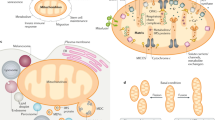
Key Points
-
The regulation of mitochondrial Ca2+ transport in physiological and pathological conditions is controlled by channels and exchangers that are located in the outer and inner mitochondrial membrane (OMM and IMM, respectively). Whereas the OMM is permeable to solutes and ions, Ca2+ transport across the IMM is highly regulated.
-
The strategic positioning of mitochondria in close proximity to Ca2+ release channels of the endoplasmic reticulum (ER) and the sarcoplasmic reticulum explains the high rate of mitochondrial Ca2+ uptake in stimulated cells, despite the low affinity of the mitochondrial Ca2+ uniporter (MCU), which mediates Ca2+ transfer through the IMM.
-
The sites of contacts between mitochondria and the ER or the sarcoendoplasmic reticulum (called mitochondria associated membranes (MAMs)) are microdomains of high Ca2+ concentration. Several proteins and chaperones are responsible for the maintenance of these structures and for efficient uptake of Ca2+ released from the ER and the sarcoendoplasmic reticulum.
-
Mitochondria act as intracellular Ca2+ buffers in the proximity of Ca2+ channels at the ER or the sarcoendoplasmic reticulum and the plasma membrane, thus affecting the Ca2+ feedback regulation of channel activity. Some cell types exhibit a defined distribution of mitochondria, and this affects the diffusion of Ca2+ waves through the cytosol.
-
Ca2+ accumulation into the mitochondria stimulates aerobic metabolism and thus ATP production by modulating the activity of the enzymes of the tricarboxylic acid cycle (TCA cycle) and other effectors.
-
Mitochondrial Ca2+ waves control cell fate. Increased levels of intracellular Ca2+ may trigger cell death by necrosis or apoptosis by causing the sustained or transient opening of a high-conductance channel of the IMM, termed the permeability transition pore (PTP). Conversely, low Ca2+ concentration levels in mitochondria cause a decrease in ATP production, which promotes pro-survival autophagy.
Abstract
During the past two decades calcium (Ca2+) accumulation in energized mitochondria has emerged as a biological process of utmost physiological relevance. Mitochondrial Ca2+ uptake was shown to control intracellular Ca2+ signalling, cell metabolism, cell survival and other cell-type specific functions by buffering cytosolic Ca2+ levels and regulating mitochondrial effectors. Recently, the identity of mitochondrial Ca2+ transporters has been revealed, opening new perspectives for investigation and molecular intervention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Clapham, D. E. Calcium signaling. Cell 131, 1047–1058 (2007).
Berridge, M. J., Bootman, M. D. & Roderick, H. L. Calcium signalling: dynamics, homeostasis and remodelling. Nature Rev. Mol. Cell Biol. 4, 517–529 (2003).
Ringer, S. A third contribution regarding the influence of the inorganic constituents of the blood on the ventricular contraction. J. Physiol. 4, 222–225 (1883).
Cobbold, P. H. & Cuthbertson, K. S. Calcium oscillations: phenomena, mechanisms and significance. Semin. Cell Biol. 1, 311–321 (1990).
Hajnoczky, G., Robb-Gaspers, L. D., Seitz, M. B. & Thomas, A. P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell 82, 415–424 (1995).
Dolmetsch, R. E., Xu, K. & Lewis, R. S. Calcium oscillations increase the efficiency and specificity of gene expression. Nature 392, 933–936 (1998).
Rizzuto, R. & Pozzan, T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. 86, 369–408 (2006).
Pozzan, T., Rizzuto, R., Volpe, P. & Meldolesi, J. Molecular and cellular physiology of intracellular calcium stores. Physiol. Rev. 74, 595–636 (1994).
Streb, H., Irvine, R. F., Berridge, M. J. & Schulz, I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature 306, 67–69 (1983).
Pinton, P., Pozzan, T. & Rizzuto, R. The Golgi apparatus is an inositol 1,4,5-trisphosphate-sensitive Ca2+ store, with functional properties distinct from those of the endoplasmic reticulum. EMBO J. 17, 5298–5308 (1998).
Calcraft, P. J. et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 (2009).
Berridge, M. J. The versatility and complexity of calcium signalling. Novartis Found. Symp. 239, 52–64 (2001).
Vasington, F. D. & Murphy, J. V. Ca ion uptake by rat kidney mitochondria and its dependence on respiration and phosphorylation. J. Biol. Chem. 237, 2670–2677 (1962).
Deluca, H. F. & Engstrom, G. W. Calcium uptake by rat kidney mitochondria. Proc. Natl Acad. Sci. USA 47, 1744–1750 (1961). Demonstrates, together with reference 13, that energized mitochondria accumulate Ca2+.
Lehninger, A. L., Rossi, C. S. & Greenawalt, J. W. Respiration-dependent accumulation of inorganic phosphate and Ca ions by rat liver mitochondria. Biochem. Biophys. Res. Commun. 10, 444–448 (1963).
Mitchell, P. & Moyle, J. Chemiosmotic hypothesis of oxidative phosphorylation. Nature 213, 137–139 (1967).
Stock, D., Leslie, A. G. & Walker, J. E. Molecular architecture of the rotary motor in ATP synthase. Science 286, 1700–1705 (1999).
Bernardi, P., Paradisi, V., Pozzan, T. & Azzone, G. F. Pathway for uncoupler-induced calcium efflux in rat liver mitochondria: inhibition by ruthenium red. Biochemistry 23, 1645–1651 (1984).
