Key Points
-
The glucose transporter GLUT4 facilitates insulin-stimulated glucose uptake into muscle and adipose tissue. Defects in glucose uptake represent an early step in the development of type 2 diabetes mellitus.
-
GLUT4 is distributed between the plasma membrane, the trans-Golgi network (TGN), endosomes and small heterogeneous vesicles that consist of sorting intermediates of the endosomal system and GLUT4 storage vesicles (GSVs). Treatment of muscle or adipose cells with insulin stimulates exocytosis of GLUT4 from multiple intracellular compartments, which results in increased GLUT4 levels at the plasma membrane for shuttling of glucose into the cell.
-
In the absence of insulin, at least 50% of GLUT4 is sequestered in specialized immobile GSVs. Stimulation with insulin results in regulated exocytosis of GSVs.
-
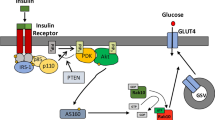
GSV mobilization, targeting and fusion at the plasma membrane requires coordinated control of the trafficking machinery by insulin. Trafficking proteins that are regulated by insulin include multiple small GTPases of the RAB, RAL and RHO families, molecular motor proteins, the exocyst complex and SNARE regulatory proteins.
-
Total internal reflection fluorescence microscopy (TIRFM) studies performed in live adipocytes have helped to elucidate the mechanisms by which insulin regulates specific trafficking proteins during GLUT4 exocytosis.
Abstract
Despite daily fasting and feeding, plasma glucose levels are normally maintained within a narrow range owing to the hormones insulin and glucagon. Insulin increases glucose uptake into fat and muscle cells through the regulated trafficking of vesicles that contain glucose transporter type 4 (GLUT4). New insights into insulin signalling reveal that phosphorylation events initiated by the insulin receptor regulate key GLUT4 trafficking proteins, including small GTPases, tethering complexes and the vesicle fusion machinery. These proteins, in turn, control GLUT4 movement through the endosomal system, formation and retention of specialized GLUT4 storage vesicles and targeted exocytosis of these vesicles. Understanding these processes may help to explain the development of insulin resistance in type 2 diabetes and provide new potential therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Chieregatti, E. & Meldolesi, J. Regulated exocytosis: new organelles for non-secretory purposes. Nature Rev. Mol. Cell Biol. 6, 181–187 (2005).
Lowenstein, C. J., Morrell, C. N. & Yamakuchi, M. Regulation of Weibel–Palade body exocytosis. Trends Cardiovasc. Med. 15, 302–308 (2005).
Brown, D. The ins and outs of aquaporin-2 trafficking. Am. J. Physiol. Renal Physiol. 284, F893–F901 (2003).
Pfenninger, K. H. et al. Regulation of membrane expansion at the nerve growth cone. J. Cell Sci. 116, 1209–1217 (2003).
Pessin, J. E. & Saltiel, A. R. Signaling pathways in insulin action: molecular targets of insulin resistance. J. Clin. Invest. 106, 165–169 (2000).
Saltiel, A. R. & Kahn, C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414, 799–806 (2001).
Thorens, B. & Mueckler, M. Glucose transporters in the 21st century. Am. J. Physiol. Endocrinol. Metab. 298, e141–e145 (2010).
Slot, J. W., Geuze, H. J., Gigengack, S., Lienhard, G. E. & James, D. E. Immuno-localization of the insulin regulatable glucose transporter in brown adipose tissue of the rat. J. Cell Biol. 113, 123–135 (1991). The first to examine the localization of GLUT4 in intact adipocytes by immunostaining, showing that the transporter is distributed between the TGN, endosomes and small tubulo-vesicular compartments.
Martin, S. et al. Effects of insulin on intracellular GLUT4 vesicles in adipocytes: evidence for a secretory mode of regulation. J. Cell Sci. 113, 3427–3438 (2000).
Bogan, J. S. & Kandror, K. V. Biogenesis and regulation of insulin-responsive vesicles containing GLUT4. Curr. Opin. Cell Biol. 22, 506–512 (2010).
