Key Points
-
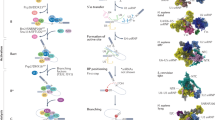
Spliceosomal snRNAs are transcribed from specialized promoters, which recruit RNA polymerase II cofactors that aid in proper 3′ end maturation of these non-polyadenylated transcripts.
-
Like most non-coding RNAs, small nuclear RNAs (snRNAs) use cognate antisense elements to interact with their nucleic acid targets via base pairing.
-
Assembly of functional small nuclear ribonucleoproteins (snRNPs) involves a series of non-functional intermediates that are often sequestered in subcellular compartments that are distinct from their sites of action.
-
snRNP function requires multiple protein partners (such as DExD/H helicases or WD box proteins) the roles of which may include modulating RNA structure or tethering an enzyme.
-
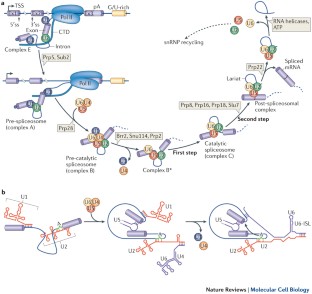
snRNPs recognize specific sequences in pre-mRNAs and assemble into the spliceosome in a stepwise manner. The splicing reaction itself is catalysed by U6/U2 snRNA complex that resembles a self-splicing ribozyme.
-
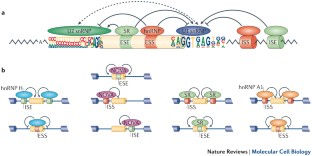
Alternative splicing is typically regulated by multiple cis-elements and trans-factors, which form complex interaction networks that may provide a great deal of regulatory plasticity.
-
Pre-mRNA splicing can be regulated throughout the entire spliceosomal assembly pathway, although the early steps are the main stages of regulation.
Abstract
One of the most amazing findings in molecular biology was the discovery that eukaryotic genes are discontinuous, with coding DNA being interrupted by stretches of non-coding sequence. The subsequent realization that the intervening regions are removed from pre-mRNA transcripts via the activity of a common set of small nuclear RNAs (snRNAs), which assemble together with associated proteins into a complex known as the spliceosome, was equally surprising. How do cells coordinate the assembly of this molecular machine? And how does the spliceosome accurately recognize exons and introns to carry out the splicing reaction? Insights into these questions have been gained by studying the life cycle of spliceosomal snRNAs from their transcription, nuclear export and re-import to their dynamic assembly into the spliceosome. This assembly process can also affect the regulation of alternative splicing and has implications for human disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Change history
11 March 2014
In table 1 (page 116) of the above article, the secondary structure of the snRNAs (small nuclear RNAs) for the U4–U6 di–snRNP (small nuclear ribonucleoprotein) was incorrect. This has now been rectified in the online version of the article. Nature Reviews Molecular Cell Biology apologizes for any confusion caused to readers.
References
Berget, S. M., Moore, C. & Sharp, P. A. Spliced segments at the 5′ terminus of adenovirus 2 late mRNA. Proc. Natl Acad. Sci. USA 74, 3171–3175 (1977).
Chow, L. T., Gelinas, R. E., Broker, T. R. & Roberts, R. J. An amazing sequence arrangement at the 5′ ends of adenovirus 2 messenger RNA. Cell 12, 1–8 (1977).
Lerner, M. R., Boyle, J. A., Mount, S. M., Wolin, S. L. & Steitz, J. A. Are snRNPs involved in splicing? Nature 283, 220–224 (1980).
Will, C. L. & Luhrmann, R. Spliceosome structure and function. Cold Spring Harb. Perspect. Biol. 3, a003707 (2011).
Jurica, M. S. & Moore, M. J. Pre-mRNA splicing: awash in a sea of proteins. Mol. Cell 12, 5–14 (2003).
Matera, A. G., Terns, R. M. & Terns, M. P. Non-coding RNAs: lessons from the small nuclear and small nucleolar RNAs. Nature Rev. Mol. Cell Biol. 8, 209–220 (2007).
Henry, R. W., Mittal, V., Ma, B., Kobayashi, R. & Hernandez, N. SNAP19 mediates the assembly of a functional core promoter complex (SNAPc) shared by RNA polymerases II and III. Genes Dev. 12, 2664–2672 (1998).
Hung, K. H. & Stumph, W. E. Regulation of snRNA gene expression by the Drosophila melanogaster small nuclear RNA activating protein complex (DmSNAPc). Crit. Rev. Biochem. Mol. Biol. 46, 11–26 (2011).
Hernandez, N. & Weiner, A. M. Formation of the 3′ end of U1 snRNA requires compatible snRNA promoter elements. Cell 47, 249–258 (1986).
Egloff, S. et al. The integrator complex recognizes a new double mark on the RNA polymerase II carboxyl-terminal domain. J. Biol. Chem. 285, 20564–20569 (2010).
Egloff, S. et al. Serine-7 of the RNA polymerase II CTD is specifically required for snRNA gene expression. Science 318, 1777–1779 (2007).
Baillat, D. et al. Integrator, a multiprotein mediator of small nuclear RNA processing, associates with the C-terminal repeat of RNA polymerase II. Cell 123, 265–276 (2005). Identifies the complex that carries out pre-snRNA 3′-end processing.
Chen, J. et al. An RNAi screen identifies additional members of the Drosophila Integrator complex and a requirement for cyclin C/Cdk8 in snRNA 3′-end formation. RNA 18, 2148–2156 (2012).
Weiner, A. M. E Pluribus Unum: 3′ end formation of polyadenylated mRNAs, histone mRNAs, and U snRNAs. Mol. Cell 20, 168–170 (2005).