Mela, L. Inhibition and activation of calcium transport in mitochondria. Effect of lanthanides and local anesthetic drugs. Biochemistry 8, 2481–2486 (1969).
Bragadin, M., Pozzan, T. & Azzone, G. F. Activation energies and enthalpies during Ca2+ transport in rat liver mitochondria. FEBS Lett. 104, 347–351 (1979).
Crompton, M., Kunzi, M. & Carafoli, E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur. J. Biochem. 79, 549–558 (1977). Identifies a Na+/Ca2+ exchange mechanism by showing that addition of Na+ to mitochondria promotes Ca2+ efflux.
Pozzan, T., Bragadin, M. & Azzone, G. F. Disequilibrium between steady-state Ca2+ accumulation ratio and membrane potential in mitochondria. Pathway and role of Ca2+ efflux. Biochemistry 16, 5618–5625 (1977). Demonstrates that Ca2+ accumulation in mitochondria does not reach a thermodynamic equilibrium but depends on the kinetic balance of influx and efflux pathways.
Cox, D. A., Conforti, L., Sperelakis, N. & Matlib, M. A. Selectivity of inhibition of Na+-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J. Cardiovasc. Pharmacol. 21, 595–599 (1993).
Rizzuto, R., Simpson, A. W., Brini, M. & Pozzan, T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature 358, 325–327 (1992).
Miyawaki, A., Griesbeck, O., Heim, R. & Tsien, R. Y. Dynamic and quantitative Ca2+ measurements using improved cameleons. Proc. Natl Acad. Sci. USA 96, 2135–2140 (1999).
Nagai, T., Sawano, A., Park, E. S. & Miyawaki, A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc. Natl Acad. Sci. USA 98, 3197–3202 (2001).
Rizzuto, R., Brini, M., Murgia, M. & Pozzan, T. Microdomains with high Ca2+ close to IP3-sensitive channels that are sensed by neighboring mitochondria. Science 262, 744–747 (1993). Demonstrates, together with reference 24, that mitochondria rapidly accumulate Ca2+ when a cell is stimulated with an Ins(1,4,5)P 3 -generating agonist, despite the low affinity of the MCU.
Szabadkai, G., Simoni, A. M. & Rizzuto, R. Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum. J. Biol. Chem. 278, 15153–15161 (2003).
Montero, M. et al. Chromaffin-cell stimulation triggers fast millimolar mitochondrial Ca2+ transients that modulate secretion. Nature Cell Biol. 2, 57–61 (2000).
Rizzuto, R. et al. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280, 1763–1766 (1998). Demonstrates by using high resolution imaging the close contacts between the ER and mitochondria and importance of these contacts for rapid mitochondrial Ca2+ uptake.
Csordas, G., Thomas, A. P. & Hajnoczky, G. Quasi-synaptic calcium signal transmission between endoplasmic reticulum and mitochondria. EMBO J. 18, 96–108 (1999). Estimates that after cell stimulation the [Ca2+] in the ER–mitochondria microdomain is >20-fold higher than the increase in [Ca2+]c.
Szalai, G., Csordas, G., Hantash, B. M., Thomas, A. P. & Hajnoczky, G. Calcium signal transmission between ryanodine receptors and mitochondria. J. Biol. Chem. 275, 15305–15313 (2000).
Mannella, C. A., Buttle, K., Rath, B. K. & Marko, M. Electron microscopic tomography of rat-liver mitochondria and their interaction with the endoplasmic reticulum. Biofactors 8, 225–228 (1998).
Parekh, A. B. & Putney, J. W. Jr. Store-operated calcium channels. Physiol. Rev. 85, 757–810 (2005).
Glitsch, M. D., Bakowski, D. & Parekh, A. B. Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake. EMBO J. 21, 6744–6754 (2002).
David, G., Barrett, J. N. & Barrett, E. F. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J. Physiol. 509, 59–65 (1998).
Young, K. W., Bampton, E. T., Pinon, L., Bano, D. & Nicotera, P. Mitochondrial Ca2+ signalling in hippocampal neurons. Cell Calcium 43, 296–306 (2008).
Giacomello, M. et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol. Cell 38, 280–290 (2010).
Csordas, G. et al. Imaging interorganelle contacts and local calcium dynamics at the ER–mitochondrial interface. Mol. Cell 39, 121–132 (2010).
Madesh, M. & Hajnoczky, G. VDAC-dependent permeabilization of the outer mitochondrial membrane by superoxide induces rapid and massive cytochrome c release. J. Cell Biol. 155, 1003–1015 (2001).
Rapizzi, E. et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 159, 613–624 (2002).
Tan, W. & Colombini, M. VDAC closure increases calcium ion flux. Biochim. Biophys. Acta 1768, 2510–2515 (2007).
Rostovtseva, T. K. & Bezrukov, S. M. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J. Bioenerg. Biomembr. 40, 163–170 (2008).
Xu, X., Decker, W., Sampson, M. J., Craigen, W. J. & Colombini, M. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J. Membr. Biol. 170, 89–102 (1999).
De Stefani, D. et al. VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Cell Death Differ. 19, 267–273 (2012).
Roy, S. S. et al. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol. Cell 33, 377–388 (2009).
Roy, S. S., Ehrlich, A. M., Craigen, W. J. & Hajnoczky, G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 10, 1341–1347 (2009).
