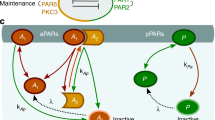
Abstract
A hallmark of cell polarity in metazoans is the distribution of partitioning defective (PAR) proteins into two domains on the membrane. Domain boundaries are set by the collective integration of mechanical, biochemical and biophysical signals, and the resulting PAR domains define areas of cytosol specialization. However, the complexity of the signals acting on PAR proteins has been a barrier to uncovering the general principles of PAR polarity. We propose that physical studies, when combined with genetic data, provide new understanding of the mechanisms of polarity establishment in the Caenorhabditis elegans embryo and other organisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Goldstein, B. & Macara, I. G. The PAR proteins: fundamental players in animal cell polarization. Dev. Cell 13, 609–622 (2007).
Knoblich, J. A. Asymmetric cell division during animal development. Nature Rev. Mol. Cell Biol. 2, 11–20 (2001).
Kemphues, K. J., Priess, J. R., Morton, D. G. & Cheng, N. S. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52, 311–320 (1988).
Cowan, C. R. & Hyman, A. A. Acto-myosin reorganization and PAR polarity in C. elegans. Development 134, 1035–1043 (2007).
Gönczy, P. & Hyman, A. A. Cortical domains and the mechanisms of asymmetric cell division. Trends Cell Biol. 6, 382–387 (1996).
Schneider, S. Q. & Bowerman, B. Cell polarity and the cytoskeleton in the Caenorhabditis elegans zygote. Annu. Rev. Genet. 37, 221–249 (2003).
Beatty, A., Morton, D. & Kemphues, K. The C. elegans homolog of Drosophila Lethal giant larvae functions redundantly with PAR-2 to maintain polarity in the early embryo. Development 137, 3995–4004 (2010).
Boyd, L., Guo, S., Levitan, D., Stinchcomb, D. T. & Kemphues, K. J. PAR-2 is asymmetrically distributed and promotes association of P granules and PAR-1 with the cortex in C. elegans embryos. Development 122, 3075–3084 (1996).
Etemad-Moghadam, B., Guo, S. & Kemphues, K. J. Asymmetrically distributed PAR-3 protein contributes to cell polarity and spindle alignment in early C. elegans embryos. Cell 83, 743–752 (1995).
Guo, S. & Kemphues, K. J. par-1, a gene required for establishing polarity in C. elegans embryos, encodes a putative Ser/Thr kinase that is asymmetrically distributed. Cell 81, 611–620 (1995).
Hoege, C. et al. LGL can partition the cortex of one-cell Caenorhabditis elegans embryos into two domains. Curr. Biol. 20, 1296–1303 (2010).
Tabuse, Y. et al. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development 125, 3607–3614 (1998).
Watts, J. L. et al. par-6, a gene involved in the establishment of asymmetry in early C. elegans embryos, mediates the asymmetric localization of PAR-3. Development 122, 3133–3140 (1996).
Goehring, N. W., Hoege, C., Grill, S. W. & Hyman, A. A. PAR proteins diffuse freely across the anterior-posterior boundary in polarized C. elegans embryos. J. Cell Biol. 193, 583–594 (2011).
Petrásek, Z. et al. Characterization of protein dynamics in asymmetric cell division by scanning fluorescence correlation spectroscopy. Biophys. J. 95, 5476–5486 (2008).
Goehring, N. W. et al. Polarization of PAR proteins by advective triggering of a pattern-forming system. Science 334, 1137–1141 (2011).
Lippincott-Schwartz, J., Altan-Bonnet, N. & Patterson, G. H. Photobleaching and photoactivation: following protein dynamics in living cells. Nature Cell Biol. 5, S7–S14 (2003).
Aceto, D., Beers, M. & Kemphues, K. J. Interaction of PAR-6 with CDC-42 is required for maintenance but not establishment of PAR asymmetry in C. elegans. Dev. Biol. 299, 386–397 (2006).
Joberty, G., Petersen, C., Gao, L. & Macara, I. G. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nature Cell Biol. 2, 531–539 (2000).
Krahn, M. P. Klopfenstein, D. R., Fischer, N. & Wodarz, A. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr. Biol. 20, 636–642 (2010).
Moravcevic, K. et al. Kinase associated-1 domains drive MARK/PAR1 kinases to membrane targets by binding acidic phospholipids. Cell 143, 966–977 (2010).