Mandel, C. R. et al. Polyadenylation factor CPSF-73 is the pre-mRNA 3′-end-processing endonuclease. Nature 444, 953–956 (2006).
Ezzeddine, N. et al. A subset of Drosophila integrator proteins is essential for efficient U7 snRNA and spliceosomal snRNA 3′-end formation. Mol. Cell. Biol. 31, 328–341 (2011).
Boon, K. L. et al. prp8 mutations that cause human retinitis pigmentosa lead to a U5 snRNP maturation defect in yeast. Nature Struct. Mol. Biol. 14, 1077–1083 (2007).
Murphy, M. W., Olson, B. L. & Siliciano, P. G. The yeast splicing factor Prp40p contains functional leucine-rich nuclear export signals that are essential for splicing. Genetics 166, 53–65 (2004).
Tkacz, I. D. et al. Identification of novel snRNA-specific Sm proteins that bind selectively to U2 and U4 snRNAs in Trypanosoma brucei. RNA 13, 30–43 (2007).
Palfi, Z. et al. SMN-assisted assembly of snRNP-specific Sm cores in trypanosomes. Genes Dev. 23, 1650–1664 (2009).
Jae, N. et al. snRNA-specific role of SMN in trypanosome snRNP biogenesis in vivo. RNA Biol. 8, 90–100 (2011).
Hernandez-Verdun, D., Roussel, P., Thiry, M., Sirri, V. & Lafontaine, D. L. The nucleolus: structure/function relationship in RNA metabolism. Wiley Interdiscip. Rev. RNA 1, 415–431 (2010).
Ohno, M. Size matters in RNA export. RNA Biol. 9, 1413–1417 (2012).
Cullen, B. R. Nuclear RNA export. J. Cell Sci. 116, 587–597 (2003).
Ohno, M., Segref, A., Kuersten, S. & Mattaj, I. W. Identity elements used in export of mRNAs. Mol. Cell 9, 659–671 (2002).
Masuyama, K., Taniguchi, I., Kataoka, N. & Ohno, M. RNA length defines RNA export pathway. Genes Dev. 18, 2074–2085 (2004).
Fuke, H. & Ohno, M. Role of poly (A) tail as an identity element for mRNA nuclear export. Nucleic Acids Res. 36, 1037–1049 (2008).
McCloskey, A. Taniguchi, I., Shinmyozu, K. & Ohno, M. hnRNP C tetramer measures RNA length to classify RNA polymerase II transcripts for export. Science 335, 1643–1646 (2012). First identification of a specific function for the non-shuttling hnRNP C-type proteins in RNA export.
Izaurralde, E. et al. A nuclear cap binding protein complex involved in pre-mRNA splicing. Cell 78, 657–668 (1994).
Ohno, M., Segref, A., Bachi, A., Wilm, M. & Mattaj, I. W. PHAX, a mediator of U snRNA nuclear export whose activity is regulated by phosphorylation. Cell 101, 187–198 (2000).
Hallais, M. et al. CBC–ARS2 stimulates 3′-end maturation of multiple RNA families and favors cap-proximal processing. Nature Struct. Mol. Biol. 20, 1358–1366 (2013). Shows that Ars2 forms 5′ cap-binding subcomplexes that participate in 3′-end processing of three distinct classes of transcript.
Fornerod, M., Ohno, M., Yoshida, M. & Mattaj, I. W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell 90, 1051–1060 (1997).
Smith, K. P. & Lawrence, J. B. Interactions of U2 gene loci and their nuclear transcripts with Cajal (coiled) bodies: evidence for PreU2 within Cajal bodies. Mol. Biol. Cell 11, 2987–2998 (2000).
Suzuki, T., Izumi, H. & Ohno, M. Cajal body surveillance of U snRNA export complex assembly. J. Cell Biol. 190, 603–612 (2010).
Boulon, S. et al. PHAX and CRM1 are required sequentially to transport U3 snoRNA to nucleoli. Mol. Cell 16, 777–787 (2004).
Frey, M. R. & Matera, A. G. RNA-mediated interaction of Cajal bodies and U2 snRNA genes. J. Cell Biol. 154, 499–509 (2001).
Lemm, I. et al. Ongoing U snRNP biogenesis is required for the integrity of Cajal bodies. Mol. Biol. Cell 17, 3221–3231 (2006).
Matera, A. G., Izaguire-Sierra, M., Praveen, K. & Rajendra, T. K. Nuclear bodies: random aggregates of sticky proteins or crucibles of macromolecular assembly? Dev. Cell 17, 639–647 (2009).
Kitao, S. et al. A compartmentalized phosphorylation/dephosphorylation system that regulates U snRNA export from the nucleus. Mol. Cell. Biol. 28, 487–497 (2008).
Meister, G., Buhler, D., Pillai, R., Lottspeich, F. & Fischer, U. A multiprotein complex mediates the ATP-dependent assembly of spliceosomal U snRNPs. Nature Cell Biol. 3, 945–949 (2001).
Pellizzoni, L., Yong, J. & Dreyfuss, G. Essential role for the SMN complex in the specificity of snRNP assembly. Science 298, 1775–1779 (2002).
Massenet, S., Pellizzoni, L., Paushkin, S., Mattaj, I. W. & Dreyfuss, G. The SMN complex is associated with snRNPs throughout their cytoplasmic assembly pathway. Mol. Cell. Biol. 22, 6533–6541 (2002).
Narayanan, U., Ospina, J. K., Frey, M. R., Hebert, M. D. & Matera, A. G. SMN, the spinal muscular atrophy protein, forms a pre-import snRNP complex with snurportin1 and importin β. Hum. Mol. Genet. 11, 1785–1795 (2002).
Mouaikel, J. et al. Interaction between the small-nuclear-RNA cap hypermethylase and the spinal muscular atrophy protein, survival of motor neuron. EMBO Rep. 4, 616–622 (2003).