Cheng, E. H., Sheiko, T. V., Fisher, J. K., Craigen, W. J. & Korsmeyer, S. J. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513–517 (2003).
Kirichok, Y., Krapivinsky, G. & Clapham, D. E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 (2004). Demonstrates in isolated mitochondria that the MCU is a highly selective, inward rectifying Ca2+ channel.
De Stefani, D., Raffaello, A., Teardo, E., Szabo, I. & Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 (2011). As reference 51, identifies the MCU as the channel component of the 'mitochondrial uniporter' and demonstrates that the protein, inserted in lipid bilayers, is sufficient for channel activity with electrophysiological properties and inhibitor sensitivity of the uniporter.
Baughman, J. M. et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 (2011).
Perocchi, F. et al. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 467, 291–296 (2010). Identifies an essential component of the mitochondrial uniporter that is not the channel itself.
Gunter, T. E. & Pfeiffer, D. R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 258, C755–C786 (1990).
Bernardi, P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol. Rev. 79, 1127–1155 (1999).
Palty, R. et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl Acad. Sci. USA 107, 436–441 (2010).
Cai, X. & Lytton, J. Molecular cloning of a sixth member of the K+-dependent Na+/Ca2+ exchanger gene family, NCKX6. J. Biol. Chem. 279, 5867–5876 (2004).
Kim, B., Takeuchi, A., Koga, O., Hikida, M. & Matsuoka, S. Pivotal role of mitochondrial Na+-Ca2+ exchange in antigen receptor mediated Ca2+ signalling in DT40 and A20 B lymphocytes. J. Physiol. 590, 459–474 (2012).
Pfeiffer, D. R., Gunter, T. E., Eliseev, R., Broekemeier, K. M. & Gunter, K. K. Release of Ca2+ from mitochondria via the saturable mechanisms and the permeability transition. IUBMB Life 52, 205–212 (2001).
Ichas, F., Jouaville, L. S. & Mazat, J. P. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell 89, 1145–1153 (1997).
Elrod, J. W. et al. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 120, 3680–3687 (2010).
Barsukova, A. et al. Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur. J. Neurosci. 33, 831–842 (2011).
Barsukova, A. G., Bourdette, D. & Forte, M. Mitochondrial calcium and its regulation in neurodegeneration induced by oxidative stress. Eur. J. Neurosci. 34, 437–447 (2011).
Vance, J. E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 265, 7248–7256 (1990).
Rusinol, A. E., Cui, Z., Chen, M. H. & Vance, J. E. A unique mitochondria-associated membrane fraction from rat liver has a high capacity for lipid synthesis and contains pre-Golgi secretory proteins including nascent lipoproteins. J. Biol. Chem. 269, 27494–27502 (1994).
Bionda, C., Portoukalian, J., Schmitt, D., Rodriguez-Lafrasse, C. & Ardail, D. Subcellular compartmentalization of ceramide metabolism: MAM (mitochondria-associated membrane) and/or mitochondria? Biochem. J. 382, 527–533 (2004).
Lewin, T. M., Kim, J. H., Granger, D. A., Vance, J. E. & Coleman, R. A. Acyl-CoA synthetase isoforms 1, 4, and 5 are present in different subcellular membranes in rat liver and can be inhibited independently. J. Biol. Chem. 276, 24674–24679 (2001).
Lebiedzinska, M., Szabadkai, G., Jones, A. W., Duszynski, J. & Wieckowski, M. R. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int. J. Biochem. Cell Biol. 41, 1805–1816 (2009).
Giorgi, C., De Stefani, D., Bononi, A., Rizzuto, R. & Pinton, P. Structural and functional link between the mitochondrial network and the endoplasmic reticulum. Int. J. Biochem. Cell Biol. 41, 1817–1827 (2009).
Csordas, G. et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell Biol. 174, 915–921 (2006).
Murgia, M., Giorgi, C., Pinton, P. & Rizzuto, R. Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J. Mol. Cell. Cardiol. 46, 781–788 (2009).
Rizzuto, R. et al. Ca2+ transfer from the ER to mitochondria: when, how and why. Biochim. Biophys. Acta 1787, 1342–1351 (2009).
de Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008). Identifies MFN2 as the ER–mitochondria tether that allows the formation of the Ca2+ signalling microdomain.
Szabadkai, G. et al. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 16, 59–68 (2004).
Saotome, M. et al. Bidirectional Ca2+-dependent control of mitochondrial dynamics by the Miro GTPase. Proc. Natl Acad. Sci. USA 105, 20728–20733 (2008).
Frieden, M. et al. Ca2+ homeostasis during mitochondrial fragmentation and perinuclear clustering induced by hFis1. J. Biol. Chem. 279, 22704–22714 (2004).
Cerqua, C. et al. Trichoplein/mitostatin regulates endoplasmic reticulum-mitochondria juxtaposition. EMBO Rep. 11, 854–860 (2010).
Garcia-Perez, C., Schneider, T. G., Hajnoczky, G. & Csordas, G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am. J. Physiol. Heart Circ. Physiol. 301, H1907–H1915 (2011).
Hayashi, T., Rizzuto, R., Hajnoczky, G. & Su, T. P. MAM: more than just a housekeeper. Trends Cell Biol. 19, 81–88 (2009).
de Brito, O. M. & Scorrano, L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J. 29, 2715–2723 (2010).
Hayashi, T. & Su, T. P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131, 596–610 (2007).