Motegi, F. et al. Microtubules induce self-organization of polarized PAR domains in Caenorhabditis elegans zygotes. Nature Cell Biol. 13, 1361–1367 (2011).
Panbianco, C. et al. A casein kinase 1 and PAR proteins regulate asymmetry of a PIP2 synthesis enzyme for asymmetric spindle positioning. Dev. Cell 15, 198–208 (2008).
Feng, W., Wu, H., Chan, L. N. & Zhang, M. The Par-3 NTD adopts a PB1-like structure required for Par-3 oligomerization and membrane localization. EMBO J. 26, 2786–2796 (2007).
Cuenca, A. A., Schetter, A., Aceto, D., Kemphues, K. & Seydoux, G. Polarization of the C. elegans zygote proceeds via distinct establishment and maintenance phases. Development 130, 1255–1265 (2003).
Motegi, F. & Sugimoto, A. Sequential functioning of the ECT-2 RhoGEF, RHO-1 and CDC-42 establishes cell polarity in Caenorhabditis elegans embryos. Nature Cell Biol. 8, 978–985 (2006).
Nakayama, Y. et al. Dynamin participates in the maintenance of anterior polarity in the Caenorhabditis elegans embryo. Dev. Cell 16, 889–900 (2009).
Schenk, C., Bringmann, H., Hyman, A. A. & Cowan, C. R. Cortical domain correction repositions the polarity boundary to match the cytokinesis furrow in C. elegans embryos. Development 137, 1743–1753 (2010).
Schonegg, S. CDC-42 and RHO-1 coordinate acto-myosin contractility and PAR protein localization during polarity establishment in C. elegans embryos. Development 133, 3507–3516 (2006).
Hao, Y., Boyd, L. & Seydoux, G. Stabilization of cell polarity by the C. elegans RING protein PAR-2. Dev. Cell 10, 199–208 (2006).
Goehring, N. W., Chowdhury, D., Hyman, A. A. & Grill, S. W. FRAP analysis of membrane-associated proteins: lateral diffusion and membrane-cytoplasmic exchange. Biophys. J. 99, 2443–2452 (2010).
Kondo, S. & Miura, T. Reaction-diffusion model as a framework for understanding biological pattern formation. Science 329, 1616–1620 (2010).
Betschinger, J., Mechtler, K. & Knoblich, J. A. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature 422, 326–330 (2003).
Yamanaka, T. et al. Mammalian Lgl forms a protein complex with PAR-6 and aPKC independently of PAR-3 to regulate epithelial cell polarity. Curr. Biol. 13, 734–743 (2003).
Munro, E., Nance, J. & Priess, J. R. Cortical flows powered by asymmetrical contraction transport PAR proteins to establish and maintain anterior-posterior polarity in the early C. elegans embryo. Dev. Cell 7, 413–424 (2004).
Hird, S. N. & White, J. G. Cortical and cytoplasmic flow polarity in early embryonic cells of Caenorhabditis elegans. J. Cell Biol. 121, 1343–1355 (1993).
Cowan, C. & Hyman, A. Centrosomes direct cell polarity independently of microtubule assembly in C. elegans embryos. Nature 431, 92–96 (2004).
Decker, M. et al. Limiting amounts of centrosome material set centrosome size in C. elegans embryos. Curr. Biol. 21, 1259–1267 (2011).
Goldstein, B. & Hird, S. N. Specification of the anteroposterior axis in Caenorhabditis elegans. Development 122, 1467–1474 (1996).
O'Connell, K. F., Maxwell, K. N. & White, J. G. The spd-2 gene is required for polarization of the anteroposterior axis and formation of the sperm asters in the Caenorhabditis elegans zygote. Dev. Biol. 222, 55–70 (2000).
Hamill, D. R., Severson, A. F., Carter, J. C. & Bowerman, B. Centrosome maturation and mitotic spindle assembly in C. elegans require SPD-5, a protein with multiple coiled-coil domains. Dev. Cell 3, 673–684 (2002).
Bienkowska, D. & Cowan, C. R. Centrosomes can initiate a polarity axis from any position within one-cell C. elegans embryos. Curr. Biol. 22, 583–589 (2012).
Fortin, S. M. et al. The PAM-1 aminopeptidase regulates centrosome positioning to ensure anterior-posterior axis specification in one-cell C. elegans embryos. Dev. Biol. 344, 992–1000 (2010).
