Key Points
-
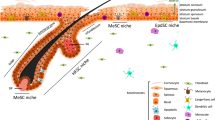
Epidermal stem cells are essential for maintaining a constant supply of cells that ensure proper hair cycling and for the formation of the barrier provided by the skin.
-
Epidermal stem cells reside in specialized niches at the basal layer of the interfollicular epidermis and at the permanent area of the hair follicle.
-
There is an unexpected heterogeneity among the hair follicle stem cells, generating specialized populations that display functional differences and differential lineage preference.
-
The microenvironment of the hair follicle contributes to the regulation of stem cell function, but hair follicle stem cells also instruct the behaviour other cell types and structures around the hair follicle.
Abstract
In the past years, our view of the molecular and cellular mechanisms that ensure the self-renewal of the skin has dramatically changed. Several populations of stem cells have been identified that differ in their spatio-temporal contribution to their compartment in steady-state and damaged conditions, suggesting that epidermal stem cell heterogeneity is far greater than previously anticipated. There is also increasing evidence that these different stem cells require a tightly controlled spatial and temporal communication between other skin residents to carry out their function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature Rev. Mol. Cell Biol. 10, 207–217 (2009).
Cotsarelis, G., Sun, T. T. & Lavker, R. M. Label-retaining cells reside in the bulge area of pilosebaceous unit: implications for follicular stem cells, hair cycle, and skin carcinogenesis. Cell 61, 1329–1337 (1990).
Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000).
Braun, K. M. et al. Manipulation of stem cell proliferation and lineage commitment: visualisation of label-retaining cells in wholemounts of mouse epidermis. Development 130, 5241–5255 (2003).
Tumbar, T. et al. Defining the epithelial stem cell niche in skin. Science 303, 359–363 (2004).
Morris, R. J. et al. Capturing and profiling adult hair follicle stem cells. Nature Biotech. 22, 411–417 (2004).
Ghazizadeh, S. & Taichman, L. B. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20, 1215–1222 (2001).
Ito, M. et al. Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nature Med. 11, 1351–1354 (2005).
Levy, V., Lindon, C., Zheng, Y., Harfe, B. D. & Morgan, B. A. Epidermal stem cells arise from the hair follicle after wounding. FASEB J. 21, 1358–1366 (2007).
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–245 (2001).
Potten, C. S. & Hendry, J. H. Letter: clonogenic cells and stem cells in epidermis. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 24, 537–540 (1973).
Liu, Y., Lyle, S., Yang, Z. & Cotsarelis, G. Keratin 15 promoter targets putative epithelial stem cells in the hair follicle bulge. J. Invest. Dermatol. 121, 963–968 (2003).
Blanpain, C., Lowry, W. E., Geoghegan, A., Polak, L. & Fuchs, E. Self-renewal, multipotency, and the existence of two cell populations within an epithelial stem cell niche. Cell 118, 635–648 (2004).
Potten, C. S. & Morris, R. J. Epithelial stem cells in vivo. J. Cell Sci. Suppl. 10, 45–62 (1988).
Potten, C. S. The epidermal proliferative unit: the possible role of the central basal cell. Cell Tissue Kinet. 7, 77–88 (1974).
Jones, P. & Simons, B. D. Epidermal homeostasis: do committed progenitors work while stem cells sleep? Nature Rev. Mol. Cell Biol. 9, 82–88 (2008).
Clayton, E. et al. A single type of progenitor cell maintains normal epidermis. Nature 446, 185–189 (2007). Shows, through lineage-tracing experiments, that committed progenitors undertake homeostatic and regenerative tasks by switching between two states, without the help of a stem cell population.
Doupe, D. P., Klein, A. M., Simons, B. D. & Jones, P. H. The ordered architecture of murine ear epidermis is maintained by progenitor cells with random fate. Dev. Cell 18, 317–323 (2010).
Doupe, D. P. et al. A single progenitor population switches behavior to maintain and repair esophageal epithelium. Science 337, 1091–1093 (2012).
Lopez-Garcia, C., Klein, A. M., Simons, B. D. & Winton, D. J. Intestinal stem cell replacement follows a pattern of neutral drift. Science 330, 822–825 (2010).