Myhill, N. et al. The subcellular distribution of calnexin is mediated by PACS-2. Mol. Biol. Cell 19, 2777–2788 (2008).
Camacho, P. & Lechleiter, J. D. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell 82, 765–771 (1995).
Li, Y. & Camacho, P. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J. Cell Biol. 164, 35–46 (2004).
Roderick, H. L., Lechleiter, J. D. & Camacho, P. Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J. Cell Biol. 149, 1235–1248 (2000).
Szabadkai, G. et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 175, 901–911 (2006).
Giorgi, C. et al. PML regulates apoptosis at endoplasmic reticulum by modulating calcium release. Science 330, 1247–1251 (2010).
Bezprozvanny, I., Watras, J. & Ehrlich, B. E. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature 351, 751–754 (1991).
Jouaville, L. S., Ichas, F., Holmuhamedov, E. L., Camacho, P. & Lechleiter, J. D. Synchronization of calcium waves by mitochondrial substrates in Xenopus laevis oocytes. Nature 377, 438–441 (1995).
Hajnoczky, G., Hager, R. & Thomas, A. P. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+. J. Biol. Chem. 274, 14157–14162 (1999).
Boitier, E., Rea, R. & Duchen, M. R. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell Biol. 145, 795–808 (1999). Demonstrates, together with references 88 and 89, that mitochondria alter the feedback control of Ca2+ on the Ins(1,4,5)P 3 R by clearing Ca2+ at the mouth of the receptor and thereby modify the spatiotemporal properties of cytosolic Ca2+ waves.
Pacher, P., Thomas, A. P. & Hajnoczky, G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc. Natl Acad. Sci. USA 99, 2380–2385 (2002).
Quintana, A. et al. Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation. EMBO J. 30, 3895–3912 (2011).
Hoth, M., Fanger, C. M. & Lewis, R. S. Mitochondrial regulation of store-operated calcium signaling in T lymphocytes. J. Cell Biol. 137, 633–648 (1997).
Hoth, M., Button, D. C. & Lewis, R. S. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc. Natl Acad. Sci. USA 97, 10607–10612 (2000).
Singaravelu, K. et al. Mitofusin 2 regulates STIM1 migration from the Ca2+ store to the plasma membrane in cells with depolarized mitochondria. J. Biol. Chem. 286, 12189–12201 (2011).
Gilabert, J. A. & Parekh, A. B. Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca2+ current I(CRAC). EMBO J. 19, 6401–6407 (2000). Shows, together with reference 93, that mitochondrial Ca2+ uptake regulates the activity of store-operated Ca2+ channels of the plasma membrane.
Gilabert, J. A., Bakowski, D. & Parekh, A. B. Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx. EMBO J. 20, 2672–2679 (2001).
Pivovarova, N. B., Hongpaisan, J., Andrews, S. B. & Friel, D. D. Depolarization-induced mitochondrial Ca accumulation in sympathetic neurons: spatial and temporal characteristics. J. Neurosci. 19, 6372–6384 (1999).
Tang, Y. & Zucker, R. S. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron 18, 483–491 (1997).
Billups, B. & Forsythe, I. D. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J. Neurosci. 22, 5840–5847 (2002).
Medler, K. & Gleason, E. L. Mitochondrial Ca2+ buffering regulates synaptic transmission between retinal amacrine cells. J. Neurophysiol. 87, 1426–1439 (2002).
Talbot, J. D., David, G. & Barrett, E. F. Inhibition of mitochondrial Ca2+ uptake affects phasic release from motor terminals differently depending on external Ca2+. J. Neurophysiol. 90, 491–502 (2003).
David, G. & Barrett, E. F. Mitochondrial Ca2+ uptake prevents desynchronization of quantal release and minimizes depletion during repetitive stimulation of mouse motor nerve terminals. J. Physiol. 548, 425–438 (2003).
Sung, J. Y. et al. WAVE1 controls neuronal activity-induced mitochondrial distribution in dendritic spines. Proc. Natl Acad. Sci. USA 105, 3112–3116 (2008).
Kang, J. S. et al. Docking of axonal mitochondria by syntaphilin controls their mobility and affects short-term facilitation. Cell 132, 137–148 (2008).
Macaskill, A. F. et al. Miro1 is a calcium sensor for glutamate receptor-dependent localization of mitochondria at synapses. Neuron 61, 541–555 (2009).
Chang, K. T., Niescier, R. F. & Min, K. T. Mitochondrial matrix Ca2+ as an intrinsic signal regulating mitochondrial motility in axons. Proc. Natl Acad. Sci. USA 108, 15456–15461 (2011).
Fluegge, D. et al. Mitochondrial Ca2+ mobilization is a key element in olfactory signaling. Nature Neurosci. 15, 754–762 (2012).
Sheng, Z. H. & Cai, Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nature Rev. Neurosci. 13, 77–93 (2012).
Voronina, S. et al. Correlation of NADH and Ca2+ signals in mouse pancreatic acinar cells. J. Physiol. 539, 41–52 (2002).
Cancela, J. M., Van Coppenolle, F., Galione, A., Tepikin, A. V. & Petersen, O. H. Transformation of local Ca2+ spikes to global Ca2+ transients: the combinatorial roles of multiple Ca2+ releasing messengers. EMBO J. 21, 909–919 (2002).
Sutton, R. et al. Signal transduction, calcium and acute pancreatitis. Pancreatology 3, 497–505 (2003).