Zonies, S., Motegi, F., Hao, Y. & Seydoux, G. Symmetry breaking and polarization of the C. elegans zygote by the polarity protein PAR-2. Development 137, 1669–1677 (2010).
McCloskey, R. J. & Kemphues, K. J. Deubiquitylation machinery is required for embryonic polarity in Caenorhabditis elegans. PLoS Genet. 8, e1003092 (2012).
Bois, J., Jülicher, F. & Grill, S. Pattern formation in active fluids. Phys. Rev. Lett. 106, 1–4 (2011).
Bray, D. & White, J. G. Cortical flow in animal cells. Science 239, 883–888 (1988).
Mayer, M., Depken, M., Bois, J. S., Jülicher, F. & Grill, S. W. Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467, 617–621 (2010).
Cheeks, R. J. et al. C. elegans PAR proteins function by mobilizing and stabilizing asymmetrically localized protein complexes. Curr. Biol. 14, 851–862 (2004).
Gonczy, P. Mechanisms of asymmetric cell division: flies and worms pave the way. Nature Rev. Mol. Cell Biol. 9, 355–366 (2008).
Schubert, C. M., Lin, R., de Vries, C. J., Plasterk, R. H. & Priess, J. R. MEX-5 and MEX-6 function to establish soma/germline asymmetry in early C. elegans embryos. Mol. Cell 5, 671–682 (2000).
Daniels, B. R., Dobrowsky, T. M., Perkins, E. M., Sun, S. X. & Wirtz, D. MEX-5 enrichment in the C. elegans early embryo mediated by differential diffusion. Development 137, 2579–2585 (2010).
Griffin, E. E., Odde, D. J. & Seydoux, G. Regulation of the MEX-5 gradient by a spatially segregated kinase/phosphatase cycle. Cell 146, 955–968 (2011).
Mello, C. C. et al. The PIE-1 protein and germline specification in C. elegans embryos. Nature 382, 710–712 (1996).
Tabara, H., Hill, R. J., Mello, C. C., Priess, J. R. & Kohara, Y. pos-1 encodes a cytoplasmic zinc-finger protein essential for germline specification in C. elegans. Development 126, 1–11 (1999).
Hyman, A. A. & Brangwynne, C. P. Beyond stereospecificity: liquids and mesoscale organization of cytoplasm. Dev. Cell 21, 14–16 (2012).
Updike, D. & Strome, S. P granule assembly and function in Caenorhabditis elegans germ cells. J. Androl. 31, 53–60 (2010).
Gallo, C. M., Wang, J. T., Motegi, F. & Seydoux, G. Cytoplasmic partitioning of P granule components is not required to specify the germline in C. elegans. Science 330, 1685–1689 (2010).
Hird, S. N., Paulsen, J. E. & Strome, S. Segregation of germ granules in living Caenorhabditis elegans embryos: cell-type-specific mechanisms for cytoplasmic localisation. Development 122, 1303–1312 (1996).
Hanazawa, M., Yonetani, M. & Sugimoto, A. PGL proteins self associate and bind RNPs to mediate germ granule assembly in C. elegans. J. Cell Biol. 192, 929–937 (2011).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Morton, D. G. et al. The Caenorhabditis elegans par-5 gene encodes a 14-3-3 protein required for cellular asymmetry in the early embryo. Dev. Biol. 241, 47–58 (2002).
Hurov, J., Watkins, J. & Piwnicaworms, H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr. Biol. 14, 736–741 (2004).
Suzuki, A. et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr. Biol. 14, 1425–1435 (2004).
Li, B., Kim, H., Beers, M. & Kemphues, K. Different domains of C. elegans PAR-3 are required at different times in development. Dev. Biol. 344, 745–757 (2010).
Morais-de-Sá, E. Mirouse, V. & Johnston, D. S. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141, 509–523 (2010).
Benton, R. & Johnston, D. S. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell 115, 691–704 (2003).
Atwood, S. X. & Prehoda, K. E. aPKC phosphorylates Miranda to polarize fate determinants during neuroblast asymmetric cell division. Curr. Biol. 19, 723–729 (2009).
Hurd, T. W. et al. Phosphorylation-dependent binding of 14-3-3 to the polarity protein Par3 regulates cell polarity in mammalian epithelia. Curr. Biol. 13, 2082–2090 (2003).
