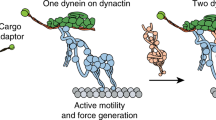
Key Points
-
Cell biological studies have identified roles for dynein motors in many in vivo processes. These include transporting diverse intracellular cargo along microtubules, organizing microtubules within the cell division machinery and powering the beating of cilia and flagella.
-
Unlike myosin and kinesin, which share an ancestry with G proteins, dynein evolved from the AAA+ superfamily of ring-shaped ATPases.
-
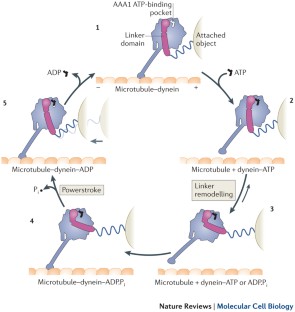
In outline, the mechanochemical cycle of dynein is similar to that of myosin, but the underlying mechanism of its movement is quite different.
-
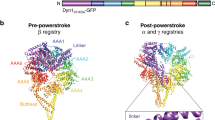
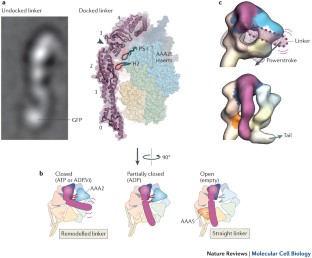
Recent structural studies point towards a model in which nucleotide-driven flexing motions in the dynein AAA+ ring are coupled to the remodelling of a mechanical element called the linker domain.
-
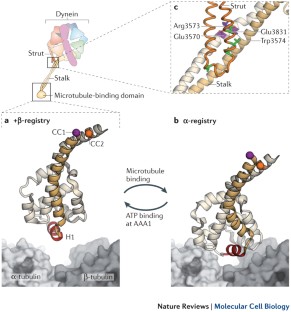
The ATPase and microtubule-binding domains of dynein are spatially separated by a coiled-coil stalk, which is thought to mediate allosteric communication via small sliding movements between its constituent α-helices.
-
Single-molecule studies are starting to reveal how the paired motor domains in cytoplasmic dynein dimers move along microtubules, but the extent to which the motor domains communicate with each other and how much force they produce are controversial.
Abstract
Fuelled by ATP hydrolysis, dyneins generate force and movement on microtubules in a wealth of biological processes, including ciliary beating, cell division and intracellular transport. The large mass and complexity of dynein motors have made elucidating their mechanisms a sizable task. Yet, through a combination of approaches, including X-ray crystallography, cryo-electron microscopy, single-molecule assays and biochemical experiments, important progress has been made towards understanding how these giant motor proteins work. From these studies, a model for the mechanochemical cycle of dynein is emerging, in which nucleotide-driven flexing motions within the AAA+ ring of dynein alter the affinity of its microtubule-binding stalk and reshape its mechanical element to generate movement.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Moughamian, A. J. & Holzbaur, E. in Dyneins: Structure, Biology and Disease (ed. King, S. M) 584–601 (Elsevier Inc., 2011).
Fliegauf, M., Benzing, T. & Omran, H. When cilia go bad: cilia defects and ciliopathies. Nature Rev. Mol. Cell Biol. 8, 880–893 (2007).
Gibbons, I. R. Studies on the protein components of cilia from Tetrahymena pyriformis. Proc. Natl Acad. Sci. USA 50, 1002–1010 (1963).
Gibbons, I. & Rowe, A. Dynein: a protein with adenosine triphosphatase activity from cilia. Science 149, 424–426 (1965). References 3 and 4 mark the discovery of dynein motor proteins.
Paschal, B. M., Shpetner, H. S. & Vallee, R. B. MAP 1C is a microtubule-activated ATPase which translocates microtubules in vitro and has dynein-like properties. J. Cell Biol. 105, 1273–1282 (1987). Describes the isolation and characterization of cytoplasmic dynein.
Paschal, B. M. & Vallee, R. B. Retrograde transport by the microtubule-associated protein MAP 1C. Nature 330, 181–183 (1987).
Schroer, T. A., Steuer, E. R. & Sheetz, M. P. Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell 56, 937–946 (1989).
Vale, R. D., Reese, T. S. & Sheetz, M. P. Identification of a novel force-generating protein, kinesin, involved in microtubule-based motility. Cell 42, 39–50 (1985).