Murphy, J. A., et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 135, 632–641 (2008).
Tinel, H. et al. Active mitochondria surrounding the pancreatic acinar granule region prevent spreading of inositol trisphosphate-evoked local cytosolic Ca2+ signals. EMBO J. 18, 4999–5008 (1999).
McCormack, J. G., Halestrap, A. P. & Denton, R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 70, 391–425 (1990).
Hansford, R. G. Physiological role of mitochondrial Ca2+ transport. J. Bioenerg. Biomembr. 26, 495–508 (1994).
McCormack, J. G. & Denton, R. M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem. J. 180, 533–544 (1979). Demonstrates the stimulatory effect of Ca2+ on a Krebs cycle dehydrogenase.
Jouaville, L. S., Pinton, P., Bastianutto, C., Rutter, G. A. & Rizzuto, R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl Acad. Sci. USA 96, 13807–13812 (1999).
Brini, M. et al. A calcium signaling defect in the pathogenesis of a mitochondrial DNA inherited oxidative phosphorylation deficiency. Nature Med. 5, 951–954 (1999).
Visch, H. J. et al. Inhibition of mitochondrial Na+-Ca2+ exchange restores agonist-induced ATP production and Ca2+ handling in human complex I deficiency. J. Biol. Chem. 279, 40328–40336 (2004).
Voronina, S. G. et al. Dynamic changes in cytosolic and mitochondrial ATP levels in pancreatic acinar cells. Gastroenterology 138, 1976–1987 (2010).
Balaban, R. S. The role of Ca2+ signaling in the coordination of mitochondrial ATP production with cardiac work. Biochim. Biophys. Acta 1787, 1334–1341 (2009).
Lasorsa, F. M. et al. Recombinant expression of the Ca2+-sensitive aspartate/glutamate carrier increases mitochondrial ATP production in agonist-stimulated Chinese hamster ovary cells. J. Biol. Chem. 278, 38686–38692 (2003).
Contreras, L. et al. Ca2+ activation kinetics of the two aspartate-glutamate mitochondrial carriers, aralar and citrin: role in the heart malate-aspartate NADH shuttle. J. Biol. Chem. 282, 7098–7106 (2007).
Satrustegui, J., Pardo, B. & Del Arco, A. Mitochondrial transporters as novel targets for intracellular calcium signaling. Physiol. Rev. 87, 29–67 (2007).
Orrenius, S., Zhivotovsky, B. & Nicotera, P. Regulation of cell death: the calcium-apoptosis link. Nature Rev. Mol. Cell Biol. 4, 552–565 (2003).
O'Rourke, B. Pathophysiological and protective roles of mitochondrial ion channels. J. Physiol. 529, 23–36 (2000).
Di Lisa, F. & Bernardi, P. A. CaPful of mechanisms regulating the mitochondrial permeability transition. J. Mol. Cell. Cardiol. 46, 775–780 (2009).
Nicholls, D. G. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim. Biophys. Acta 1787, 1416–1424 (2009).
Pivovarova, N. B. & Andrews, S. B. Calcium-dependent mitochondrial function and dysfunction in neurons. FEBS J. 277, 3622–3636 (2010).
Bano, D. et al. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120, 275–285 (2005).
Rasola, A. & Bernardi, P. Mitochondrial permeability transition in Ca2+-dependent apoptosis and necrosis. Cell Calcium 50, 222–233 (2011).
Szalai, G., Krishnamurthy, R. & Hajnoczky, G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 18, 6349–6361 (1999).
Jacobson, J. & Duchen, M. R. Mitochondrial oxidative stress and cell death in astrocytes--requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 115, 1175–1188 (2002).
Davidson, S. M., Yellon, D. M., Murphy, M. P. & Duchen, M. R. Slow calcium waves and redox changes precede mitochondrial permeability transition pore opening in the intact heart during hypoxia and reoxygenation. Cardiovasc. Res. 93, 445–453 (2012).
Scorrano, L. et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev. Cell 2, 55–67 (2002).
Cereghetti, G. M. et al. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc. Natl Acad. Sci. USA 105, 15803–15808 (2008).
Cribbs, J. T. & Strack, S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 8, 939–944 (2007).
Frank, S. et al. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev. Cell 1, 515–525 (2001).
Martinou, J. C. & Youle, R. J. Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ. 13, 1291–1295 (2006).
Pinton, P. et al. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J. Cell Biol. 148, 857–862 (2000).
Pinton, P. et al. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 20, 2690–2701 (2001).
Palmer, A. E., Jin, C., Reed, J. C. & Tsien, R. Y. Bcl-2-mediated alterations in endoplasmic reticulum Ca2+ analyzed with an improved genetically encoded fluorescent sensor. Proc. Natl Acad. Sci. USA 101, 17404–17409 (2004).
Foyouzi-Youssefi, R. et al. Bcl-2 decreases the free Ca2+ concentration within the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 97, 5723–5728 (2000).
Scorrano, L. et al. BAX and BAK regulation of endoplasmic reticulum Ca2+: a control point for apoptosis. Science 300, 135–139 (2003). Demonstrates, together with references 133 and 142, the role of [Ca2+]mt increases in sensitizing mitochondria to apoptotic challenges.
Rimessi, A. et al. Intramitochondrial calcium regulation by the FHIT gene product sensitizes to apoptosis. Proc. Natl Acad. Sci. USA 106, 12753–12758 (2009).