Snippert, H. J. et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell 143, 134–144 (2010). Shows that LGR6 labels a stem cell subpopulation residing at the isthmus of the hair follicle. The contribution of this population changes with age, restricting it to sebaceous gland and interfollicular epidermis in adults.
Driessens, G., Beck, B., Caauwe, A., Simons, B. D. & Blanpain, C. Defining the mode of tumour growth by clonal analysis. Nature 488, 527–530 (2012).
Janich, P. et al. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 480, 209–214 (2011). Shows that one of the unsuspected mechanisms used to generate heterogeneity among bulge stem cells is through circadian clock oscillations, leading to different responses to activating signals.
Gaddameedhi, S., Selby, C. P., Kaufmann, W. K., Smart, R. C. & Sancar, A. Control of skin cancer by the circadian rhythm. Proc. Natl Acad. Sci. USA 108, 18790–18795 (2011).
Sporl, F. et al. Kruppel-like factor 9 is a circadian transcription factor in human epidermis that controls proliferation of keratinocytes. Proc. Natl Acad. Sci. USA 109, 10903–10908 (2012).
Geyfman, M. et al. Brain and muscle Arnt-like protein-1 (BMAL1) controls circadian cell proliferation and susceptibility to UVB-induced DNA damage in the epidermis. Proc. Natl Acad. Sci. USA 109, 11758–11763 (2012).
Albrecht, U. Timing to perfection: the biology of central and peripheral circadian clocks. Neuron 74, 246–260 (2012).
Mascre, G. et al. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature 489, 257–262 (2012). Defines, with the use of two different lineage-tracing strategies, a committed progenitor and a quiescent stem cell population in the interfollicular epidermis. These two cell types are hierarchically organized and show different regenerative and homeostatic properties.
Jones, P. H. & Watt, F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724 (1993).
Jones, P. H., Harper, S. & Watt, F. M. Stem cell patterning and fate in human epidermis. Cell 80, 83–93 (1995).
Li, A., Simmons, P. J. & Kaur, P. Identification and isolation of candidate human keratinocyte stem cells based on cell surface phenotype. Proc. Natl Acad. Sci. USA 95, 3902–3907 (1998).
Lowell, S., Jones, P., Le Roux, I., Dunne, J. & Watt, F. M. Stimulation of human epidermal differentiation by Delta–Notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 10, 491–500 (2000).
Legg, J., Jensen, U. B., Broad, S., Leigh, I. & Watt, F. M. Role of melanoma chondroitin sulphate proteoglycan in patterning stem cells in human interfollicular epidermis. Development 130, 6049–6063 (2003).
Jensen, K. B. & Watt, F. M. Single-cell expression profiling of human epidermal stem and transit-amplifying cells: Lrig1 is a regulator of stem cell quiescence. Proc. Natl Acad. Sci. USA 103, 11958–11963 (2006).
Luis, N. M. et al. Regulation of human epidermal stem cell proliferation and senescence requires polycomb- dependent and -independent functions of Cbx4. Cell Stem Cell 9, 233–246 (2011).
Wan, H. et al. Desmosomal proteins, including desmoglein 3, serve as novel negative markers for epidermal stem cell-containing population of keratinocytes. J. Cell Sci. 116, 4239–4248 (2003).
Fortunel, N. O. et al. Long-term expansion of human functional epidermal precursor cells: promotion of extensive amplification by low TGF-β1 concentrations. J. Cell Sci. 116, 4043–4052 (2003).
Schluter, H., Paquet-Fifield, S., Gangatirkar, P., Li, J. & Kaur, P. Functional characterization of quiescent keratinocyte stem cells and their progeny reveals a hierarchical organization in human skin epidermis. Stem Cells 29, 1256–1268 (2011).
Webb, A., Li, A. & Kaur, P. Location and phenotype of human adult keratinocyte stem cells of the skin. Differentiation 72, 387–395 (2004).
Jensen, U. B., Lowell, S. & Watt, F. M. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development 126, 2409–2418 (1999).
Ghazizadeh, S. & Taichman, L. B. Organization of stem cells and their progeny in human epidermis. J. Invest. Dermatol. 124, 367–372 (2005).