Vale, R. D. & Milligan, R. A. The way things move: looking under the hood of molecular motor proteins. Science 288, 88–95 (2000).
Billington, N. & Sellers, J. R. Dynein struts its stuff. Nature Struct. Mol. Biol. 18, 635–636 (2011).
Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. AAA+: a class of chaperone-like ATPases associated with the assembly, operation, and disassembly of protein complexes. Genome Res. 9, 27–43 (1999).
Vallee, R. B., McKenney, R. J. & Ori-McKenney, K. M. Multiple modes of cytoplasmic dynein regulation. Nature Cell Biol. 14, 224–230 (2012).
Kardon, J. & Vale, R. Regulators of the cytoplasmic dynein motor. Nature Rev. Mol. Cell Biol. 10, 854–865 (2009).
Allan, V. J. Cytoplasmic dynein. Biochem. Soc. Trans. 39, 1169–1178 (2011).
Akhmanova, A. & Hammer, J. A. 3rd. Linking molecular motors to membrane cargo. Curr. Opin. Cell Biol. 22, 479–487 (2010).
Riedel-Kruse, I. H., Hilfinger, A., Howard, J. & Jülicher, F. How molecular motors shape the flagellar beat. HFSP J. 1, 192–208 (2007).
Brokaw, C. Thinking about flagellar oscillation. Cell. Motil. Cytoskeleton 66, 425–436 (2009).
Lindemann, C. B. & Lesich, K. A. Flagellar and ciliary beating: the proven and the possible. J. Cell Sci. 123, 519–528 (2010).
King, S. M. Integrated control of axonemal dynein AAA+ motors. J. Struct. Biol. 179, 222–228 (2012).
Wickstead, B. & Gull, K. Dyneins across eukaryotes: a comparative genomic analysis. Traffic 8, 1708–1721 (2007).
Vale, R. D. The molecular motor toolbox for intracellular transport. Cell 112, 467–480 (2003).
Yagi, T. Bioinformatic approaches to dynein heavy chain classification. Methods Cell Biol. 92, 1–9 (2009).
Moore, J., Stuchell-Brereton, M. & Cooper, J. Function of dynein in budding yeast: mitotic spindle positioning in a polarized cell. Cell. Motil. Cytoskeleton 66, 546–555 (2009).
Egan, M. J., McClintock, M. A. & Reck-Peterson, S. L. Microtubule-based transport in filamentous fungi. Curr. Opin. Microbiol. 15, 637–645 (2012).
Koonce, M. P. Dictyostelium, a model organism for microtubule-based transport. Protist 151, 17–25 (2000).
Pfister, K. K. et al. Genetic analysis of the cytoplasmic dynein subunit families. PLoS Genet. 2, e1 (2006).
Trokter, M., Mücke, N. & Surrey, T. Reconstitution of the human cytoplasmic dynein complex. Proc. Natl Acad. Sci. USA 109, 20895–20900 (2012). Describes the reconstitution of the human cytoplasmic dynein complex from recombinant subunits. Surprisingly, despite driving robust microtubule gliding, the complexes do not show processive motility, suggesting that additional factors might be required for this behaviour.
Schroer, T. A. Dynactin. Annu. Rev. Cell Dev. Biol. 20, 759–779 (2004).
Driskell, O. J., Mironov, A., Allan, V. J. & Woodman, P. G. Dynein is required for receptor sorting and the morphogenesis of early endosomes. Nature Cell Biol. 9, 113–120 (2007).
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680–1685 (2001).
Blocker, A. et al. Molecular requirements for bi-directional movement of phagosomes along microtubules. J. Cell Biol. 137, 113–129 (1997).
Gross, S. P. et al. Interactions and regulation of molecular motors in Xenopus melanophores. J. Cell Biol. 156, 855–865 (2002).
Kural, C. et al. Kinesin and dynein move a peroxisome in vivo: a tug-of-war or coordinated movement? Science 308, 1469–1472 (2005).
Gross, S. P., Welte, M. A., Block, S. M. & Wieschaus, E. F. Dynein-mediated cargo transport in vivo. A switch controls travel distance. J. Cell Biol. 148, 945–956 (2000).
Pilling, A. D., Horiuchi, D., Lively, C. M. & Saxton, W. M. Kinesin-1 and dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons. Mol. Biol. Cell 17, 2057–2068 (2006).