Jones, R. G. et al. The proapoptotic factors Bax and Bak regulate T cell proliferation through control of endoplasmic reticulum Ca2+ homeostasis. Immunity 27, 268–280 (2007).
White, C. et al. The endoplasmic reticulum gateway to apoptosis by Bcl-XL modulation of the InsP3R. Nature Cell Biol. 7, 1021–1028 (2005).
Hanson, C. J., Bootman, M. D., Distelhorst, C. W., Wojcikiewicz, R. J. & Roderick, H. L. Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium 44, 324–338 (2008).
Chen, R. et al. Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 166, 193–203 (2004).
Bassik, M. C., Scorrano, L., Oakes, S. A., Pozzan, T. & Korsmeyer, S. J. Phosphorylation of BCL-2 regulates ER Ca2+ homeostasis and apoptosis. EMBO J. 23, 1207–1216 (2004).
Oakes, S. A. et al. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc. Natl Acad. Sci. USA 102, 105–110 (2005).
Rong, Y. P. et al. The BH4 domain of Bcl-2 inhibits ER calcium release and apoptosis by binding the regulatory and coupling domain of the IP3 receptor. Proc. Natl Acad. Sci. USA 106, 14397–14402 (2009).
Rong, Y. P. et al. Targeting Bcl-2-IP3 receptor interaction to reverse Bcl-2's inhibition of apoptotic calcium signals. Mol. Cell 31, 255–265 (2008).
Chami, M., Ferrari, D., Nicotera, P., Paterlini-Brechot, P. & Rizzuto, R. Caspase-dependent alterations of Ca2+ signaling in the induction of apoptosis by hepatitis B virus X protein. J. Biol. Chem. 278, 31745–31755 (2003).
Campanella, M. et al. The coxsackievirus 2B protein suppresses apoptotic host cell responses by manipulating intracellular Ca2+ homeostasis. J. Biol. Chem. 279, 18440–18450 (2004).
Baines, C. P. et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 434, 658–662 (2005).
Hoyer-Hansen, M. et al. Control of macroautophagy by calcium, calmodulin-dependent kinase kinase-β, and Bcl-2. Mol. Cell 25, 193–205 (2007).
Gastaldello, A., Callaghan, H., Gami, P. & Campanella, M. Ca2+-dependent autophagy is enhanced by the pharmacological agent PK11195. Autophagy 6, 607–613 (2010).
Williams, A. et al. Novel targets for Huntington's disease in an mTOR-independent autophagy pathway. Nature Chem. Biol. 4, 295–305 (2008).
Sarkar, S. et al. Lithium induces autophagy by inhibiting inositol monophosphatase. J. Cell Biol. 170, 1101–1111 (2005).
Vicencio, J. M. et al. The inositol 1,4,5-trisphosphate receptor regulates autophagy through its interaction with Beclin 1. Cell Death Differ. 16, 1006–1017 (2009).
Criollo, A. et al. Regulation of autophagy by the inositol trisphosphate receptor. Cell Death Differ. 14, 1029–1039 (2007).
Cardenas, C., et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283 (2010). Shows that Ins(1,4,5)P 3 -mediated Ca2+ transfer to mitochondria exerts an inhibitory effect on macroautophagy.
Khan, M. T. & Joseph, S. K. Role of inositol trisphosphate receptors in autophagy in DT40 cells. J. Biol. Chem. 285, 16912–16920 (2010).
Decuypere, J. P. et al. Ins(1,4,5)P3 receptor-mediated Ca2+ signaling and autophagy induction are interrelated. Autophagy 7, 1472–1489 (2011).
Panfili, E. et al. Specific inhibition of mitochondrial Ca2+ transport by antibodies directed to the Ca2+-binding glycoprotein. Nature 264, 185–186 (1976).
Beutner, G., Sharma, V. K., Giovannucci, D. R., Yule, D. I. & Sheu, S. S. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 276, 21482–21488 (2001).
Ryu, S. Y., Beutner, G., Kinnally, K. W., Dirksen, R. T. & Sheu, S. S. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J. Biol. Chem. 286, 21324–21329 (2011).
Trenker, M., Malli, R., Fertschai, I., Levak-Frank, S. & Graier, W. F. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nature Cell Biol. 9, 445–452 (2007).
Brookes, P. S. et al. UCPs-unlikely calcium porters. Nature Cell Biol. 10, 1235–1237 (2008).
De Marchi, U., Castelbou, C. & Demaurex, N. Uncoupling protein 3 (UCP3) modulates the activity of Sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by decreasing mitochondrial ATP production. J. Biol. Chem. 286, 32533–32541 (2011).
Jiang, D., Zhao, L. & Clapham, D. E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science 326, 144–147 (2009).
Dimmer, K. S. et al. LETM1, deleted in Wolf–Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability. Hum. Mol. Genet. 17, 201–214 (2008).
Brand, M. D. The stoichiometry of the exchange catalysed by the mitochondrial calcium/sodium antiporter. Biochem. J. 229, 161–166 (1985).
Dash, R. K. & Beard, D. A. Analysis of cardiac mitochondrial Na+-Ca2+ exchanger kinetics with a biophysical model of mitochondrial Ca2+ handling suggests a 3:1 stoichiometry. J. Physiol. 586, 3267–3285 (2008).
Pagliarini, D. J. et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell 134, 112–123 (2008).