Meister, G. et al. Methylation of Sm proteins by a complex containing PRMT5 and the putative U snRNP assembly factor pICln. Curr. Biol. 11, 1990–1994 (2001).
Friesen, W. J. et al. The methylosome, a 20S complex containing JBP1 and pICln, produces dimethylarginine-modified Sm proteins. Mol. Cell. Biol. 21, 8289–8300 (2001).
Grimm, C. et al. Structural basis of assembly chaperone-mediated snRNP formation. Mol. Cell 49, 692–703 (2013).
Chari, A. et al. An assembly chaperone collaborates with the SMN complex to generate spliceosomal snRNPs. Cell 135, 497–509 (2008).
Yong, J., Kasim, M., Bachorik, J. L., Wan, L. & Dreyfuss, G. Gemin5 delivers snRNA precursors to the SMN complex for snRNP biogenesis. Mol. Cell 38, 551–562 (2010).
Raker, V. A., Plessel, G. & Luhrmann, R. The snRNP core assembly pathway: identification of stable core protein heteromeric complexes and an snRNP subcore particle in vitro. EMBO J. 15, 2256–2269 (1996).
Kambach, C. et al. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell 96, 375–387 (1999).
Leung, A. K., Nagai, K. & Li, J. Structure of the spliceosomal U4 snRNP core domain and its implication for snRNP biogenesis. Nature 473, 536–539 (2011). Co-crystal structure of U4 snRNA construct with an Sm core definitively shows that the RNA passes through the hole in the Sm ring.
Kroiss, M. et al. Evolution of an RNP assembly system: a minimal SMN complex facilitates formation of UsnRNPs in Drosophila melanogaster. Proc. Natl Acad. Sci. USA 105, 10045–10050 (2008). Shows that both human and fruitfly SMN–GEMIN2 heterodimers are sufficient for mediating Sm core assembly in vitro.
Zhang, R. et al. Structure of a key intermediate of the SMN complex reveals Gemin2's crucial function in snRNP assembly. Cell 146, 384–395 (2011). Together with reference 46, these papers identify key intermediates in the Sm core assembly pathway, highlighting an unexpected role for GEMIN2.
Liu, Q., Fischer, U., Wang, F. & Dreyfuss, G. The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90, 1013–1021 (1997).
Buhler, D., Raker, V., Luhrmann, R. & Fischer, U. Essential role for the tudor domain of SMN in spliceosomal U snRNP assembly: implications for spinal muscular atrophy. Hum. Mol. Genet. 8, 2351–2357 (1999).
Pellizzoni, L., Charroux, B. & Dreyfuss, G. SMN mutants of spinal muscular atrophy patients are defective in binding to snRNP proteins. Proc. Natl Acad. Sci. USA 96, 11167–11172 (1999).
Hannus, S., Buhler, D., Romano, M., Seraphin, B. & Fischer, U. The Schizosaccharomyces pombe protein Yab8p and a novel factor, Yip1p, share structural and functional similarity with the spinal muscular atrophy-associated proteins SMN and SIP1. Hum. Mol. Genet. 9, 663–674 (2000).
Rajendra, T. K. et al. A Drosophila melanogaster model of spinal muscular atrophy reveals a function for SMN in striated muscle. J. Cell Biol. 176, 831–841 (2007).
Shpargel, K. B. & Matera, A. G. Gemin proteins are required for efficient assembly of Sm-class ribonucleoproteins. Proc. Natl Acad. Sci. USA 102, 17372–17377 (2005). Assays individual Gemins, as well as a panel of SMN missense mutants for ability to carry out Sm core assembly, showing that certain SMA-causing alleles are functional, whereas others are not.
Selenko, P. et al. SMN Tudor domain structure and its interaction with the Sm proteins. Nature Struct. Biol. 8, 27–31 (2001).
Lorson, C. L. et al. SMN oligomerization defect correlates with spinal muscular atrophy severity. Nature Genet. 19, 63–66 (1998).
Martin, R., Gupta, K., Ninan, N. S., Perry, K. & Van Duyne, G. D. The survival motor neuron protein forms soluble glycine zipper oligomers. Structure 20, 1929–1939 (2012).
Fischer, U. & Luhrmann, R. An essential signaling role for the m3G cap in the transport of U1 snRNP to the nucleus. Science 249, 786–790 (1990).
Narayanan, U., Achsel, T., Luhrmann, R. & Matera, A. G. Coupled in vitro import of U snRNPs and SMN, the spinal muscular atrophy protein. Mol. Cell 16, 223–234 (2004).
Fischer, U., Sumpter, V., Sekine, M., Satoh, T. & Luhrmann, R. Nucleo-cytoplasmic transport of U snRNPs: definition of a nuclear location signal in the Sm core domain that binds a transport receptor independently of the m3G cap. EMBO J. 12, 573–583 (1993).
Huber, J. et al. Snurportin1, an m3G-cap-specific nuclear import receptor with a novel domain structure. EMBO J. 17, 4114–4126 (1998).
Palacios, I., Hetzer, M., Adam, S. A. & Mattaj, I. W. Nuclear import of U snRNPs requires importin β. EMBO J. 16, 6783–6792 (1997).
Fischer, U., Liu, Q. & Dreyfuss, G. The SMN–SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell 90, 1023–1029 (1997).
Neubauer, G. et al. Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nature Genet. 20, 46–50 (1998).
Trinkle-Mulcahy, L. et al. Identifying specific protein interaction partners using quantitative mass spectrometry and bead proteomes. J. Cell Biol. 183, 223–239 (2008).
Herold, N. et al. Conservation of the protein composition and electron microscopy structure of Drosophila melanogaster and human spliceosomal complexes. Mol. Cell. Biol. 29, 281–301 (2009).