Presley, J. F. et al. ER-to-Golgi transport visualized in living cells. Nature 389, 81–85 (1997).
Heerssen, H. M., Pazyra, M. F. & Segal, R. A. Dynein motors transport activated Trks to promote survival of target-dependent neurons. Nature Neurosci. 7, 596–604 (2004).
Young, A., Dictenberg, J. B., Purohit, A., Tuft, R. & Doxsey, S. J. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Mol. Biol. Cell 11, 2047–2056 (2000).
Harrell, J. M. et al. Evidence for glucocorticoid receptor transport on microtubules by dynein. J. Biol. Chem. 279, 54647–54654 (2004).
Shah, J. V., Flanagan, L. A., Janmey, P. A. & Leterrier, J. F. Bidirectional translocation of neurofilaments along microtubules mediated in part by dynein/dynactin. Mol. Biol. Cell 11, 3495–3508 (2000).
Wilkie, G. S. & Davis, I. Drosophila wingless and pair-rule transcripts localize apically by dynein-mediated transport of RNA particles. Cell 105, 209–219 (2001).
Johnston, J. A., Illing, M. E. & Kopito, R. R. Cytoplasmic dynein/dynactin mediates the assembly of aggresomes. Cell. Motil. Cytoskeleton 53, 26–38 (2002).
Maday, S., Wallace, K. E. & Holzbaur, E. L. F. Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J. Cell Biol. 196, 407–417 (2012).
Dodding, M. P. & Way, M. Coupling viruses to dynein and kinesin-1. EMBO J. 30, 3527–3539 (2011).
Sato, A. et al. Cytoskeletal forces span the nuclear envelope to coordinate meiotic chromosome pairing and synapsis. Cell 139, 907–919 (2009).
Steinberg, G. et al. Motor-driven motility of fungal nuclear pores organizes chromosomes and fosters nucleocytoplasmic transport. J. Cell Biol. 198, 343–355 (2012).
Schnyder, T. et al. B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity 34, 905–918 (2011).
Hashimoto-Tane, A. et al. Dynein-driven transport of T cell receptor microclusters regulates immune synapse formation and T cell activation. Immunity 34, 919–931 (2011).
Laan, L. et al. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell 148, 502–514 (2012).
Hendricks, A. G. et al. Dynein tethers and stabilizes dynamic microtubule plus ends. Curr. Biol. 22, 632–637 (2012).
Tsai, J.-W., Bremner, K. H. & Vallee, R. B. Dual subcellular roles for LIS1 and dynein in radial neuronal migration in live brain tissue. Nature Neurosci. 10, 970–979 (2007).
Dujardin, D. L. et al. A role for cytoplasmic dynein and LIS1 in directed cell movement. J. Cell Biol. 163, 1205–1211 (2003).
Combs, J. et al. Recruitment of dynein to the Jurkat immunological synapse. Proc. Natl Acad. Sci. USA 103, 14883–14888 (2006).
McNally, F. J. Mechanisms of spindle positioning. J. Cell Biol. 200, 131–140 (2013).
Kiyomitsu, T. & Cheeseman, I. M. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nature Cell Biol. 14, 311–317 (2012).
Collins, E. S., Balchand, S. K., Faraci, J. L., Wadsworth, P. & Lee, W.-L. Cell cycle-regulated cortical dynein/dynactin promotes symmetric cell division by differential pole motion in anaphase. Mol. Biol. Cell 23, 3380–3390 (2012).
Mitchison, T. et al. Growth, interaction, and positioning of microtubule asters in extremely large vertebrate embryo cells. Cytoskeleton (Hoboken) 69, 738–750 (2012).
Kimura, K. & Kimura, A. Intracellular organelles mediate cytoplasmic pulling force for centrosome centration in the Caenorhabditis elegans early embryo. Proc. Natl Acad. Sci. USA 108, 137–142 (2011).
Longoria, R. A. & Shubeita, G. T. Cargo transport by cytoplasmic dynein can center embryonic centrosomes. PLoS ONE 8, e67710 (2013).
Corthesy-Theulaz, I., Pauloin, A. & Pfeffer, S. R. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J. Cell Biol. 118, 1333–1345 (1992).
Levy, J. R. & Holzbaur, E. L. Dynein drives nuclear rotation during forward progression of motile fibroblasts. J. Cell Sci. 121, 3187–3195 (2008).