Acknowledgements
The experimental work in the authors' laboratory is supported by grants from the Italian Ministry of Health, the Italian Ministry of Education, Universities and Research, the European Union (ERC mitoCalcium, no. 294777 and FP7 'MyoAGE', no. 223576), the US National Institues of Health (NIH; grant #1P01AG025532-01A1), the Cariparo Foundation (Padua), the Italian Association for Cancer Research (AIRC) and Telethon-Italy (GPP10005A and GGP11082B).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Related links
Glossary
- Immunological synapse
-
The site of functional apposition between an antigen-presenting cell (APC) and a T cell. During antigen presentation, the T cell undergoes a gross morphological rearrangement, with T cell receptors, adhesion molecules, cytoskeletal elements and organelles (including mitochondria) spatially relocating at clusters at the contact site with the APC.
- Energized mitochondria
-
When oxidizable substrates are provided to mitochondria, electrons are fed into the respiratory chain, which couples electron flow to proton pumps across the inner mitochondrial membrane. An electrochemical proton gradient is established that drives ATP synthesis and provides the thermodynamic force for Ca2+ accumulation in these energized mitochondria.
- Necrosis
-
A common form of cell death that frequently results from toxic injury, hypoxia or stress. Necrosis involves cell swelling, dysregulation of cell-membrane ion and water fluxes, mitochondrial swelling and the eventual release of cell contents into the interstitium. This form of cell death usually causes tissue inflammation.
- Autophagy
-
A cellular process (also known as macroautophagy) that mediates the bulk degradation of cytosolic components. Molecules and organelles are surrounded by a double-membrane vesicle (the autophagosome). After fusion with a lysosome, an autolysosome (also known as autophagolysosome) is formed, and the content is degraded by lysosomal hydrolases. Basal autophagy has a function in quality control, whereas higher levels of autophagy are induced during starvation and other stress conditions.
- Chemiosmotic theory
-
The energy that is required for the endergonic synthesis of ATP by the F1F0 ATPase is provided by the electrochemical gradient that is generated by the respiratory chain across the inner mitochondrial membrane. In respiring mitochondria, reducing equivalents are transported by carrier molecules (such as NADH and FADH2) to the electron transport chain, whereas matrix protons are transported outwards. Re-equilibration of H+ into the matrix down its electrochemical gradient is coupled to ATP production.
- Electrochemical proton gradient
-
Gradient generated by the activity of the electron transport chain that translocates H+ across the inner mitochondrial membrane. It is composed of a membrane potential difference (ΔΨ) and a cation concentration difference (ΔpH), but the ΔΨ component is predominant. The electrochemical proton gradient represents the driving force for H+ entry and thus ATP synthesis.
- Membrane potential
-
The charge difference (measured in mV) between the two surfaces of a biological membrane that arises from the different concentrations of ions such as H+, Na+ or K+ on either side. The Na+/K+-ATPase creates a membrane potential by using the energy stored in ATP to maintain a low concentration of Na+ and a high concentration of K+ inside the cell, and a high concentration of Na+ and a low concentration of K+ on the outside.
- Nernst equation
-

Calculates the equilibrium potential for an ion on the basis of the charge of the ion and the concentration gradient.
Veq is the equilibrium potential, R the universal gas constant, T the temperature, z the valence of the ion, F the Faraday's constant and [CATz+] is the concentration of any cation.
- Mitochondrial Ca2+ uniporter
-
(MCU). The inner mitochondrial membrane channel responsible for mitochondrial Ca2+ uptake. Early biochemical work in the1960s showed that energized mitochondria rapidly accumulate Ca2+ via an electrogenic mechanism with a net charge transfer of 2. Because of the lack of evidence for other ions being co-transported or exchanged during this process, this channel has been defined as a 'uniporter'.
- Na+/Ca2+ exchangers
-
(NCX). Antiporters that exchange Na+ with Ca2+ at the membrane. Although fully reversible, the function of NCX at the plasma membrane is to extrude Ca2+ from the cytosol, while Na+ enters down its concentration gradient. At the inner mitochondrial membrane, mitochondrial Ca2+ is extruded while Na+ enters into the matrix.
- H+/Ca2+ exchangers
-
(HCX). Na+-independent antiporter membrane protein that permits the extrusion of Ca2+ from the matrix and the entrance of H+.
- Aequorin
-
Ca2+-sensitive photoprotein isolated from luminescent jellyfish that is used to detect the Ca2+ content in different subcellular compartments.
- Inositol-1,4,5-trisphosphate-sensitive channels
-
(Ins(1,4,5)P3-sensitive channels). Ca2+-selective channels that are activated by the second messenger Ins(1,4,5)P3 and are mainly located in the endoplasmic reticulum (ER) membrane. Given the difference in the Ca2+ concentration between the ER lumen and the cytosol, opening of this channel results in a transient increase in intracellular Ca2+ concentration.
- Ryanodine-sensitive channels
-
Ca2+-selective channels that are mainly located in the membrane of the sarcoplasmic reticulum of skeletal muscle and heart, but they are also expressed in the endoplasmic reticulum of other tissues (in particular in the brain). They are activated by Ca2+ and blocked by the plant alkaloid ryanodine.
- Voltage-operated channels
-
Plasma membrane located, highly selective Ca2+ channels that are activated by membrane depolarization. They are divided in several different subfamilies on the basis of their subunit composition, which also reflects their different tissue distribution, Ca2+-current type and pharmacological profile. Present nomenclature uses a numerical system (that is, Cav1.x, Cav2.x and Cav3.x), but older nomenclature uses an alphabetical system (that is, L-, P/Q-, R-, N- and T-type) according to their Ca2+-current features.