Matera, A. G. & Shpargel, K. B. Pumping RNA: nuclear bodybuilding along the RNP pipeline. Curr. Opin. Cell Biol. 18, 317–324 (2006).
Stanek, D. & Neugebauer, K. M. The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze. Chromosoma 115, 343–354 (2006).
Sleeman, J. E. & Lamond, A. I. Newly assembled snRNPs associate with coiled bodies before speckles, suggesting a nuclear snRNP maturation pathway. Curr. Biol. 9, 1065–1074 (1999).
Lamond, A. I. & Spector, D. L. Nuclear speckles: a model for nuclear organelles. Nature Rev. Mol. Cell Biol. 4, 605–612 (2003).
Ospina, J. K. et al. Cross-talk between snurportin1 subdomains. Mol. Biol. Cell 16, 4660–4671 (2005).
Jady, B. E. et al. Modification of Sm small nuclear RNAs occurs in the nucleoplasmic Cajal body following import from the cytoplasm. EMBO J. 22, 1878–1888 (2003).
Nesic, D., Tanackovic, G. & Kramer, A. A role for Cajal bodies in the final steps of U2 snRNP biogenesis. J. Cell Sci. 117, 4423–4433 (2004).
Schaffert, N., Hossbach, M., Heintzmann, R., Achsel, T. & Luhrmann, R. RNAi knockdown of hPrp31 leads to an accumulation of U4/U6 di-snRNPs in Cajal bodies. EMBO J. 23, 3000–3009 (2004).
Novotny, I., Blazikova, M., Stanek, D., Herman, P. & Malinsky, J. In vivo kinetics of U4/U6. U5 tri-snRNP formation in Cajal bodies. Mol. Biol. Cell 22, 513–523 (2011).
Stanek, D. & Neugebauer, K. M. Detection of snRNP assembly intermediates in Cajal bodies by fluorescence resonance energy transfer. J. Cell Biol. 166, 1015–1025 (2004).
Stanek, D., Rader, S. D., Klingauf, M. & Neugebauer, K. M. Targeting of U4/U6 small nuclear RNP assembly factor SART3/p110 to Cajal bodies. J. Cell Biol. 160, 505–516 (2003).
Strzelecka, M., Oates, A. C. & Neugebauer, K. M. Dynamic control of Cajal body number during zebrafish embryogenesis. Nucleus 1, 96–108 (2010).
Takata, H., Nishijima, H., Maeshima, K. & Shibahara, K. The integrator complex is required for integrity of Cajal bodies. J. Cell Sci. 125, 166–175 (2012).
Tucker, K. E. et al. Residual Cajal bodies in coilin knockout mice fail to recruit Sm snRNPs and SMN, the spinal muscular atrophy gene product. J. Cell Biol. 154, 293–307 (2001).
Liu, J. L. et al. Coilin is essential for Cajal body organization in Drosophila melanogaster. Mol. Biol. Cell 20, 1661–1670 (2009).
Walker, M. P., Tian, L. & Matera, A. G. Reduced viability, fertility and fecundity in mice lacking the cajal body marker protein, coilin. PLoS ONE 4, e6171 (2009).
Strzelecka, M. et al. Coilin-dependent snRNP assembly is essential for zebrafish embryogenesis. Nature Struct. Mol. Biol. 17, 403–409 (2010).
Spector, D. L. & Lamond, A. I. Nuclear speckles. Cold Spring Harb. Perspect. Biol. 3, a000646 (2011).
Hall, L. L., Smith, K. P., Byron, M. & Lawrence, J. B. Molecular anatomy of a speckle. Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 288, 664–675 (2006).
Girard, C. et al. Post-transcriptional spliceosomes are retained in nuclear speckles until splicing completion. Nature Commun. 3, 994 (2012).
Valadkhan, S. Role of the snRNAs in spliceosomal active site. RNA Biol. 7, 345–353 (2010).
Du, H. & Rosbash, M. The U1 snRNP protein U1C recognizes the 5′ splice site in the absence of base pairing. Nature 419, 86–90 (2002).
Wiesner, S., Stier, G., Sattler, M. & Macias, M. J. Solution structure and ligand recognition of the WW domain pair of the yeast splicing factor Prp40. J. Mol. Biol. 324, 807–822 (2002).
Morris, D. P. & Greenleaf, A. L. The splicing factor, Prp40, binds the phosphorylated carboxyl-terminal domain of RNA polymerase II. J. Biol. Chem. 275, 39935–39943 (2000).
Gornemann, J. et al. Cotranscriptional spliceosome assembly and splicing are independent of the Prp40p WW domain. RNA 17, 2119–2129 (2011).
Staknis, D. & Reed, R. SR proteins promote the first specific recognition of Pre-mRNA and are present together with the U1 small nuclear ribonucleoprotein particle in a general splicing enhancer complex. Mol. Cell. Biol. 14, 7670–7682 (1994).
Cho, S. et al. Interaction between the RNA binding domains of Ser-Arg splicing factor 1 and U1–70K snRNP protein determines early spliceosome assembly. Proc. Natl Acad. Sci. USA 108, 8233–8238 (2011).
Pabis, M. et al. The nuclear cap-binding complex interacts with the U4/U6. U5 tri-snRNP and promotes spliceosome assembly in mammalian cells. RNA 19, 1054–1063 (2013).
Fox-Walsh, K. L. et al. The architecture of pre-mRNAs affects mechanisms of splice-site pairing. Proc. Natl Acad. Sci. USA 102, 16176–16181 (2005).
Xiao, X., Wang, Z., Jang, M. & Burge, C. B. Coevolutionary networks of splicing cis-regulatory elements. Proc. Natl Acad. Sci. USA 104, 18583–18588 (2007).
