996).
Seeley, T. W., Wang, L. & Zhen, J. Y. Phosphorylation of human MAD1 by the BUB1 kinase in vitro. Biochem. Biophys. Res. Commun. 257, 589–595 (1999).
Campbell, M. S., Chan, G. K. & Yen, T. J. Mitotic checkpoint proteins HsMAD1 and HsMAD2 are associated with nuclear pore complexes in interphase. J. Cell Sci. 114, 953–963 (2001).
Fang, G., Yu, H. & Kirschner, M. W. The checkpoint protein MAD2 and the mitotic regulator CDC20 form a ternary complex with the anaphase-promoting complex to control anaphase initiation. Genes Dev. 12, 1871–1883 (1998).
Michel, L. S. et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature 409, 355–359 (2001).
Geley, S. et al. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J. Cell Biol. 153, 137–148 (2001).
Rieder, C. L. et al. Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage. Proc. Natl Acad. Sci. USA 94, 5107–5112 (1997).
Fang, G. Checkpoint protein BubR1 acts synergistically with Mad2 to inhibit anaphase-promoting complex. Mol. Biol. Cell 13, 755–766 (2002).
Kallio, M. et al. Mammalian p55CDC mediates association of the spindle checkpoint protein Mad2 with the cyclosome/anaphase-promoting complex, and is involved in regulating anaphase onset and late mitotic events. J. Cell Biol. 141, 1393–1406 (1998).
Li, Y. et al. MAD2 associates with the cyclosome/anaphase-promoting complex and inhibits its activity. Proc. Natl Acad. Sci. USA 94, 12431–12436 (1997).
Wassmann, K. & Benezra, R. Mad2 transiently associates with an APC/p55Cdc complex during mitosis. Proc. Natl Acad. Sci. USA 95, 11193–11198 (1998).
Wu, H. et al. p55CDC/hCDC20 is associated with BUBR1 and may be a downstream target of the spindle checkpoint kinase. Oncogene 19, 4557–4562 (2000).
Rudner, A. D. & Murray, A. W. Phosphorylation by Cdc28 activates the Cdc20-dependent activity of the anaphase-promoting complex. J. Cell Biol. 149, 1377–1390 (2000).
Huang, J.-Y. & Raff, J. W. The dynamic localisation of the Drosophila APC/C: evidence for the existence of multiple complexes that perform distinct functions and are differentially localised. J. Cell Sci. 115, 2847–2856 (2002).
Uhlmann, F., Lottspeich, F. & Nasmyth, K. Sister-chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37–42 (1999).
Alexandru, G. et al. Phosphorylation of the cohesin subunit Scc1 by Polo/Cdc5 kinase regulates sister chromatid separation in yeast. Cell 105, 459–472 (2001).
Stemmann, O. et al. Dual inhibition of sister chromatid separation at metaphase. Cell 107, 715–726 (2001).
Weinert, T. A. & Hartwell, L. H. The RAD9 gene controls the cell cycle response to DNA damage in Saccharomyces cerevisiae. Science 241, 317–322 (1988).
Hartwell, L. H. & Weinert, T. A. Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629–634 (1989).
Fraschini, R., Formenti, E., Lucchini, G. & Piatti, S. Budding yeast Bub2 is localized at spindle pole bodies and activates the mitotic checkpoint via a different pathway from Mad2. J. Cell Biol. 145, 979–991 (1999).
Fesquet, D. et al. A Bub2p-dependent spindle checkpoint pathway regulates the Dbf2p kinase in budding yeast. EMBO J. 18, 2424–2434 (1999).
Pereira, G. et al. The Bub2p spindle checkpoint links nuclear migration with mitotic exit. Mol. Cell 6, 1–10 (2000).
Bloecher, A., Venturi, G. M. & Tatchell, K. Anaphase spindle position is monitored by the BUB2 checkpoint. Nature Cell Biol. 2, 556–558 (2000).