- Store-operated channels
-
Highly selective Ca2+ channels located at the plasma membrane that open in response to depletion of internal Ca2+ stores. Upon endoplasmic reticulum (ER) Ca2+ depletion, the Ca2+ sensors of the STIM family, which are located at the ER surface, cause the opening of plasma membrane store-operated channels, termed ORAI. The ORAI family includes three members (ORAI1, ORAI2 and ORAI3), and the STIM family is composed of STIM1 and STIM2.
- Voltage-dependent anion channels
-
(VDACs). Abundant and highly conserved proteins of the outer mitochondrial membrane. VDAC exists in three isoforms in mammals (termed VDAC1, VDAC2 and VDAC3) and is permeable to many solutes below 5 kDa.
- Mitocarta
-
Inventory of 1098 mouse genes encoding proteins that are likely to be localized at mitochondria. The data were generated by mass spectrometry of mitochondria isolated from 14 tissues and protein localization was confirmed by large-scale tagging of candidate proteins with GFP and microscopy. The data were integrated with six other genome-scale data sets of mitochondrial localization.
- Permeability transition pore
-
(PTP). High conductance inner mitochondrial membrane (IMM) channel that requires a permissive load of matrix Ca2+ for opening and is specifically inhibited by cyclosporin A. Persistent PTP opening irreversibly commits cells to death by causing IMM depolarization (which blocks of oxidative phosphorylation and reactive oxygen species production), matrix swelling and cristae unfolding and results in the release of stored Ca2+ and of apoptogenic proteins.
- Cyclophilin D
-
(CYPD). Major inducer of the opening of the permeability transition pore (PTP). CYPD binds to the inner mitochondrial membrane (IMM) in a process that is regulated by Ca2+, inorganic phosphate and reactive oxygen species. This interaction is prevented by cyclosporin A and by other CYPD-interacting molecules that are usually described as PTP inhibitors.
- Ca2+ buffers
-
Molecules and organelles that bind or sequester Ca2+ and thereby act as Ca2+ sponges and modulate Ca2+ concentration in subcellular subdomains.
- ORAI channels
-
Pore-forming subunits of store-operated channels. They are predicted to have four transmembrane domains and three family members have been identified thus far.
- Store-operated Ca2+ entry (SOCE)
-
The activation of a Ca2+ channel in the plasma membrane in response to the depletion of Ca2+ levels in the endoplasmic reticulum. SOCE is also known as capacitative Ca2+ entry.
- Ca2+-dependent inactivation
-
(CDI). The process whereby increases of cytosolic Ca2+ leads to inactivation of the Ca2+ release-activated Ca2+ (CRAC) channel.
- EF-hand
-
A highly conserved Ca2+-binding domain comprising two helices (that is, E and F after the 5th and 6th helices of parvalbumin) that are linked by a short acidic Ca2+-binding loop.
- Excitoxicity
-
The pathological process in which neurons undergo cell death caused by excessive stimulation. Classically, overactivation of glutamate receptors leads to excessive Ca2+ entry, which causes activation of enzymes, such as calpains, that break down key cellular components leading to cell death.
- N-methyl-D-aspartate receptors
-
(NMDRs). Subtypes of plasma membrane ionotropic glutamate receptors that are mainly involved in memory formation and excitotoxicity. Ionotropic glutamate receptors are classified on the basis of their selective agonists (such as NMDA, AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and kainate).
- L-type Ca2+ channels
-
Voltage-operated channels that belong to the Cav1.x family and that are activated by strong depolarizations. They are long-lasting and are inhibited by dihydropyridines and phenylalkylamines.
- NLRP3 inflammasome
-
(Nucleotide-binding oligomerization domain- Leu-rich repeat- and pyrin domain-containing 3 inflammasome). A high molecular weight signalling complex that consists of a family of cytoplasmic proteins, the NLRP proteins. This complex recruits pro-caspase 1, which is then activated by autocatalytic cleavage. Active caspase 1 catalyses the cleavage of pro-interleukin1β, pro-IL-18 and pro-IL-33, resulting in the secretion of biologically active forms of these pro-inflammatory cytokines.
Rights and permissions
About this article
Cite this article
Rizzuto, R., De Stefani, D., Raffaello, A. et al. Mitochondria as sensors and regulators of calcium signalling. Nat Rev Mol Cell Biol 13, 566–578 (2012). https://doi.org/10.1038/nrm3412
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3412
This article is cited by
-
Real-time imaging of mitochondrial redox reveals increased mitochondrial oxidative stress associated with amyloid β aggregates in vivo in a mouse model of Alzheimer’s disease
Molecular Neurodegeneration (2024)
-
CGI1746 targets σ1R to modulate ferroptosis through mitochondria-associated membranes
Nature Chemical Biology (2024)
-
Disruption of MAM integrity in mutant FUS oligodendroglial progenitors from hiPSCs
Acta Neuropathologica (2024)
-
Alpha-Asarone Ameliorates Neurological Dysfunction of Subarachnoid Hemorrhagic Rats in Both Acute and Recovery Phases via Regulating the CaMKII-Dependent Pathways
Translational Stroke Research (2024)
-
Mitochondria in endothelial cells angiogenesis and function: current understanding and future perspectives
Journal of Translational Medicine (2023)