Bryant, N. J., Govers, R. & James, D. E. Regulated transport of the glucose transporter GLUT4. Nature Rev. Mol. Cell Biol. 3, 267–277 (2002).
Kraegen, E. W., James, D. E., Jenkins, A. B. & Chisholm, D. J. Dose-response curves for in vivo insulin sensitivity in individual tissues in rats. Am. J. Physiol. 248, e353–e362 (1985).
Zisman, A. et al. Targeted disruption of the glucose transporter 4 selectively in muscle causes insulin resistance and glucose intolerance. Nature Med. 6, 924–928 (2000).
Abel, E. D. et al. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature 409, 729–733 (2001).
Lazar, D. F. et al. Mitogen-activated protein kinase kinase inhibition does not block the stimulation of glucose utilization by insulin. J. Biol. Chem. 270, 20801–20807 (1995).
Polak, P. et al. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 8, 399–410 (2008).
Cai, H., Reinisch, K. & Ferro-Novick, S. Coats, tethers, Rabs, and SNAREs work together to mediate the intracellular destination of a transport vesicle. Dev. Cell 12, 671–682 (2007).
Yip, M. F. et al. CaMKII-mediated phosphorylation of the myosin motor Myo1c is required for insulin-stimulated GLUT4 translocation in adipocytes. Cell Metab. 8, 384–398 (2008).
Myers, M. G. Jr & White, M. F. Insulin signal transduction and the IRS proteins. Annu. Rev. Pharmacol. Toxicol. 36, 615–658 (1996).
Lee, J. & Pilch, P. F. The insulin receptor: structure, function, and signaling. Am. J. Physiol. 266, C319–C334 (1994).
Whiteman, E. L., Cho, H. & Birnbaum, M. J. Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 (2002).
Katome, T. et al. Use of RNA interference-mediated gene silencing and adenoviral overexpression to elucidate the roles of AKT/protein kinase B isoforms in insulin actions. J. Biol. Chem. 278, 28312–28323 (2003).
Clarke, J. F., Young, P. W., Yonezawa, K., Kasuga, M. & Holman, G. D. Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, wortmannin. Biochem. J. 300, 631–635 (1994).
Quon, M. J. et al. Insulin receptor substrate 1 mediates the stimulatory effect of insulin on GLUT4 translocation in transfected rat adipose cells. J. Biol. Chem. 269, 27920–27924 (1994).
Kohn, A. D., Summers, S. A., Birnbaum, M. J. & Roth, R. A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271, 31372–31378 (1996).
Ng, Y., Ramm, G., Lopez, J. A. & James, D. E. Rapid activation of Akt2 is sufficient to stimulate GLUT4 translocation in 3T3-L1 adipocytes. Cell Metab. 7, 348–356 (2008).
Bai, L. et al. Dissecting multiple steps of GLUT4 trafficking and identifying the sites of insulin action. Cell Metab. 5, 47–57 (2007). One of the earliest studies to visualize GLUT4 trafficking in real-time by TIRFM, showing that insulin modulates the rates of GLUT4 vesicle docking and fusion.
Gonzalez, E. & McGraw, T. E. Insulin signaling diverges into Akt-dependent and -independent signals to regulate the recruitment/docking and the fusion of GLUT4 vesicles to the plasma membrane. Mol. Biol. Cell 17, 4484–4493 (2006).
Fujita, H. et al. Identification of three distinct functional sites of insulin-mediated GLUT4 trafficking in adipocytes using quantitative single molecule imaging. Mol. Biol. Cell 21, 2721–2731 (2010).
Chen, X. W. et al. A Ral GAP complex links PI 3-kinase/Akt signaling to RalA activation in insulin action. Mol. Biol. Cell 22, 141–152 (2010).
Zeigerer, A., McBrayer, M. K. & McGraw, T. E. Insulin stimulation of GLUT4 exocytosis, but not its inhibition of endocytosis, is dependent on RabGAP AS160. Mol. Biol. Cell 15, 4406–4415 (2004).
Min, J. et al. Synip: a novel insulin-regulated syntaxin 4-binding protein mediating GLUT4 translocation in adipocytes. Mol. Cell 3, 751–760 (1999).