Pereira, G., Tanaka, T. U., Nasmyth, K. & Schiebel, E. Modes of spindle pole body inheritance and segregation of the Bfa1p–Bub2p checkpoint protein complex. EMBO J. 20, 6359–6370 (2001).
Bardin, A. J. & Amon, A. MEN and SIN: what's the difference? Nature Rev. Mol. Cell Biol. 2, 815–826 (2001).
Gachet, Y., Tournier, S., Millar, J. B. & Hyams, J. S. A MAP kinase-dependent actin checkpoint ensures proper spindle orientation in fission yeast. Nature 412, 352–355 (2001).
Weiss, E. & Winey, M. The Saccharomyces cerevisiae spindle pole body duplication gene MPS1 is part of a mitotic checkpoint. J. Cell Biol. 132, 111–123 (1996).
Roberts, B. T., Farr, K. A. & Hoyt, M. A. The Saccharomyces cerevisiae checkpoint gene BUB1 encodes a novel protein kinase. Mol. Cell. Biol. 14, 8282–8291 (1994).
Farr, K. A. & Hoyt, M. A. Bub1p kinase activates the Saccharomyces cerevisiae spindle assembly checkpoint. Mol. Cell. Biol. 18, 2738–2747 (1998).
Warren, C. D. et al. Distinct chromosome segregation roles for spindle checkpoint proteins. Mol. Biol. Cell (in the press).
Stucke, V. M., Sillje, H. H. W., Arnaud, L. & Nigg, E. A. Human Mps1 kinase is required for the spindle assembly checkpoint but not for centrosome duplication. EMBO J. 21, 1723–1732 (2002).
Hardwick, K. G. & Murray, A. W. Mad1p, a phosphoprotein component of the spindle assembly checkpoint in budding yeast. J. Cell Biol. 131, 709–720 (1995).
Schonn, M. A., McCarroll, R. & Murray, A. W. M. Requirement of the spindle checkpoint for proper chromosome segregation in budding yeast meiosis. Science 289, 300–303 (2000).
Bernard, P., Maure, J. F. & Javerzat, J. P. Fission yeast Bub1 is essential in setting up the meiotic pattern of chromosome segregation. Nature Cell Biol. 3, 522–526 (2001).
Dobles, M. et al. Chromosome missegregation and apoptosis in mice lacking the mitotic checkpoint protein Mad2. Cell 101, 635–645 (2000).
Kalitsis, P., Earle, E., Fowler, K. J. & Choo, K. H. Bub3 gene disruption in mice reveals essential mitotic spindle checkpoint function during early embryogenesis. Genes Dev. 14, 2277–2282 (2000).
Kitagawa, R. & Rose, A. M. Components of the spindle-assembly checkpoint are essential in Caenorhabditis elegans. Nature Cell Biol. 1, 514–521 (1999).
Lee, H. et al. Mitotic checkpoint inactivation fosters transformation in cells lacking the breast cancer susceptibility gene, Brca2. Mol. Cell 4, 1–10 (1999).
Cahill, D. P. et al. Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303 (1998).
Tighe, A., Johnson, V. L., Albertella, M. & Taylor, S. S. Aneuploid colon cancer cells have a robust spindle checkpoint. EMBO Rep. 2, 609–614 (2001).
Fodde, R. et al. Mutations in the APC tumour suppressor gene cause chromosomal instability. Nature Cell Biol. 3, 433–438 (2001).
Kaplan, K. B. et al. A role for the adenomatous polyposis coli protein in chromosome segregation. Nature Cell Biol. 3, 429–432 (2001).
Aravind, L. & Koonin, E. V. The HORMA domain: a common structural denominator in mitotic checkpoints, chromosome synapsis and DNA repair. Trends Biochem. Sci. 23, 284–286 (1998).