Benitah, S. A. & Frye, M. Stem cells in ectodermal development. J. Mol. Med. (Berl.) 90, 783–790 (2012).
Nowak, J. A., Polak, L., Pasolli, H. A. & Fuchs, E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 3, 33–43 (2008).
Vidal, V. P. et al. Sox9 is essential for outer root sheath differentiation and the formation of the hair stem cell compartment. Curr. Biol. 15, 1340–1351 (2005).
Mardaryev, A. N. et al. Lhx2 differentially regulates Sox9, Tcf4 and Lgr5 in hair follicle stem cells to promote epidermal regeneration after injury. Development 138, 4843–4852 (2011).
Rhee, H., Polak, L. & Fuchs, E. Lhx2 maintains stem cell character in hair follicles. Science 312, 1946–1949 (2006).
Horsley, V., Aliprantis, A. O., Polak, L., Glimcher, L. H. & Fuchs, E. NFATc1 balances quiescence and proliferation of skin stem cells. Cell 132, 299–310 (2008).
Merrill, B. J., Gat, U., DasGupta, R. & Fuchs, E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15, 1688–1705 (2001).
Nguyen, H. et al. Tcf3 and Tcf4 are essential for long-term homeostasis of skin epithelia. Nature Genet. 41, 1068–1075 (2009).
Chen, T. et al. An RNA interference screen uncovers a new molecule in stem cell self-renewal and long-term regeneration. Nature 485, 104–108 (2012).
Paus, R. & Cotsarelis, G. The biology of hair follicles. N. Engl. J. Med. 341, 491–497 (1999).
DasGupta, R. & Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 (1999).
Greco, V. et al. A two-step mechanism for stem cell activation during hair regeneration. Cell Stem Cell 4, 155–169 (2009). Shows that hair germ cells are the first cells to respond to activating signals generated by the dermal papilla, which fuel the first stages of anagen.
Plikus, M. V. et al. Cyclic dermal BMP signalling regulates stem cell activation during hair regeneration. Nature 451, 340–344 (2008). Describes long-range communication between tissues: the signals secreted by adipose tissue affect hair follicle stem cell behaviour. The inhibitory signals secreted by the adipocytes are out of phase with activating signals and generate different telogen sub-phases.
Lyle, S. et al. Human hair follicle bulge cells are biochemically distinct and possess an epithelial stem cell phenotype. J. Investig. Dermatol. Symp. Proc. 4, 296–301 (1999).
Hsu, Y. C., Pasolli, H. A. & Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 144, 92–105 (2011). Shows, using a combination of advanced lineage-tracing techniques, that stem cell progeny that has exited the bulge can go back to the stem cell niche and be spared for the next hair follicle cycle.
Panteleyev, A. A., Jahoda, C. A. & Christiano, A. M. Hair follicle predetermination. J. Cell Sci. 114, 3419–3431 (2001).
Kobielak, K., Stokes, N., de la Cruz, J., Polak, L. & Fuchs, E. Loss of a quiescent niche but not follicle stem cells in the absence of bone morphogenetic protein signaling. Proc. Natl Acad. Sci. USA 104, 10063–10068 (2007).
Lowry, W. E. et al. Defining the impact of β-catenin/Tcf transactivation on epithelial stem cells. Genes Dev. 19, 1596–1611 (2005).
Trempus, C. S. et al. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Invest. Dermatol. 120, 501–511 (2003).
Claudinot, S., Nicolas, M., Oshima, H., Rochat, A. & Barrandon, Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc. Natl Acad. Sci. USA 102, 14677–14682 (2005).
Lin, K. K. et al. Circadian clock genes contribute to the regulation of hair follicle cycling. PLoS Genet. 5, e1000573 (2009). Reports for the first time a role of circadian clock in controlling mouse hair follicle cycling.
Waghmare, S. K. et al. Quantitative proliferation dynamics and random chromosome segregation of hair follicle stem cells. EMBO J. 27, 1309–1320 (2008).
Zhang, Y. V., Cheong, J., Ciapurin, N., McDermitt, D. J. & Tumbar, T. Distinct self-renewal and differentiation phases in the niche of infrequently dividing hair follicle stem cells. Cell Stem Cell 5, 267–278 (2009).