Xie, X. et al. C2 domain-containing phosphoprotein CDP138 regulates GLUT4 insertion into the plasma membrane. Cell Metab. 14, 378–389 (2011).
Yamada, E. et al. Akt2 phosphorylates Synip to regulate docking and fusion of GLUT4-containing vesicles. J. Cell Biol. 168, 921–928 (2005).
Hu, J., Liu, J., Ghirlando, R., Saltiel, A. R. & Hubbard, S. R. Structural basis for recruitment of the adaptor protein APS to the activated insulin receptor. Mol. Cell 12, 1379–1389 (2003).
Ribon, V., Printen, J. A., Hoffman, N. G., Kay, B. K. & Saltiel, A. R. A novel, multifuntional c-Cbl binding protein in insulin receptor signaling in 3T3-L1 adipocytes. Mol. Cell. Biol. 18, 872–879 (1998).
Liu, J., Kimura, A., Baumann, C. A. & Saltiel, A. R. APS facilitates c-Cbl tyrosine phosphorylation and GLUT4 translocation in response to insulin in 3T3-L1 adipocytes. Mol. Cell. Biol. 22, 3599–3609 (2002).
Ribon, V., Hubbell, S., Herrera, R. & Saltiel, A. R. The product of the cbl oncogene forms stable complexes in vivo with endogenous Crk in a tyrosine phosphorylation-dependent manner. Mol. Cell. Biol. 16, 45–52 (1996).
Knudsen, B. S., Feller, S. M. & Hanafusa, H. Four proline-rich sequences of the guanine-nucleotide exchange factor C3G bind with unique specificity to the first Src homology 3 domain of Crk. J. Biol. Chem. 269, 32781–32787 (1994).
Chiang, S. H. et al. Insulin-stimulated GLUT4 translocation requires the CAP-dependent activation of TC10. Nature 410, 944–948 (2001).
Okada, T., Kawano, Y., Sakakibara, T., Hazeki, O. & Ui, M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J. Biol. Chem. 269, 3568–3573 (1994).
Ahn, M. Y., Katsanakis, K. D., Bheda, F. & Pillay, T. S. Primary and essential role of the adaptor protein APS for recruitment of both c-Cbl and its associated protein CAP in insulin signaling. J. Biol. Chem. 279, 21526–21532 (2004).
Chang, L., Chiang, S. H. & Saltiel, A. R. TC10α is required for insulin-stimulated glucose uptake in adipocytes. Endocrinology 148, 27–33 (2007).
Sharma, P. M. et al. Inhibition of phosphatidylinositol 3-kinase activity by adenovirus-mediated gene transfer and its effect on insulin action. J. Biol. Chem. 273, 18528–18537 (1998).
Schultze, S. M., Jensen, J., Hemmings, B. A., Tschopp, O. & Niessen, M. Promiscuous affairs of PKB/AKT isoforms in metabolism. Arch. Physiol. Biochem. 117, 70–77 (2011).
Lesniewski, L. A. et al. Bone marrow-specific Cap gene deletion protects against high-fat diet-induced insulin resistance. Nature Med. 13, 455–462 (2007).
Zhang, M., Kimura, A. & Saltiel, A. R. Cloning and characterization of Cbl-associated protein splicing isoforms. Mol. Med. 9, 18–25 (2003).
Satoh, S. et al. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells. Evidence that exocytosis is a critical site of hormone action. J. Biol. Chem. 268, 17820–17829 (1993).
Muretta, J. M., Romenskaia, I. & Mastick, C. C. Insulin releases Glut4 from static storage compartments into cycling endosomes and increases the rate constant for Glut4 exocytosis. J. Biol. Chem. 283, 311–323 (2008).
Ros-Baro, A. et al. Lipid rafts are required for GLUT4 internalization in adipose cells. Proc. Natl Acad. Sci. USA 98, 12050–12055 (2001).
Blot, V. & McGraw, T. E. GLUT4 is internalized by a cholesterol-dependent nystatin-sensitive mechanism inhibited by insulin. EMBO J. 25, 5648–5658 (2006).