Raaijmakers, J. A. et al. Nuclear envelope-associated dynein drives prophase centrosome separation and enables Eg5-independent bipolar spindle formation. EMBO J. 31, 4179–4190 (2012).
Salina, D. et al. Cytoplasmic dynein as a facilitator of nuclear envelope breakdown. Cell 108, 97–107 (2002).
Heald, R. et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996).
Merdes, A., Ramyar, K., Vechio, J. D. & Cleveland, D. W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87, 447–458 (1996).
Foley, E. A. & Kapoor, T. M. Microtubule attachment and spindle assembly checkpoint signalling at the kinetochore. Nature Rev. Mol. Cell Biol. 14, 25–37 (2012).
Howell, B. J. et al. Cytoplasmic dynein/dynactin drives kinetochore protein transport to the spindle poles and has a role in mitotic spindle checkpoint inactivation. J. Cell Biol. 155, 1159–1172 (2001).
Wojcik, E. et al. Kinetochore dynein: its dynamics and role in the transport of the Rough deal checkpoint protein. Nature Cell Biol. 3, 1001–1007 (2001).
Varma, D., Monzo, P., Stehman, S. A. & Vallee, R. B. Direct role of dynein motor in stable kinetochore–microtubule attachment, orientation, and alignment. J. Cell Biol. 182, 1045–1054 (2008).
Yang, Z., Tulu, U. S., Wadsworth, P. & Rieder, C. L. Kinetochore dynein is required for chromosome motion and congression independent of the spindle checkpoint. Curr. Biol. 17, 973–980 (2007).
Ishikawa, H. & Marshall, W. F. Ciliogenesis: building the cell's antenna. Nature Rev. Mol. Cell Biol. 12, 222–234 (2011).
Pazour, G. J., Dickert, B. L. & Witman, G. B. The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J. Cell Biol. 144, 473–481 (1999).
Porter, M. E., Bower, R., Knott, J. A., Byrd, P. & Dentler, W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol. Biol. Cell 10, 693–712 (1999).
Mikami, A. et al. Molecular structure of cytoplasmic dynein 2 and its distribution in neuronal and ciliated cells. J. Cell Sci. 115, 4801–4808 (2002).
Perrone, C. A. et al. A novel dynein light intermediate chain colocalizes with the retrograde motor for intraflagellar transport at sites of axoneme assembly in Chlamydomonas and mammalian cells. Mol. Biol. Cell 14, 2041–2056 (2003).
Ichikawa, M., Watanabe, Y., Murayama, T. & Toyoshima, Y. Y. Recombinant human cytoplasmic dynein heavy chain 1 and 2: observation of dynein-2 motor activity in vitro. FEBS Lett. 585, 2419–2423 (2011).
Pigino, G. et al. Electron-tomographic analysis of intraflagellar transport particle trains in situ. J. Cell Biol. 187, 135–148 (2009).
Laib, J. A., Marin, J. A., Bloodgood, R. A. & Guilford, W. H. The reciprocal coordination and mechanics of molecular motors in living cells. Proc. Natl Acad. Sci. USA 106, 3190–3195 (2009).
Shih, S. M. et al. Intraflagellar transport drives flagellar surface motility. Elife 2, e00744 (2013).
Mallik, R., Rai, A. K., Barak, P., Rai, A. & Kunwar, A. Teamwork in microtubule motors. Trends Cell Biol. http://dx.doi.org/10.1016/j.tcb.2013.06.003 (2013).
Koonce, M. P. & Samsó, M. Overexpression of cytoplasmic dynein's globular head causes a collapse of the interphase microtubule network in Dictyostelium. Mol. Biol. Cell 7, 935–948 (1996). Details a recombinant expression system for the D. discoideum cytoplasmic dynein motor domain, forming the foundation for numerous structural and functional analyses. Also demonstrates that the motor domain includes the globular head structure seen in earlier electron microscopy images.
Nishiura, M. et al. A single-headed recombinant fragment of Dictyostelium cytoplasmic dynein can drive the robust sliding of microtubules. J. Biol. Chem. 279, 22799–22802 (2004).
Reck-Peterson, S. L. et al. Single-molecule analysis of dynein processivity and stepping behavior. Cell 126, 335–348 (2006). Establishes S. cerevisiae as another important cytoplasmic dynein expression system and provides insight into the requirements for processive dynein motion.