Zecevic, M. et al. Active MAP kinase in mitosis: localization at kinetochores and association with the motor protein CENP-E. J. Cell Biol. 142, 1547–1558 (1998).
Schwab, M. S. et al. Bub1 is activated by the protein kinase p90Rsk during Xenopus oocyte maturation. Curr. Biol. 11, 141–150 (2001).
Scaerou, F. et al. The ZW10 and Rough Deal checkpoint proteins function together in a large, evolutionarily conserved complex targeted to the kinetochore. J. Cell Sci. 114, 3103–3114 (2001).
Daum, J. R. et al. The 3F3/2 anti-phosphoepitope antibody binds mitotically phosphorylated anaphase-promoting complex/cyclosome. Curr. Biol. 10, 850–852 (2000).
Shannon, K. B., Canman, J. C. & Salmon, E. D. . Mad2 and BubR1 function in a single checkpoint pathway that responds to a loss of tension. Mol. Biol. Cell, 2002 August 6 (DOI 10.1091/mbc.E02-03-0137).
Martin-Lluesma, S., Stucke, V. M. & Nigg, E. A. . Role of Hec1 in spindle checkpoint signaling and kinetochore recruitment of Mad1/Mad2. Science (in the press).
Acknowledgements
We thank S. Piatti, A. Pidoux and V. Vanoosthuyse for critical reading of the manuscript. A.M. is an EMBO Young Investigator and a Scholar of the Italian Foundation for Cancer Research. K.G.H. is a Senior Research Fellow of the Wellcome Trust.
Author information
Authors and Affiliations
Glossary
- KINETOCHORE
-
Complex protein assembly that links chromosomes to the mitotic spindle. Kinetochores assemble around specialized chromosomal regions known as centromeres, whose sequence and overall structures vary considerably between different organisms.
- APC
-
Anaphase-promoting complex. A multiprotein complex with ubiquitin-ligase activity that is responsible for the ubiquitylation of several key cell-cycle regulators. Also known as the cyclosome.
- CDC20
-
Positive regulator of the APC and a target of the spindle checkpoint. Also known as p55Cdc20, Fizzy and Slp1.
- CONGRESSION
-
The microtubule-dependent movement of paired sister chromatids, whereby they become aligned at the metaphase plate.
- 3F3/2 PHOSPHOANTIGENS
-
Unknown antigens that are phosphorylated in prometaphase, and become dephosphorylated when tension is exerted on sister chromatids. There are probably several distinct antigens, including APC subunits126.
- ANEUPLOID
-
Cell in which one or more chromosomes are missing or present in more than their usual copy number.
- HAPLOINSUFFICIENCY
-
Loss of a functional allele from a diploid cell results in haploinsufficiency if the product of the remaining allele is insufficient to perform its function correctly.
- RNA INTERFERENCE
-
The process by which an introduced double-stranded RNA specifically silences the expression of genes through degradation of their cognate mRNAs.
Rights and permissions
About this article
Cite this article
Musacchio, A., Hardwick, K. The spindle checkpoint: structural insights into dynamic signalling. Nat Rev Mol Cell Biol 3, 731–741 (2002). https://doi.org/10.1038/nrm929
Issue Date:
DOI: https://doi.org/10.1038/nrm929
This article is cited by
-
FOXM1 is critical for the fitness recovery of chromosomally unstable cells
Cell Death & Disease (2023)
-
Unique progerin C-terminal peptide ameliorates Hutchinson–Gilford progeria syndrome phenotype by rescuing BUBR1
Nature Aging (2023)
-
CCHCR1-astrin interaction promotes centriole duplication through recruitment of CEP72
BMC Biology (2022)
-
Knockout of caspase-7 gene improves the expression of recombinant protein in CHO cell line through the cell cycle arrest in G2/M phase
Biological Research (2022)
-
Biallelic mutations in CDC20 cause female infertility characterized by abnormalities in oocyte maturation and early embryonic development
Protein & Cell (2020)