Shigematsu, S., Watson, R. T., Khan, A. H. & Pessin, J. E. The adipocyte plasma membrane caveolin functional/structural organization is necessary for the efficient endocytosis of GLUT4. J. Biol. Chem. 278, 10683–10690 (2003).
Lajoie, P. & Nabi, I. R. Lipid rafts, caveolae, and their endocytosis. Int. Rev. Cell. Mol. Biol. 282, 135–163 (2010).
Al-Hasani, H. et al. Roles of the N- and C-termini of GLUT4 in endocytosis. J. Cell Sci. 115, 131–140 (2002).
Owen, D. J., Collins, B. M. & Evans, P. R. Adaptors for clathrin coats: structure and function. Annu. Rev. Cell Dev. Biol. 20, 153–191 (2004).
Kandror, K. V., Stephens, J. M. & Pilch, P. F. Expression and compartmentalization of caveolin in adipose cells: coordinate regulation with and structural segregation from GLUT4. J. Cell Biol. 129, 999–1006 (1995).
Malide, D., Ramm, G., Cushman, S. W. & Slot, J. W. Immunoelectron microscopic evidence that GLUT4 translocation explains the stimulation of glucose transport in isolated rat white adipose cells. J. Cell Sci. 113 (Pt. 23), 4203–4210 (2000).
Mettlen, M., Pucadyil, T., Ramachandran, R. & Schmid, S. L. Dissecting dynamin's role in clathrin-mediated endocytosis. Biochem. Soc. Trans. 37, 1022–1026 (2009).
Kao, A. W., Ceresa, B. P., Santeler, S. R. & Pessin, J. E. Expression of a dominant interfering dynamin mutant in 3T3L1 adipocytes inhibits GLUT4 endocytosis without affecting insulin signaling. J. Biol. Chem. 273, 25450–25457 (1998).
Guilherme, A. et al. Perinuclear localization and insulin responsiveness of GLUT4 requires cytoskeletal integrity in 3T3-L1 adipocytes. J. Biol. Chem. 275, 38151–38159 (2000).
Huang, J., Imamura, T. & Olefsky, J. M. Insulin can regulate GLUT4 internalization by signaling to Rab5 and the motor protein dynein. Proc. Natl Acad. Sci. USA 98, 13084–13089 (2001).
Foster, L. J., Li, D., Randhawa, V. K. & Klip, A. Insulin accelerates inter-endosomal GLUT4 traffic via phosphatidylinositol 3-kinase and protein kinase B. J. Biol. Chem. 276, 44212–44221 (2001).
Jhun, B. H., Rampal, A. L., Liu, H., Lachaal, M. & Jung, C. Y. Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes. Evidence of constitutive GLUT4 recycling. J. Biol. Chem. 267, 17710–17715 (1992).
Czech, M. P. & Buxton, J. M. Insulin action on the internalization of the GLUT4 glucose transporter in isolated rat adipocytes. J. Biol. Chem. 268, 9187–9190 (1993).
Habtemichael, E. N., Brewer, P. D., Romenskaia, I. & Mastick, C. C. Kinetic evidence that Glut4 follows different endocytic pathways than the receptors for transferrin and α2-macroglobulin. J. Biol. Chem. 286, 10115–10125 (2011).
Govers, R., Coster, A. C. & James, D. E. Insulin increases cell surface GLUT4 levels by dose dependently discharging GLUT4 into a cell surface recycling pathway. Mol. Cell. Biol. 24, 6456–6466 (2004). The authors show that adipocytes contain a recycling pool of GLUT4 and a pool of GLUT4 that is sequestered in GSVs, which are mobilized to the plasma membrane only after stimulation with insulin.
Maxfield, F. R. & McGraw, T. E. Endocytic recycling. Nature Rev. Mol. Cell Biol. 5, 121–132 (2004).