Höök, P. et al. Long range allosteric control of cytoplasmic dynein ATPase activity by the stalk and C-terminal domains. J. Biol. Chem. 280, 33045–33054 (2005).
Imamula, K., Kon, T., Ohkura, R. & Sutoh, K. The coordination of cyclic microtubule association/dissociation and tail swing of cytoplasmic dynein. Proc. Natl Acad. Sci. USA 104, 16134–16139 (2007).
Mogami, T., Kon, T., Ito, K. & Sutoh, K. Kinetic characterization of tail swing steps in the ATPase cycle of Dictyostelium cytoplasmic dynein. J. Biol. Chem. 282, 21639–21644 (2007).
Johnson, K. A. Pathway of the microtubule–dynein ATPase and the structure of dynein: a comparison with actomyosin. Annu. Rev. Biophys. Biophys. Chem. 14, 161–188 (1985).
Samsó, M., Radermacher, M., Frank, J. & Koonce, M. P. Structural characterization of a dynein motor domain. J. Mol. Biol. 276, 927–937 (1998).
Gee, M. A., Heuser, J. E. & Vallee, R. B. An extended microtubule-binding structure within the dynein motor domain. Nature 390, 636–639 (1997).
Roberts, A. J. et al. ATP-driven remodeling of the linker domain in the dynein motor. Structure 20, 1670–1680 (2012). A 2D and 3D electron microscopy study of an axonemal and cytoplasmic dynein, revealing that the linker domain is a stable structural entity that is remodelled by the AAA+ ring.
Roberts, A. et al. AAA+ ring and linker swing mechanism in the dynein motor. Cell 136, 485–495 (2009).
Burgess, S. A., Walker, M. L., Sakakibara, H., Knight, P. J. & Oiwa, K. Dynein structure and power stroke. Nature 421, 715–718 (2003).
Kon, T. et al. The 2.8 Å crystal structure of the dynein motor domain. Nature 484, 345–350 (2012). Reports a high-resolution crystal structure of the D. discoideum dynein motor domain in the ADP-bound state. Structure-guided mutagenesis provides insight into how the linker domain moves and the roles of the AAA+ modules, strut and C-terminal sequence in allosteric communication.
Kon, T., Sutoh, K. & Kurisu, G. X-ray structure of a functional full-length dynein motor domain. Nature Struct. Mol. Biol. 18, 638–642 (2011).
Schmidt, H., Gleave, E. S. & Carter, A. P. Insights into dynein motor domain function from a 3.3-Å crystal structure. Nature Struct. Mol. Biol. 19, 492–497 (2012). Reports a high-resolution structure of the S. cerevisiae dynein motor domain, crystallized in the absence of nucleotide. Crystal-soaking experiments reveal distinct nucleotide-binding behaviours in AAA1–AAA4, and mutagenesis suggests a functional role for linker docking at AAA5.
Carter, A. P., Cho, C., Jin, L. & Vale, R. D. Crystal structure of the dynein motor domain. Science 331, 1159–1165 (2011).
Glynn, S. E., Martin, A., Nager, A. R., Baker, T. A. & Sauer, R. T. Structures of asymmetric ClpX hexamers reveal nucleotide-dependent motions in a AAA+ protein-unfolding machine. Cell 139, 744–756 (2009).
Wang, J. et al. Nucleotide-dependent conformational changes in a protease-associated ATPase HsIU. Structure 9, 1107–1116 (2001).
Gibbons, I. R. et al. Photosensitized cleavage of dynein heavy chains. Cleavage at the “V1 site” by irradiation at 365 nm in the presence of ATP and vanadate. J. Biol. Chem. 262, 2780–2786 (1987).
Kon, T., Nishiura, M., Ohkura, R., Toyoshima, Y. Y. & Sutoh, K. Distinct functions of nucleotide-binding/hydrolysis sites in the four AAA modules of cytoplasmic dynein. Biochemistry 43, 11266–11274 (2004).
Enemark, E. J. & Joshua-Tor, L. Mechanism of DNA translocation in a replicative hexameric helicase. Nature 442, 270–275 (2006).
Cho, C., Reck-Peterson, S. & Vale, R. Cytoplasmic dynein's regulatory ATPase sites affect processivity and force generation. J. Biol. Chem. 283, 25839–25845 (2008).