Jaks, V. et al. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nature Genet. 40, 1291–1299 (2008).
Barker, N. et al. Very long-term self-renewal of small intestine, colon, and hair follicles from cycling Lgr5+ve stem cells. Cold Spring Harb. Symp. Quant. Biol. 73, 351–356 (2008).
Brownell, I., Guevara, E., Bai, C. B., Loomis, C. A. & Joyner, A. L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 8, 552–565 (2011). Shows that signals from nerve fibres surrounding the hair follicles can change their long-term contribution in interfollicular epidermis wound repair.
Ito, M., Kizawa, K., Hamada, K. & Cotsarelis, G. Hair follicle stem cells in the lower bulge form the secondary germ, a biochemically distinct but functionally equivalent progenitor cell population, at the termination of catagen. Differentiation 72, 548–557 (2004).
Frye, M. & Benitah, S. A. Chromatin regulators in mammalian epidermis. Semin. Cell Dev. Biol. 23, 897–905 (2012).
Zhang, J., Bardot, E. & Ezhkova, E. Epigenetic regulation of skin: focus on the Polycomb complex. Cell. Mol. Life Sci. 69, 2161–2172 (2012).
Snippert, H. J. et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science 327, 1385–1389 (2010).
Nijhof, J. G. et al. The cell-surface marker MTS24 identifies a novel population of follicular keratinocytes with characteristics of progenitor cells. Development 133, 3027–3037 (2006).
Jensen, U. B. et al. A distinct population of clonogenic and multipotent murine follicular keratinocytes residing in the upper isthmus. J. Cell Sci. 121, 609–617 (2008).
Jensen, K. B. et al. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 4, 427–439 (2009). Identifies a new population of stem cells residing at the hair follicle junctional zone that contributes to sebaceous gland and interfollicular epidermis homeostasis.
Wong, V. W. et al. Lrig1 controls intestinal stem-cell homeostasis by negative regulation of ErbB signalling. Nature Cell Biol. 14, 401–408 (2012).
Powell, A. E. et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell 149, 146–158 (2012).
Horsley, V. et al. Blimp1 defines a progenitor population that governs cellular input to the sebaceous gland. Cell 126, 597–609 (2006).
Lu, C. P. et al. Identification of stem cell populations in sweat glands and ducts reveals roles in homeostasis and wound repair. Cell 150, 136–150 (2012).
Rittie, L., Sachs, D. L., Orringer, J. S., Voorhees, J. J. & Fisher, G. J. Eccrine sweat glands are major contributors to reepithelialization of human wounds. Am. J. Pathol. 182, 163–171 (2013).
Toyoshima, K. E. et al. Fully functional hair follicle regeneration through the rearrangement of stem cells and their niches. Nature Commun. 3, 784 (2012).
Watt, F. M. & Fujiwara, H. Cell–extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 3, a005124 (2011).
Benitah, S. A., Frye, M., Glogauer, M. & Watt, F. M. Stem cell depletion through epidermal deletion of Rac1. Science 309, 933–935 (2005).
Castilho, R. M. et al. Requirement of Rac1 distinguishes follicular from interfollicular epithelial stem cells. Oncogene 26, 5078–5085 (2007).
Chrostek, A. et al. Rac1 is crucial for hair follicle integrity but is not essential for maintenance of the epidermis. Mol. Cell. Biol. 26, 6957–6970 (2006).
Lechler, T. & Fuchs, E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437, 275–280 (2005).
Takeda, N. et al. Hopx expression defines a subset of multipotent hair follicle stem cells and a progenitor population primed to give rise to K6+ niche cells. Development 140, 1655–1664 (2013).
Jahoda, C. A., Horne, K. A. & Oliver, R. F. Induction of hair growth by implantation of cultured dermal papilla cells. Nature 311, 560–562 (1984).
Chi, W., Wu, E. & Morgan, B. A. Dermal papilla cell number specifies hair size, shape and cycling and its reduction causes follicular decline. Development 140, 1676–1683 (2013).