Livingstone, C., James, D. E., Rice, J. E., Hanpeter, D. & Gould, G. W. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3-L1 adipocytes. Biochem. J. 315, 487–495 (1996). By chemically ablating compartments that contain TfR, this study demonstrates that a large portion of cellular GLUT4 is found in a vesicle population that is distinct from endosomes.
Martin, S. et al. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J. Cell Biol. 134, 625–635 (1996).
Tanner, L. I. & Lienhard, G. E. Insulin elicits a redistribution of transferrin receptors in 3T3-L1 adipocytes through an increase in the rate constant for receptor externalization. J. Biol. Chem. 262, 8975–8980 (1987).
Kupriyanova, T. A., Kandror, V. & Kandror, K. V. Isolation and characterization of the two major intracellular Glut4 storage compartments. J. Biol. Chem. 277, 9133–9138 (2002).
Slot, J. W., Geuze, H. J., Gigengack, S., James, D. E. & Lienhard, G. E. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Proc. Natl Acad. Sci. USA 88, 7815–7819 (1991).
Martin, S. et al. The glucose transporter GLUT4 and the aminopeptidase vp165 colocalise in tubulo-vesicular elements in adipocytes and cardiomyocytes. J. Cell Sci. 110, 2281–2291 (1997).
Lin, B. Z., Pilch, P. F. & Kandror, K. V. Sortilin is a major protein component of Glut4-containing vesicles. J. Biol. Chem. 272, 24145–24147 (1997).
Jedrychowski, M. P. et al. Proteomic analysis of GLUT4 storage vesicles reveals LRP1 to be an important vesicle component and target of insulin signaling. J. Biol. Chem. 285, 104–114 (2010). Uses proteomics to characterize the protein components of immuno-isolated GSVs and provides evidence supporting a 'mass action' model of GSV formation.
Shi, J., Huang, G. & Kandror, K. V. Self-assembly of Glut4 storage vesicles during differentiation of 3T3-L1 adipocytes. J. Biol. Chem. 283, 30311–30321 (2008).
Martin, L. B., Shewan, A., Millar, C. A., Gould, G. W. & James, D. E. Vesicle-associated membrane protein 2 plays a specific role in the insulin-dependent trafficking of the facilitative glucose transporter GLUT4 in 3T3-L1 adipocytes. J. Biol. Chem. 273, 1444–1452 (1998).
Rea, S. et al. Syndet, an adipocyte target SNARE involved in the insulin-induced translocation of GLUT4 to the cell surface. J. Biol. Chem. 273, 18784–18792 (1998).
Zeigerer, A. et al. GLUT4 retention in adipocytes requires two intracellular insulin-regulated transport steps. Mol. Biol. Cell 13, 2421–2435 (2002).
Xu, Y. et al. Dual-mode of insulin action controls GLUT4 vesicle exocytosis. J. Cell Biol. 193, 643–653 (2011). The authors use a novel TIRFM approach to distinguish vesicles by size and show that immediately after insulin stimulation GLUT4 is mainly exocytosed from GSVs, but after prolonged insulin treatment GLUT4 arrives at the plasma membrane in endosomes.
Shewan, A. M. et al. GLUT4 recycles via a trans-Golgi network (TGN) subdomain enriched in Syntaxins 6 and 16 but not TGN38: involvement of an acidic targeting motif. Mol. Biol. Cell 14, 973–986 (2003).
Karylowski, O., Zeigerer, A., Cohen, A. & McGraw, T. E. GLUT4 is retained by an intracellular cycle of vesicle formation and fusion with endosomes. Mol. Biol. Cell 15, 870–882 (2004). Demonstrates that futile cycling between GLUT4 vesicles and endosomes is part of a mechanism that retains GLUT4 within non-stimulated cells.
Miinea, C. P. et al. AS160, the Akt substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain. Biochem. J. 391, 87–93 (2005). Biochemical characterization of the AS160 RAB GAP activity identifies RAB8, RAB10 and RAB14 as targets of AS160.
Lodhi, I. J. et al. Insulin stimulates phosphatidylinositol 3-phosphate production via the activation of Rab5. Mol. Biol. Cell 19, 2718–2728 (2008).