Huang, J., Roberts, A. J., Leschziner, A. E. & Reck-Peterson, S. L. Lis1 acts as a “clutch” between the ATPase and microtubule-binding domains of the dynein motor. Cell 150, 975–986 (2012).
McKenney, R. J., Vershinin, M., Kunwar, A., Vallee, R. B. & Gross, S. P. LIS1 and NudE induce a persistent dynein force-producing state. Cell 141, 304–314 (2010).
Yamada, M. et al. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. EMBO J. 27, 2471–2483 (2008).
Cho, C. & Vale, R. D. The mechanism of dynein motility: insight from crystal structures of the motor domain. Biochim. Biophys. Acta 1823, 182–191 (2011).
Numata, N., Shima, T., Ohkura, R., Kon, T. & Sutoh, K. C-Sequence of the Dictyostelium cytoplasmic dynein participates in processivity modulation. FEBS Lett. 585, 1185–1190 (2011).
Hwang, W. & Lang, M. J. Nucleotide-dependent control of internal strains in ring-shaped AAA+ motors. Cell. Mol. Bioeng. 6, 65–73 (2013).
Gibbons, I. R. et al. The affinity of the dynein microtubule-binding domain is modulated by the conformation of its coiled-coil stalk. J. Biol. Chem. 280, 23960–23965 (2005).
Carter, A. P. et al. Structure and functional role of dynein's microtubule-binding domain. Science 322, 1691–1695 (2008).
Kon, T. et al. Helix sliding in the stalk coiled coil of dynein couples ATPase and microtubule binding. Nature Struct. Mol. Biol. 16, 325–333 (2009).
Redwine, W. B. et al. Structural basis for microtubule binding and release by dynein. Science 337, 1532–1536 (2012). References 109–112 provide evidence for the helix-sliding hypothesis for allosteric communication through the stalk of dynein, which was put forward in reference 109. Reference 112 also provides insight into the structural changes within the microtubule-binding domain of dynein.
Sweeney, H. L. & Houdusse, A. The motor mechanism of myosin V: insights for muscle contraction. Philo. Trans. R. Soc. Lond. B 359, 1829–1841 (2004).
Xi, Z., Gao, Y., Sirinakis, G., Guo, H. & Zhang, Y. Single-molecule observation of helix staggering, sliding, and coiled coil misfolding. Proc. Natl Acad. Sci. USA 109, 5711–5716 (2012).
Burgess, S. A., Walker, M. L., Sakakibara, H., Oiwa, K. & Knight, P. J. The structure of dynein-c by negative stain electron microscopy. J. Struct. Biol. 146, 205–216 (2004).
Burgess, S. A. & Knight, P. J. Is the dynein motor a winch? Curr. Opin. Struct. Biol. 14, 138–146 (2004).
Gennerich, A., Carter, A. P., Reck-Peterson, S. L. & Vale, R. D. Force-induced bidirectional stepping of cytoplasmic dynein. Cell 131, 952–965 (2007).
Kon, T., Mogami, T., Ohkura, R., Nishiura, M. & Sutoh, K. ATP hydrolysis cycle-dependent tail motions in cytoplasmic dynein. Nature Struct. Mol. Biol. 12, 513–519 (2005).
Shima, T., Kon, T., Imamula, K., Ohkura, R. & Sutoh, K. Two modes of microtubule sliding driven by cytoplasmic dynein. Proc. Natl Acad. Sci. USA 103, 17736–17740 (2006).
Ueno, H., Yasunaga, T., Shingyoji, C. & Hirose, K. Dynein pulls microtubules without rotating its stalk. Proc. Natl Acad. Sci. USA 105, 19702–19707 (2008).
Movassagh, T., Bui, K. H., Sakakibara, H., Oiwa, K. & Ishikawa, T. Nucleotide-induced global conformational changes of flagellar dynein arms revealed by in situ analysis. Nature Struct. Mol. Biol. 17, 761–767 (2010).
Qiu, W. et al. Dynein achieves processive motion using both stochastic and coordinated stepping. Nature Struct. Mol. Biol. 19, 193–200 (2012).
Dewitt, M. A., Chang, A. Y., Combs, P. A. & Yildiz, A. Cytoplasmic dynein moves through uncoordinated stepping of the AAA+ ring domains. Science 335, 221–225 (2011). References 122 and 123 use two-colour single-molecule experiments to reveal that S. cerevisiae cytoplasmic dynein dimers can move processively along the microtubule without following a strict stepping pattern. Analysis of the stepping traces provides insight into the spatial configuration of the motor domains and their loose, tension-based coordination.