Sterner, D. A., Carlo, T. & Berget, S. M. Architectural limits on split genes. Proc. Natl Acad. Sci. USA 93, 15081–15085 (1996).
De Conti, L., Baralle, M. & Buratti, E. Exon and intron definition in pre-mRNA splicing. Wiley Interdiscip. Rev. RNA 4, 49–60 (2013).
Bonnal, S. et al. RBM5/Luca-15/H37 regulates Fas alternative splice site pairing after exon definition. Mol. Cell 32, 81–95 (2008).
Sharma, S., Kohlstaedt, L. A., Damianov, A., Rio, D. C. & Black, D. L. Polypyrimidine tract binding protein controls the transition from exon definition to an intron defined spliceosome. Nature Struct. Mol. Biol. 15, 183–191 (2008). Demonstrates that an early step in spliceosome assembly (transition from exon definition to intron definition complex) is a key stage for splicing regulation.
Sun, J. S. & Manley, J. L. A novel U2–U6 snRNA structure is necessary for mammalian mRNA splicing. Genes Dev. 9, 843–854 (1995).
Raghunathan, P. L. & Guthrie, C. RNA unwinding in U4/U6 snRNPs requires ATP hydrolysis and the DEIH-box splicing factor Brr2. Curr. Biol. 8, 847–855 (1998).
Ilagan, J. O., Chalkley, R. J., Burlingame, A. L. & Jurica, M. S. Rearrangements within human spliceosomes captured after exon ligation. RNA 19, 400–412 (2013)
Schwer, B. & Gross, C. H. Prp22, a DExH-box RNA helicase, plays two distinct roles in yeast pre-mRNA splicing. EMBO J. 17, 2086–2094 (1998).
Fourmann, J. B. et al. Dissection of the factor requirements for spliceosome disassembly and the elucidation of its dissociation products using a purified splicing system. Genes Dev. 27, 413–428 (2013).
Abelson, J. et al. Conformational dynamics of single pre-mRNA molecules during in vitro splicing. Nature Struct. Mol. Biol. 17, 504–512 (2010).
Hoskins, A. A. et al. Ordered and dynamic assembly of single spliceosomes. Science 331, 1289–1295 (2011).
Tseng, C. K. & Cheng, S. C. Both catalytic steps of nuclear pre-mRNA splicing are reversible. Science 320, 1782–1784 (2008).
Yang, F. et al. Splicing proofreading at 5′ splice sites by ATPase Prp28p. Nucleic Acids Res. 41, 4660–4670 (2013).
Malca, H., Shomron, N. & Ast, G. The U1 snRNP base pairs with the 5′ splice site within a penta–snRNP complex. Mol. Cell. Biol. 23, 3442–3455 (2003).
Stevens, S. W. et al. Composition and functional characterization of the yeast spliceosomal penta–snRNP. Mol. Cell 9, 31–44 (2002).
Gornemann, J., Kotovic, K. M., Hujer, K. & Neugebauer, K. M. Cotranscriptional spliceosome assembly occurs in a stepwise fashion and requires the cap binding complex. Mol. Cell 19, 53–63 (2005). Development of a novel chromatin immunoprecipitation assay to investigate co-transcriptional spliceosome assembly, demonstrating a role for the CBC in recruitment of snRNPs to nascent pre-mRNA transcripts.
Behzadnia, N., Hartmuth, K., Will, C. L. & Luhrmann, R. Functional spliceosomal A complexes can be assembled in vitro in the absence of a penta–snRNP. RNA 12, 1738–1746 (2006).
Schneider, M. et al. Exon definition complexes contain the tri-snRNP and can be directly converted into B-like precatalytic splicing complexes. Mol. Cell 38, 223–235 (2010). Together with reference 116, these studies suggest the existence of alternative spliceosome assembly pathways.
Madhani, H. D. & Guthrie, C. Dynamic RNA–RNA interactions in the spliceosome. Annu. Rev. Genet. 28, 1–26 (1994).
Valadkhan, S., Mohammadi, A., Wachtel, C. & Manley, J. L. Protein-free spliceosomal snRNAs catalyze a reaction that resembles the first step of splicing. RNA 13, 2300–2311 (2007).
Valadkhan, S., Mohammadi, A., Jaladat, Y. & Geisler, S. Protein-free small nuclear RNAs catalyze a two-step splicing reaction. Proc. Natl Acad. Sci. USA 106, 11901–11906 (2009). Together with reference 122, demonstrates that protein-free U6/U2 snRNA constructs can recognize 5′ splice site and branch point sequence to carry out the first and second steps of splicing.
Marcia, M. & Pyle, A. M. Visualizing group II intron catalysis through the stages of splicing. Cell 151, 497–507 (2012).
Toor, N., Keating, K. S. & Pyle, A. M. Structural insights into RNA splicing. Curr. Opin. Struct. Biol. 19, 260–266 (2009).
Toor, N., Keating, K. S., Taylor, S. D. & Pyle, A. M. Crystal structure of a self-spliced group II intron. Science 320, 77–82 (2008).
Fica, S. M. et al. RNA catalyses nuclear pre-mRNA splicing. Nature 503, 229–234 (2013).
Butcher, S. E. The spliceosome and its metal ions. Met. Ions Life Sci. 9, 235–251 (2011).
Cordin, O., Hahn, D. & Beggs, J. D. Structure, function and regulation of spliceosomal RNA helicases. Curr. Opin. Cell Biol. 24, 431–438 (2012).
Small, E. C., Leggett, S. R., Winans, A. A. & Staley, J. P. The EF-G-like GTPase Snu114p regulates spliceosome dynamics mediated by Brr2p, a DExD/H box ATPase. Mol. Cell 23, 389–399 (2006).