Zerial, M. & McBride, H. Rab proteins as membrane organizers. Nature Rev. Mol. Cell Biol. 2, 107–117 (2001).
Zoncu, R. et al. A phosphoinositide switch controls the maturation and signaling properties of APPL endosomes. Cell 136, 1110–1121 (2009).
Cormont, M. et al. Potential role of Rab4 in the regulation of subcellular localization of Glut4 in adipocytes. Mol. Cell. Biol. 16, 6879–6886 (1996).
Mari, M. et al. The Rab4 effector Rabip4 plays a role in the endocytotic trafficking of Glut 4 in 3T3-L1 adipocytes. J. Cell Sci. 119, 1297–1306 (2006).
Sun, Y., Bilan, P. J., Liu, Z. & Klip, A. Rab8A and Rab13 are activated by insulin and regulate GLUT4 translocation in muscle cells. Proc. Natl Acad. Sci. USA 107, 19909–19914 (2010).
Sano, H. et al. Rab10, a target of the AS160 Rab GAP, is required for insulin-stimulated translocation of GLUT4 to the adipocyte plasma membrane. Cell Metab. 5, 293–303 (2007).
Ishikura, S. & Klip, A. Muscle cells engage Rab8A and myosin Vb in insulin-dependent GLUT4 translocation. Am. J. Physiol. Cell Physiol. 295, C1016–C1025 (2008).
Sano, H., Roach, W. G., Peck, G. R., Fukuda, M. & Lienhard, G. E. Rab10 in insulin-stimulated GLUT4 translocation. Biochem. J. 411, 89–95 (2008).
Kane, S. et al. A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase-activating protein (GAP) domain. J. Biol. Chem. 277, 22115–22118 (2002).
Sano, H. et al. Insulin-stimulated phosphorylation of a Rab GTPase-activating protein regulates GLUT4 translocation. J. Biol. Chem. 278, 14599–14602 (2003).
Sano, H., Peck, G. R., Kettenbach, A. N., Gerber, S. A. & Lienhard, G. E. Insulin-stimulated GLUT4 protein translocation in adipocytes requires the Rab10 guanine nucleotide exchange factor Dennd4C. J. Biol. Chem. 286, 16541–16545 (2011).
Imamura, T. et al. Insulin-induced GLUT4 translocation involves protein kinase C-λ-mediated functional coupling between Rab4 and the motor mrotein kinesin. Mol. Cell. Biol. 23, 4892–4900 (2003).
Schwenk, R. W. & Eckel, J. A novel method to monitor insulin-stimulated GTP-loading of Rab11a in cardiomyocytes. Cell. Signal. 19, 825–830 (2007).
Shi, J. & Kandror, K. V. The luminal Vps10p domain of sortilin plays the predominant role in targeting to insulin-responsive Glut4-containing vesicles. J. Biol. Chem. 282, 9008–9016 (2007).
Li, J. et al. An ACAP1-containing clathrin coat complex for endocytic recycling. J. Cell Biol. 178, 453–464 (2007).
Gillingham, A. K., Koumanov, F., Pryor, P. R., Reaves, B. J. & Holman, G. D. Association of AP1 adaptor complexes with GLUT4 vesicles. J. Cell Sci. 112, 4793–4800 (1999).
Li, L. V. & Kandror, K. V. Golgi-localized, γ-ear-containing, Arf-binding protein adaptors mediate insulin-responsive trafficking of glucose transporter 4 in 3T3-L1 adipocytes. Mol. Endocrinol. 19, 2145–2153 (2005).
Blot, V. & McGraw, T. E. Molecular mechanisms controlling GLUT4 intracellular retention. Mol. Biol. Cell 19, 3477–3487 (2008).
Lodhi, I. J. et al. Gapex-5, a Rab31 guanine nucleotide exchange factor that regulates Glut4 trafficking in adipocytes. Cell Metab. 5, 59–72 (2007).
Rodriguez-Gabin, A. G., Cammer, M., Almazan, G., Charron, M. & Larocca, J. N. Role of rRAB22b, an oligodendrocyte protein, in regulation of transport of vesicles from trans Golgi to endocytic compartments. J. Neurosci. Res. 66, 1149–1160 (2001).