Veigel, C., Wang, F., Bartoo, M. L., Sellers, J. R. & Molloy, J. E. The gated gait of the processive molecular motor, myosin V. Nature Cell Biol. 4, 59–65 (2002).
Shima, T., Imamula, K., Kon, T., Ohkura, R. & Sutoh, K. Head-head coordination is required for the processive motion of cytoplasmic dynein, an AAA+ molecular motor. J. Struct. Biol. 156, 182–189 (2006).
Stinson, B. M. et al. Nucleotide binding and conformational switching in the hexameric ring of a AAA+ machine. Cell 153, 628–639 (2013).
Sakamoto, T., Webb, M. R., Forgacs, E., White, H. D. & Sellers, J. R. Direct observation of the mechanochemical coupling in myosin Va during processive movement. Nature 455, 128–132 (2008).
Firestone, A. J. et al. Small-molecule inhibitors of the AAA+ ATPase motor cytoplasmic dynein. Nature 484, 125–129 (2012).
Kamiya, R. Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int. Rev. Cytol. 219, 115–155 (2002).
Kikushima, K. & Kamiya, R. Clockwise translocation of microtubules by flagellar inner-arm dyneins in vitro. Biophys. J. 94, 4014–4019 (2008).
Mallik, R., Carter, B. C., Lex, S. A., King, S. J. & Gross, S. P. Cytoplasmic dynein functions as a gear in response to load. Nature 427, 649–652 (2004).
Rai, A. K., Rai, A., Ramaiya, A. J., Jha, R. & Mallik, R. Molecular adaptations allow dynein to generate large collective forces inside cells. Cell 152, 172–182 (2013).
Schroeder, H. W., Mitchell, C., Shuman, H., Holzbaur, E. L. F. & Goldman, Y. E. Motor number controls cargo switching at actin-microtubule intersections in vitro. Curr. Biol. 20, 687–696 (2010).
Toba, S., Watanabe, T. M., Yamaguchi-Okimoto, L., Toyoshima, Y. Y. & Higuchi, H. Overlapping hand-over-hand mechanism of single molecular motility of cytoplasmic dynein. Proc. Natl Acad. Sci. USA 103, 5741–5745 (2006).
Walter, W. J., Koonce, M. P., Brenner, B. & Steffen, W. Two independent switches regulate cytoplasmic dynein's processivity and directionality. Proc. Natl Acad. Sci. USA 109, 5289–5293 (2012).
Ross, J. L., Wallace, K., Shuman, H., Goldman, Y. E. & Holzbaur, E. L. F. Processive bidirectional motion of dynein-dynactin complexes in vitro. Nature Cell Biol. 8, 562–570 (2006).
Ananthanarayanan, V. et al. Dynein motion switches from diffusive to directed upon cortical anchoring. Cell 153, 1526–1536 (2013).
Nicastro, D. et al. Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. Proc. Natl Acad. Sci. USA 108, E845–E853 (2011).
Summers, K. E. & Gibbons, I. R. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc. Natl Acad. Sci. USA 68, 3092–3096 (1971).
Nicastro, D. Cryo-electron microscope tomography to study axonemal organization. Methods Cell Biol. 91, 1–39 (2009).
Hom, E. F. et al. A unified taxonomy for ciliary dyneins. Cytoskeleton (Hoboken) 68, 555–565 (2011).
Bui, K. H., Yagi, T., Yamamoto, R., Kamiya, R. & Ishikawa, T. Polarity and asymmetry in the arrangement of dynein and related structures in the Chlamydomonas axoneme. J. Cell Biol. 198, 913–925 (2012).
Kagami, O. & Kamiya, R. Translocation and rotation of microtubules caused by multiple species of Chlamydomonas inner-arm dynein. J. Cell Sci. 103, 653–664 (1992).
Yagi, T., Uematsu, K., Liu, Z. & Kamiya, R. Identification of dyneins that localize exclusively to the proximal portion of Chlamydomonas flagella. J. Cell Sci. 122, 1306–1314 (2009).
Woolley, D. M. Studies on the eel sperm flagellum. I. The structure of the inner dynein arm complex. J. Cell Sci. 110, 85–94 (1997).