Galej, W. P., Oubridge, C., Newman, A. J. & Nagai, K. Crystal structure of Prp8 reveals active site cavity of the spliceosome. Nature 493, 638–643 (2013).
Schellenberg, M. J. et al. A conformational switch in PRP8 mediates metal ion coordination that promotes pre-mRNA exon ligation. Nature Struct. Mol. Biol. 20, 728–734 (2013).
Mozaffari-Jovin, S. et al. Inhibition of RNA helicase Brr2 by the C-terminal tail of the spliceosomal protein Prp8. Science 341, 80–84 (2013).
Ohrt, T. et al. Molecular dissection of step 2 catalysis of yeast pre-mRNA splicing investigated in a purified system. RNA 19, 902–915 (2013).
Sun, H. & Chasin, L. A. Multiple splicing defects in an intronic false exon. Mol. Cell. Biol. 20, 6414–6425 (2000).
Matlin, A. J., Clark, F. & Smith, C. W. Understanding alternative splicing: towards a cellular code. Nature Rev. Mol. Cell Biol. 6, 386–398 (2005).
Wang, Z. & Burge, C. B. Splicing regulation: from a parts list of regulatory elements to an integrated splicing code. RNA 14, 802–813 (2008).
Bessonov, S., Anokhina, M., Will, C. L., Urlaub, H. & Luhrmann, R. Isolation of an active step I spliceosome and composition of its RNP core. Nature 452, 846–850 (2008).
Zhou, Z., Licklider, L. J., Gygi, S. P. & Reed, R. Comprehensive proteomic analysis of the human spliceosome. Nature 419, 182–185 (2002). Identifies more than 100 proteins in the active spliceosome, many more than the known protein components of snRNPs.
Hegele, A. et al. Dynamic protein–protein interaction wiring of the human spliceosome. Mol. Cell 45, 567–580 (2012).
Izquierdo, J. M. et al. Regulation of Fas alternative splicing by antagonistic effects of TIA-1 and PTB on exon definition. Mol. Cell 19, 475–484 (2005).
Sharma, S., Maris, C., Allain, F. H. & Black, D. L. U1 snRNA directly interacts with polypyrimidine tract-binding protein during splicing repression. Mol. Cell 41, 579–588 (2011).
Chiou, N. T., Shankarling, G. & Lynch, K. W. HnRNP L and hnRNP A1 induce extended U1 snRNA interactions with an exon to repress spliceosome assembly. Mol. Cell 49, 972–982 (2013).
House, A. E. & Lynch, K. W. An exonic splicing silencer represses spliceosome assembly after ATP-dependent exon recognition. Nature Struct. Mol. Biol. 13, 937–944 (2006).
McCullough, A. J. & Berget, S. M. G triplets located throughout a class of small vertebrate introns enforce intron borders and regulate splice site selection. Mol. Cell. Biol. 17, 4562–4571 (1997).
Chou, M. Y., Rooke, N., Turck, C. W. & Black, D. L. hnRNP H is a component of a splicing enhancer complex that activates a c-src alternative exon in neuronal cells. Mol. Cell. Biol. 19, 69–77 (1999).
Chen, C. D., Kobayashi, R. & Helfman, D. M. Binding of hnRNP H to an exonic splicing silencer is involved in the regulation of alternative splicing of the rat β-tropomyosin gene. Genes Dev. 13, 593–606 (1999).
Caputi, M. & Zahler, A. M. Determination of the RNA binding specificity of the heterogeneous nuclear ribonucleoprotein (hnRNP) H/H′/F/2H9 family. J. Biol. Chem. 276, 43850–43859 (2001).
Ule, J. et al. An RNA map predicting Nova-dependent splicing regulation. Nature 444, 580–586 (2006).
Wang, Y. et al. A complex network of factors with overlapping affinities represses splicing through intronic elements. Nature Struct. Mol. Biol. 20, 36–45 (2013). Suggests that interactions between various cis -acting elements and trans -acting factors form a complex network that controls context-dependent splicing.
Borah, S., Wong, A. C. & Steitz, J. A. Drosophila hnRNP A1 homologs Hrp36/Hrp38 enhance U2-type versus U12-type splicing to regulate alternative splicing of the prospero twintron. Proc. Natl Acad. Sci. USA 106, 2577–2582 (2009).
Wang, Z. et al. Systematic identification and analysis of exonic splicing silencers. Cell 119, 831–845 (2004).
Yu, Y. et al. Dynamic regulation of alternative splicing by silencers that modulate 5′ splice site competition. Cell 135, 1224–1236 (2008).
Donahue, C. P., Muratore, C., Wu, J. Y., Kosik, K. S. & Wolfe, M. S. Stabilization of the tau exon 10 stem loop alters pre-mRNA splicing. J. Biol. Chem. 281, 23302–23306 (2006).
Graveley, B. R. Mutually exclusive splicing of the insect Dscam pre-mRNA directed by competing intronic RNA secondary structures. Cell 123, 65–73 (2005). A great example of how RNA structures can have a leading role in controlling a complicated regimen of mutally exclusive splicing.
Yang, Y. et al. RNA secondary structure in mutually exclusive splicing. Nature Struct. Mol. Biol. 18, 159–168 (2011).
Wang, X. et al. An RNA architectural locus control region involved in Dscam mutually exclusive splicing. Nature Commun. 3, 1255 (2012).
Bleichert, F. & Baserga, S. J. The long unwinding road of RNA helicases. Mol. Cell 27, 339–352 (2007).
Honig, A., Auboeuf, D., Parker, M. M., O'Malley, B. W. & Berget, S. M. Regulation of alternative splicing by the ATP-dependent DEAD-box RNA helicase p72. Mol. Cell. Biol. 22, 5698–5707 (2002).