Yu, C., Cresswell, J., Loffler, M. G. & Bogan, J. S. The glucose transporter 4-regulating protein TUG is essential for highly insulin-responsive glucose uptake in 3T3-L1 adipocytes. J. Biol. Chem. 282, 7710–7722 (2007).
Liu, L. B., Omata, W., Kojima, I. & Shibata, H. The SUMO conjugating enzyme Ubc9 is a regulator of GLUT4 turnover and targeting to the insulin-responsive storage compartment in 3T3-L1 adipocytes. Diabetes 56, 1977–1985 (2007).
Bogan, J. S., Hendon, N., McKee, A. E., Tsao, T. S. & Lodish, H. F. Functional cloning of TUG as a regulator of GLUT4 glucose transporter trafficking. Nature 425, 727–733 (2003).
Yang, J. & Holman, G. D. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3-L1 cells. J. Biol. Chem. 268, 4600–4603 (1993).
Huang, S. et al. Insulin stimulates membrane fusion and GLUT4 accumulation in clathrin coats on adipocyte plasma membranes. Mol. Cell. Biol. 27, 3456–3469 (2007).
Lizunov, V. A., Matsumoto, H., Zimmerberg, J., Cushman, S. W. & Frolov, V. A. Insulin stimulates the halting, tethering, and fusion of mobile GLUT4 vesicles in rat adipose cells. J. Cell Biol. 169, 481–489 (2005).
Bose, A. et al. Glucose transporter recycling in response to insulin is facilitated by myosin Myo1c. Nature 420, 821–824 (2002). Reports a role for the actin motor MYO1C in GLUT4 vesicle exocytosis.
Inoue, M., Chang, L., Hwang, J., Chiang, S. H. & Saltiel, A. R. The exocyst complex is required for targeting of Glut4 to the plasma membrane by insulin. Nature 422, 629–633 (2003). Identifies the exocyst complex as a crucial component of the cellular trafficking machinery that is mobilized by insulin and facilitates GLUT4 targeting.
D'Andrea-Merrins, M., Chang, L., Lam, A. D., Ernst, S. A. & Stuenkel, E. L. Munc18c interaction with syntaxin 4 monomers and SNARE complex intermediates in GLUT4 vesicle trafficking. J. Biol. Chem. 282, 16553–16566 (2007).
Fukuda, N. et al. DOC2B: a novel syntaxin-4 binding protein mediating insulin-regulated GLUT4 vesicle fusion in adipocytes. Diabetes 58, 377–384 (2009).
Jewell, J. L. et al. Munc18c phosphorylation by the insulin receptor links cell signaling directly to SNARE exocytosis. J. Cell Biol. 193, 185–199 (2011).
Patki, V. et al. Insulin action on GLUT4 traffic visualized in single 3T3-l1 adipocytes by using ultra-fast microscopy. Mol. Biol. Cell 12, 129–141 (2001).
Fletcher, L. M., Welsh, G. I., Oatey, P. B. & Tavare, J. M. Role for the microtubule cytoskeleton in GLUT4 vesicle trafficking and in the regulation of insulin-stimulated glucose uptake. Biochem. J. 352 (Pt. 2), 267–276 (2000).
Oatey, P. B., Van Weering, D. H., Dobson, S. P., Gould, G. W. & Tavare, J. M. GLUT4 vesicle dynamics in living 3T3 L1 adipocytes visualized with green-fluorescent protein. Biochem. J. 327, 637–642 (1997).
Semiz, S. et al. Conventional kinesin KIF5B mediates insulin-stimulated GLUT4 movements on microtubules. EMBO J. 22, 2387–2399 (2003).
Wang, Q., Bilan, P. J., Tsakiridis, T., Hinek, A. & Klip, A. Actin filaments participate in the relocalization of phosphatidylinositol3-kinase to glucose transporter-containing compartments and in the stimulation of glucose uptake in 3T3-L1 adipocytes. Biochem. J. 331, 917–928 (1998).