Rompolas, P. et al. Live imaging of stem cell and progeny behaviour in physiological hair-follicle regeneration. Nature 487, 496–499 (2012). Breakthrough imaging of hair follicles in live mice combined with laser ablation techniques showing the same hair follicle at different stages of the cycle.
Driskell, R. R. et al. Clonal growth of dermal papilla cells in hydrogels reveals intrinsic differences between Sox2-positive and -negative cells in vitro and in vivo. J. Invest. Dermatol. 132, 1084–1093 (2012).
Tobin, D. J., Gunin, A., Magerl, M., Handijski, B. & Paus, R. Plasticity and cytokinetic dynamics of the hair follicle mesenchyme: implications for hair growth control. J. Invest. Dermatol. 120, 895–904 (2003).
Rendl, M., Polak, L. & Fuchs, E. BMP signaling in dermal papilla cells is required for their hair follicle-inductive properties. Genes Dev. 22, 543–557 (2008).
Chi, W. Y., Enshell-Seijffers, D. & Morgan, B. A. De novo production of dermal papilla cells during the anagen phase of the hair cycle. J. Invest. Dermatol. 130, 2664–2666 (2010).
Collins, C. A., Jensen, K. B., MacRae, E. J., Mansfield, W. & Watt, F. M. Polyclonal origin and hair induction ability of dermal papillae in neonatal and adult mouse back skin. Dev. Biol. 366, 290–297 (2012).
Schlake, T. Determination of hair structure and shape. Semin. Cell Dev. Biol. 18, 267–273 (2007).
Jahoda, C. A. Cellular and developmental aspects of androgenetic alopecia. Exp. Dermatol. 7, 235–248 (1998).
Sennett, R. & Rendl, M. Mesenchymal–epithelial interactions during hair follicle morphogenesis and cycling. Semin. Cell Dev. Biol. 23, 917–927 (2012).
Enshell-Seijffers, D., Lindon, C., Kashiwagi, M. & Morgan, B. A. β-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 18, 633–642 (2010).
Oshimori, N. & Fuchs, E. Paracrine TGF-β signaling counterbalances BMP-mediated repression in hair follicle stem cell activation. Cell Stem Cell 10, 63–75 (2012).
Enshell-Seijffers, D., Lindon, C. & Morgan, B. A. The serine protease Corin is a novel modifier of the Agouti pathway. Development 135, 217–225 (2008).
Driskell, R. R., Giangreco, A., Jensen, K. B., Mulder, K. W. & Watt, F. M. Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136, 2815–2823 (2009).
Grisanti, L. et al. Tbx18 targets dermal condensates for labeling, isolation, and gene ablation during embryonic hair follicle formation. J. Invest. Dermatol. 133, 344–353 (2013).
Clavel, C. et al. Sox2 in the dermal papilla niche controls hair growth by fine-tuning BMP signaling in differentiating hair shaft progenitors. Dev. Cell 23, 981–994 (2012).
Nishimura, E. K. et al. Dominant role of the niche in melanocyte stem-cell fate determination. Nature 416, 854–860 (2002).
Rabbani, P. et al. Coordinated activation of Wnt in epithelial and melanocyte stem cells initiates pigmented hair regeneration. Cell 145, 941–955 (2011).
Nishimura, E. K. et al. Key roles for transforming growth factor-β in melanocyte stem cell maintenance. Cell Stem Cell 6, 130–140 (2010).
Tanimura, S. et al. Hair follicle stem cells provide a functional niche for melanocyte stem cells. Cell Stem Cell 8, 177–187 (2011).
Collins, C. A., Kretzschmar, K. & Watt, F. M. Reprogramming adult dermis to a neonatal state through epidermal activation of β-catenin. Development 138, 5189–5199 (2011).
Chang, C. Y. et al. NFIB is a governor of epithelial-melanocyte stem cell behaviour in a shared niche. Nature 495, 98–102 (2013).
Nishimura, E. K., Granter, S. R. & Fisher, D. E. Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 307, 720–724 (2005).
Plikus, M. V. et al. Self-organizing and stochastic behaviors during the regeneration of hair stem cells. Science 332, 586–589 (2011).
Festa, E. et al. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell 146, 761–771 (2011).