Lee, C. G. RH70, a bidirectional RNA helicase, co-purifies with U1snRNP. J. Biol. Chem. 277, 39679–39683 (2002).
Weeks, K. M. Advances in RNA structure analysis by chemical probing. Curr. Opin. Struct. Biol. 20, 295–304 (2010).
Khodor, Y. L. et al. Nascent-seq indicates widespread cotranscriptional pre-mRNA splicing in Drosophila. Genes Dev. 25, 2502–2512 (2011).
Ip, J. Y. et al. Global impact of RNA polymerase II elongation inhibition on alternative splicing regulation. Genome Res. 21, 390–401 (2011).
Roberts, G. C., Gooding, C., Mak, H. Y., Proudfoot, N. J. & Smith, C. W. Co-transcriptional commitment to alternative splice site selection. Nucleic Acids Res. 26, 5568–5572 (1998).
Kornblihtt, A. R. et al. Alternative splicing: a pivotal step between eukaryotic transcription and translation. Nature Rev. Mol. Cell Biol. 14, 153–165 (2013).
Brugiolo, M., Herzel, L. & Neugebauer, K. M. Counting on co-transcriptional splicing. F1000Prime Rep. 5, 9 (2013).
Wang, Y., Ma, M., Xiao, X. & Wang, Z. Intronic splicing enhancers, cognate splicing factors and context-dependent regulation rules. Nature Struct. Mol. Biol. 19, 1044–1052 (2012).
Spellman, R., Llorian, M. & Smith, C. W. Crossregulation and functional redundancy between the splicing regulator PTB and its paralogs nPTB and ROD1. Mol. Cell 27, 420–434 (2007).
Boutz, P. L. et al. A post-transcriptional regulatory switch in polypyrimidine tract-binding proteins reprograms alternative splicing in developing neurons. Genes Dev. 21, 1636–1652 (2007).
Barash, Y. et al. Deciphering the splicing code. Nature 465, 53–59 (2010).
Nilsen, T. W. & Graveley, B. R. Expansion of the eukaryotic proteome by alternative splicing. Nature 463, 457–463 (2010).
Nilsen, T. W. The spliceosome: the most complex macromolecular machine in the cell? BioEssays 25, 1147–1149 (2003).
Singh, R. K. & Cooper, T. A. Pre-mRNA splicing in disease and therapeutics. Trends Mol. Med. 18, 472–482 (2012).
Padgett, R. A. New connections between splicing and human disease. Trends Genet. 28, 147–154 (2012).
Tanackovic, G. et al. PRPF mutations are associated with generalized defects in spliceosome formation and pre-mRNA splicing in patients with retinitis pigmentosa. Hum. Mol. Genet. 20, 2116–2130 (2011).
Utz, V. M., Beight, C. D., Marino, M. J., Hagstrom, S. A. & Traboulsi, E. I. Autosomal dominant retinitis pigmentosa secondary to pre-mRNA splicing-factor gene PRPF31 (RP11): review of disease mechanism and report of a family with a novel 3-base pair insertion. Ophthalm. Genet. 34, 183–188 (2013).
Pena, V., Liu, S., Bujnicki, J. M., Luhrmann, R. & Wahl, M. C. Structure of a multipartite protein–protein interaction domain in splicing factor prp8 and its link to retinitis pigmentosa. Mol. Cell 25, 615–624 (2007).
He, H. et al. Mutations in U4atac snRNA, a component of the minor spliceosome, in the developmental disorder MOPD I. Science 332, 238–240 (2011).
Lorson, C. L., Hahnen, E., Androphy, E. J. & Wirth, B. A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc. Natl Acad. Sci. USA 96, 6307–6311 (1999).
Schrank, B. et al. Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proc. Natl Acad. Sci. USA 94, 9920–9925 (1997).
Gabanella, F. et al. Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS ONE 2, e921 (2007).
Praveen, K., Wen, Y. & Matera, A. G. A. Drosophila model of spinal muscular atrophy uncouples snRNP biogenesis functions of survival motor neuron from locomotion and viability defects. Cell Rep. 1, 624–631 (2012).
Garcia, E. L., Lu, Z., Meers, M. P., Praveen, K. & Matera, A. G. Developmental arrest of Drosophila survival motor neuron (Smn) mutants accounts for differences in expression of minor intron-containing genes. RNA 19, 1510–1516 (2013).
Baumer, D. et al. Alternative splicing events are a late feature of pathology in a mouse model of spinal muscular atrophy. PLoS Genet. 5, e1000773 (2009). Together with references 182 and 183, these studies show that SMA phenotypes can be uncoupled from global splicing deficits. Using a missense allele that is active in Sm core assembly, reference 184 reveals a separation of SMN functions.
Cazzola, M., Rossi, M. & Malcovati, L. Biologic and clinical significance of somatic mutations of SF3B1 in myeloid and lymphoid neoplasms. Blood 121, 260–269 (2013).
Yoshida, K. et al. Frequent pathway mutations of splicing machinery in myelodysplasia. Nature 478, 64–69 (2011).
Chesnais, V. et al. Spliceosome mutations in myelodysplastic syndromes and chronic myelomonocytic leukemia. Oncotarget 3, 1284–1293 (2012).
Dhir, A., Buratti, E., van Santen, M. A., Luhrmann, R. & Baralle, F. E. The intronic splicing code: multiple factors involved in ATM pseudoexon definition. EMBO J. 29, 749–760 (2010).
Lewandowska, M. A., Stuani, C., Parvizpur, A., Baralle, F. E. & Pagani, F. Functional studies on the ATM intronic splicing processing element. Nucleic Acids Res. 33, 4007–4015 (2005).