Zhang, X. & Wigley, D. The 'glutamate switch' provides a link between ATPase activity and ligand binding in AAA+ proteins. Nature Struct. Mol. Biol. 15, 1223–1227 (2008).
Hanson, P. I. & Whiteheart, S. W. AAA+ proteins: have engine, will work. Nature Rev. Mol. Cell Biol. 6, 519–529 (2005).
Pfister, K. K. et al. Cytoplasmic dynein nomenclature. J. Cell Biol. 171, 411–413 (2005).
Mizuno, N. et al. Dynein and kinesin share an overlapping microtubule-binding site. EMBO J. 23, 2459–2467 (2004).
Acknowledgements
The authors apologize to their colleagues whose work could not be cited owing to space limitations. They thank K. Toropova, R. Hernandez-Lopez, J. Huang and S. Reck-Peterson for helpful comments on the manuscript and B. Malkova for providing electron microscopy data for figure 5a. A.J.R. is grateful to J. Iwasa for training on AutoDesk Maya software. The work in the authors laboratory was supported by: a Sir Henry Wellcome Postdoctoral Fellowship (092436/Z/10/Z) to A.J.R.; a Grant-in-Aid for Scientific Research (B) 23370073 from the Japan Society for Promotion of Science (JSPS) and a Japan Science and Technology Agency PRESTO award to T.K.; a Grant-in-Aid for Scientific Research (B) 23370075 from the JSPS to K.S., and grants BB/E00928X/1 and BB/BB/K000705/1 from the BBSRC (UK) and RGP0009/2008-C from the Human Frontiers Science Program to S.A.B.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary information S1 (table)
Motif conservation among the six AAA+ modules of dynein. (PDF 332 kb)
Glossary
- G protein-related fold
-
A characteristic arrangement of secondary structure elements and loops (such as switch I and switch II) shared by G proteins, myosins and kinesins, which indicates that these proteins originated from a common ancestor.
- Axoneme
-
The microtubule-based core of eukaryotic cilia and flagella. The terms cilia and flagella are often used interchangeably, as both describe cellular appendages with an axoneme at their core. In this Review, we use cilia for consistency.
- Autophagosomes
-
Organelles that enwrap cytoplasmic material in a double-membrane-bound structure and subsequently fuse with lysosomes, leading to degradation of the confined material.
- Immunological synapse
-
The interface formed between an antigen-presenting cell and a lymphocyte, such as a B cell or a T cell.
- Astral microtubules
-
Microtubules radiating from the spindle poles that do not contact the kinetochore or overlap with other microtubules in the spindle midzone.
- Crystal soaking
-
A technique in which a crystallized macromolecule is bathed in a ligand-containing solution. The ligand has the opportunity to bind the macromolecule of interest owing to diffusion through solvent-filled channels in the crystal.
- E1 helicase
-
A hexameric AAA+ ATPase protein from papillomavirus that encircles and translocates along single-stranded DNA, thereby unwinding DNA duplexes with a 3′ to 5′ directionality.
- Reptation
-
Snake-like movement of a polymer along a path, originally introduced by De Gennes in the field of polymer theory.
- Highly inclined and laminated optical sheet microscopy
-
A technique for visualizing fluorescently labelled single molecules in cells. To minimize background signal (which can confound single-molecule detection) the specimen is illuminated with an angled sheet of light.
Rights and permissions
About this article
Cite this article
Roberts, A., Kon, T., Knight, P. et al. Functions and mechanics of dynein motor proteins. Nat Rev Mol Cell Biol 14, 713–726 (2013). https://doi.org/10.1038/nrm3667
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3667
This article is cited by
-
Cargo specificity, regulation, and therapeutic potential of cytoplasmic dynein
Experimental & Molecular Medicine (2024)
-
DNALI1 deficiency causes male infertility with severe asthenozoospermia in humans and mice by disrupting the assembly of the flagellar inner dynein arms and fibrous sheath
Cell Death & Disease (2023)
-
Cell biology through the macroscopic lens
Nature Physics (2023)
-
Carboplatin-induced upregulation of pan β-tubulin and class III β-tubulin is implicated in acquired resistance and cross-resistance of ovarian cancer
Cellular and Molecular Life Sciences (2023)
-
Tubulin Cytoskeleton in Neurodegenerative Diseases–not Only Primary Tubulinopathies
Cellular and Molecular Neurobiology (2023)