Nishi, Y., Rogers, E., Robertson, S. M. & Lin, R. Polo kinases regulate C. elegans embryonic polarity via binding to DYRK2-primed MEX-5 and MEX-6. Development 135, 687–697 (2008).
Dahan, I., Yearim, A., Touboul, Y. & Ravid, S. The tumor suppressor Lgl1 regulates NMII-A cellular distribution and focal adhesion morphology to optimize cell migration. Mol. Biol. Cell 23, 591–601 (2012).
Strand, D. et al. The Drosophila lethal(2)giant larvae tumor suppressor protein forms homo-oligomers and is associated with nonmuscle myosin II heavy chain. J. Cell Biol. 127, 1361–1373 (1994).
Guo, S. & Kemphues, K. J. A non-muscle myosin required for embryonic polarity in Caenorhabditis elegans. Nature 382, 455–458 (1996).
Chartier, N. T. et al. PAR-4/LKB1 mobilizes nonmuscle myosin through anillin to regulate C. elegans embryonic polarization and cytokinesis. Curr. Biol. 21, 259–269 (2011).
Garrard, S. M. et al. Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. EMBO J. 22, 1125–1133 (2003).
Acknowledgements
The authors thank the anonymous reviewers, their colleagues in the laboratory and at the Max Planck Institute of Molecular Cell Biology for critical comments, suggestions and discussion on the manuscript. They apologize to their colleagues in the field for omitting their work due to space limitations. Work in the authors laboratory was supported by the Max-Planck-Gesellschaft (MPG) and the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Actomyosin
-
Crosslinked network of actin and non-muscle myosin filaments, which can contract by sliding along each other and which underlie the plasma membrane of the cell.
- Advection
-
Passive transport of molecules by drag forces in liquids.
- Anisotropic
-
Directionally dependent difference in a property of the material.
- Cellular cortex
-
Layer on the inner side of the plasma membrane that mechanically supports the membrane. It consists of actin, non-muscle myosin (see actomyosin) and several actin-binding proteins.
- Centrioles
-
Cylindrical structure in most cells that is typically composed of nine triplets of microtubules. Involved in the organization of the centrosome and the mitotic spindle.
- Centrosome
-
Organelle that organizes microtubules in the cell and contains centrioles.
- Effector
-
A product or process that operates after instructions issued by, for example, a control protein such as a kinase.
- Fluorescence recovery after photobleaching
-
(FRAP). Microscope technique in which fluorescent molecules are studied in a defined area before and after fluorescent molecules are photobleached. The kinetics of fluorescence recovery is a readout of the mobility of the fluorescent molecules in space.
- Inverse fluorescence recovery after photobleaching
-
(iFRAP). A special type of FRAP, in which fluorescent molecules are photobleached outside the region that is analysed.
- Lateral diffusion
-
Two-dimensional diffusion (for example, on a surface); that is, the motion of molecules that causes flux and mixing of molecules from high to low concentrations.
- P granules
-
Complex assembly of heterogeneous RNA and protein molecules that form spheres in the cytoplasm of the germ line, oocytes and embryos of C. elegans. Although their function is not yet solved, they seem to contribute to future germ cell fate (known as germ granules in other organisms).
- Phase separation
-
A type of partitioning between thermodynamically similar regions of space (for example, P granules partition between two different kinds of liquids in the cytosol).
- Random walks
-
The stochastic moving path of a molecule travelling through a medium.
- Reaction–diffusion system
-
In biology a patterning process in which diffusive molecules (morphogens) undergo biochemical reactions (for example, phosphorylation, degradation and synthesis) and form well-defined spatial distributions.
- Steady state
-
Dynamic equilibrium in which reactions do not change the composition of mixtures.
- Tension
-
Pulling force that acts between solid objects.
- Triggers
-
A cue or signal that initiates a process transforming an unpolarized cell into a polarized cell.
- Viscous forces
-
A measure of resistance to flow. This describes the internal friction of a moving fluid (for example, fluids are more viscous than liquids).
Rights and permissions
About this article
Cite this article
Hoege, C., Hyman, A. Principles of PAR polarity in Caenorhabditis elegans embryos. Nat Rev Mol Cell Biol 14, 315–322 (2013). https://doi.org/10.1038/nrm3558
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3558