Pagani, F. et al. A new type of mutation causes a splicing defect in ATM. Nature Genet. 30, 426–429 (2002).
Gunderson, S. I., Polycarpou-Schwarz, M. & Mattaj, I. W. U1 snRNP inhibits pre-mRNA polyadenylation through a direct interaction between U1 70K and poly(A) polymerase. Mol. Cell 1, 255–264 (1998).
Langemeier, J., Radtke, M. & Bohne, J. U1 snRNP-mediated poly(A) site suppression: beneficial and deleterious for mRNA fate. RNA Biol. 10, 180–184 (2013).
Kaida, D. et al. U1 snRNP protects pre-mRNAs from premature cleavage and polyadenylation. Nature 468, 664–668 (2010).
Almada, A. E., Wu, X., Kriz, A. J., Burge, C. B. & Sharp, P. A. Promoter directionality is controlled by U1 snRNP and polyadenylation signals. Nature 499, 360–363 (2013).
Berg, M. G. et al. U1 snRNP determines mRNA length and regulates isoform expression. Cell 150, 53–64 (2012). Together with reference 193, these genome-wide analyses illustrate a pervasive, non-splicing role for U1 snRNP in selection of the site of pre-mRNA 3′-end cleavage and polyadenylation.
Peterson, M. L., Bingham, G. L. & Cowan, C. Multiple features contribute to the use of the immunoglobulin M secretion-specific poly(A) signal but are not required for developmental regulation. Mol. Cell. Biol. 26, 6762–6771 (2006).
Hall-Pogar, T., Liang, S., Hague, L. K. & Lutz, C. S. Specific trans-acting proteins interact with auxiliary RNA polyadenylation elements in the COX-2 3′-UTR. RNA 13, 1103–1115 (2007).
Luo, W. et al. The conserved intronic cleavage and polyadenylation site of CstF-77 gene imparts control of 3′ end processing activity through feedback autoregulation and by U1 snRNP. PLoS Genet. 9, e1003613 (2013).
Michaeli, S. Trans-splicing in trypanosomes: machinery and its impact on the parasite transcriptome. Future Microbiol. 6, 459–474 (2011).
Lasda, E. L. & Blumenthal, T. Trans-splicing. Wiley Interdiscip. Rev. RNA 2, 417–434 (2011).
Bruzik, J. P. & Maniatis, T. Spliced leader RNAs from lower eukaryotes are trans-spliced in mammalian cells. Nature 360, 692–695 (1992).
Smith, E. R. et al. The little elongation complex regulates small nuclear RNA transcription. Mol. Cell 44, 954–965 (2011).
Fabrizio, P. et al. The evolutionarily conserved core design of the catalytic activation step of the yeast spliceosome. Mol. Cell 36, 593–608 (2009).
Acknowledgements
Research in the authors' laboratories is supported by US National Institutes of Health grants R01-GM053034 and R01-NS041617 (to A.G.M.), as well as R01-CA158283 and R21-AR061640 (to Z.W.). The authors apologize to those whose work could not be discussed owing to space limitations.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Splice site
-
The short sequences at exon–intron junctions of pre-mRNA, which include the 5′ splice (splice donor) site and the 3′ splice (splice acceptor) site located at the beginning and the end of an intron, respectively.
- Heterogeneous nuclear RNP
-
(hnRNP). A diverse class of ribonucleoproteins (RNPs) located in the cell nucleus, and primarily involved in post-transcriptional regulation of mRNAs. The hnRNP proteins are a class of RNA-binding factors, many of which shuttle between the nucleus and cytoplasm, that are involved in regulating the processing, stability and subcellular transport of mRNPs.
- Cajal bodies
-
Nuclear substructures that are highly enriched in pre-mRNA splicing factors. They are thought to function as sites of ribonucleoprotein assembly and remodelling.
- Tudor domain
-
A conserved protein structural motif that is thought to bind to methylated arginine or lysine residues, promoting physical interactions with its target protein.
- Nuclear speckles
-
Sub-nuclear structures highly enriched in pre-mRNA-splicing factors. At the ultrastructural level, they correspond to domains known as interchromatin granule clusters.
- SR proteins
-
Proteins that contain a domain with repeats of serine (S) and arginine (R) residues and one or more RNA-recognition motifs. SR proteins are best known for their ability to bind exonic splicing enhancers and activate splicing, although some SR proteins also regulate transcription.
- Branch point
-
A loosely conserved short sequence usually located ∼15–50 nucleotides upstream of the 3′ splice site, before a region rich in pyrimidines (cytosine and uracil). Most branch points include an adenine nucleotide as the site of lariat formation.
- Exon definition
-
One of two different modes of initial splice site pairing at the early stage of splicing (the other being intron definition). During exon definition, the U1 and U2 small nuclear ribonucleoproteins (snRNPs) interact to pair the splice sites across an exon. For some small introns, the U1 and U2 snRNPs interact to pair the splice sites across introns.
Rights and permissions
About this article
Cite this article
Matera, A., Wang, Z. A day in the life of the spliceosome. Nat Rev Mol Cell Biol 15, 108–121 (2014). https://doi.org/10.1038/nrm3742
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3742
This article is cited by
-
Splicing alterations in pancreatic ductal adenocarcinoma: a new molecular landscape with translational potential
Journal of Experimental & Clinical Cancer Research (2023)
-
Tumor educated platelet: the novel BioSource for cancer detection
Cancer Cell International (2023)
-
A dual role of RBM42 in modulating splicing and translation of CDKN1A/p21 during DNA damage response
Nature Communications (2023)
-
Posttranslational splicing modifications as a key mechanism in cytarabine resistance in acute myeloid leukemia
Leukemia (2023)
-
RNA splicing analysis using heterogeneous and large RNA-seq datasets
Nature Communications (2023)