Fujiwara, H. et al. The basement membrane of hair follicle stem cells is a muscle cell niche. Cell 144, 577–589 (2011). Describes how bulge stem cells can generate the appropriate niche at the basement membrane that directs the attachment of the APM.
Tan, D. W. et al. Single-cell gene expression profiling reveals functional heterogeneity of undifferentiated human epidermal cells. Development 140, 1433–1444 (2013).
Connelly, J. T. et al. Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nature Cell Biol. 12, 711–718 (2010).
Mannik, J., Alzayady, K. & Ghazizadeh, S. Regeneration of multilineage skin epithelia by differentiated keratinocytes. J. Invest. Dermatol. 130, 388–397 (2010).
Beck, B. et al. A vascular niche and a VEGF–Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478, 399–403 (2011).
Amoh, Y. et al. Nascent blood vessels in the skin arise from nestin-expressing hair-follicle cells. Proc. Natl Acad. Sci. USA 101, 13291–13295 (2004).
Arck, P. et al. Is there a 'gut–brain–skin axis'? Exp. Dermatol. 19, 401–405 (2010).
Giangreco, A., Goldie, S. J., Failla, V., Saintigny, G. & Watt, F. M. Human skin aging is associated with reduced expression of the stem cell markers β1 integrin and MCSP. J. Invest. Dermatol. 130, 604–608 (2010).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302–2306 (1987).
Giangreco, A., Qin, M., Pintar, J. E. & Watt, F. M. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell 7, 250–259 (2008).
Stern, M. M. & Bickenbach, J. R. Epidermal stem cells are resistant to cellular aging. Aging Cell 6, 439–452 (2007).
Doles, J., Storer, M., Cozzuto, L., Roma, G. & Keyes, W. M. Age-associated inflammation inhibits epidermal stem cell function. Genes Dev. 26, 2144–2153 (2012).
Sotiropoulou, P. A., Candi, A. & Blanpain, C. The majority of multipotent epidermal stem cells do not protect their genome by asymmetrical chromosome segregation. Stem Cells 26, 2964–2973 (2008).
Sotiropoulou, P. A. et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nature Cell Biol. 12, 572–582 (2010).
Sotiropoulou, P. A. et al. BRCA1 deficiency in skin epidermis leads to selective loss of hair follicle stem cells and their progeny. Genes Dev. 27, 39–51 (2013).
Flores, I., Cayuela, M. L. & Blasco, M. A. Effects of telomerase and telomere length on epidermal stem cell behavior. Science 309, 1253–1256 (2005).
Castilho, R. M., Squarize, C. H., Chodosh, L. A., Williams, B. O. & Gutkind, J. S. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell 5, 279–289 (2009).
Conboy, I. M. et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 433, 760–764 (2005).
Acknowledgements
G.S. was funded by the AXA Research Fund. The authors apologize to their colleagues whose work could not be cited owing to space constraints.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Cornified cells
-
Epidermal keratinocytes that have undergone a process of terminal differentiation whereby they form a mesh of crosslinked proteins that confers impermeability and barrier protection.
- Lineage-tracing techniques
-
Genetic tagging of a certain cell type that allows following its fate and that of its progeny during a particular process, such as development, homeostasis or carcinogenesis.
- Placodes
-
Condensate of embryonic epidermal cells that invaginate into the dermis to form the embryonic hair follicle structures.
Rights and permissions
About this article
Cite this article
Solanas, G., Benitah, S. Regenerating the skin: a task for the heterogeneous stem cell pool and surrounding niche. Nat Rev Mol Cell Biol 14, 737–748 (2013). https://doi.org/10.1038/nrm3675
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3675
This article is cited by
-
Role of vitamin D and calcium signaling in epidermal wound healing
Journal of Endocrinological Investigation (2022)
-
Tracing the origin of hair follicle stem cells
Nature (2021)
-
Skin-resident immune cells actively coordinate their distribution with epidermal cells during homeostasis
Nature Cell Biology (2021)
-
Decrease of laminin-511 in the basement membrane due to photoaging reduces epidermal stem/progenitor cells
Scientific Reports (2020)
-
The RNA-binding protein YBX1 regulates epidermal progenitors at a posttranscriptional level
Nature Communications (2018)