Wang, Q., Khayat, Z., Kishi, K., Ebina, Y. & Klip, A. GLUT4 translocation by insulin in intact muscle cells: detection by a fast and quantitative assay. FEBS Lett. 427, 193–197 (1998).
Omata, W., Shibata, H., Li, L., Takata, K. & Kojima, I. Actin filaments play a critical role in insulin-induced exocytotic recruitment but not in endocytosis of GLUT4 in isolated rat adipocytes. Biochem. J. 346 (Pt. 2), 321–328 (2000).
Huang, J., Imamura, T., Babendure, J. L., Lu, J. C. & Olefsky, J. M. Disruption of microtubules ablates the specificity of insulin signaling to GLUT4 translocation in 3T3-L1 adipocytes. J. Biol. Chem. 280, 42300–42306 (2005).
Bose, A. et al. Unconventional myosin Myo1c promotes membrane fusion in a regulated exocytic pathway. Mol. Cell. Biol. 24, 5447–5458 (2004).
Lopez, J. A. et al. Identification of a distal GLUT4 trafficking event controlled by actin polymerization. Mol. Biol. Cell 20, 3918–3929 (2009).
Chen, Y., Wang, Y., Ji, W., Xu, P. & Xu, T. A pre-docking role for microtubules in insulin-stimulated glucose transporter 4 translocation. FEBS J. 275, 705–712 (2008).
Yoshizaki, T. et al. Myosin 5a is an insulin-stimulated Akt2 (protein kinase Bβ) substrate modulating GLUT4 vesicle translocation. Mol. Cell. Biol. 27, 5172–5183 (2007).
Chen, X. W., Leto, D., Chiang, S. H., Wang, Q. & Saltiel, A. R. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev. Cell 13, 391–404 (2007).
Lipatova, Z. et al. Direct interaction between a myosin V motor and the Rab GTPases Ypt31/32 is required for polarized secretion. Mol. Biol. Cell 19, 4177–4187 (2008).
Boldogh, I. R., Ramcharan, S. L., Yang, H. C. & Pon, L. A. A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division. Mol. Biol. Cell 15, 3994–4002 (2004).
Jordens, I. et al. The Rab7 effector protein RILP controls lysosomal transport by inducing the recruitment of dynein-dynactin motors. Curr. Biol. 11, 1680–1685 (2001).
Hales, C. M., Vaerman, J. P. & Goldenring, J. R. Rab11 family interacting protein 2 associates with Myosin Vb and regulates plasma membrane recycling. J. Biol. Chem. 277, 50415–50421 (2002).
Inoue, M., Chiang, S. H., Chang, L., Chen, X. W. & Saltiel, A. R. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol. Biol. Cell 17, 2303–2311 (2006).
He, B. & Guo, W. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 21, 537–542 (2009).
Chen, X. W. et al. Exocyst function is regulated by effector phosphorylation. Nature Cell Biol. 13, 580–588 (2011).
He, B., Xi, F., Zhang, X., Zhang, J. & Guo, W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO J. 26, 4053–4065 (2007).
Liu, J., Zuo, X., Yue, P. & Guo, W. Phosphatidylinositol 4,5-bisphosphate mediates the targeting of the exocyst to the plasma membrane for exocytosis in mammalian cells. Mol. Biol. Cell 18, 4483–4492 (2007).
Zhang, X. et al. Membrane association and functional regulation of Sec3 by phospholipids and Cdc42. J. Cell Biol. 180, 145–158 (2008).
Martin, T. F. PI(4,5)P2 regulation of surface membrane traffic. Curr. Opin. Cell Biol. 13, 493–499 (2001).
Moskalenko, S. et al. The exocyst is a Ral effector complex. Nature Cell Biol. 4, 66–72 (2002).
Moskalenko, S. et al. Ral GTPases regulate exocyst assembly through dual subunit interactions. J. Biol. Chem. 278, 51743–51748 (2003).
Munson, M. & Novick, P. The exocyst defrocked, a framework of rods revealed. Nature Struct. Mol. Biol. 13, 577–581 (2006).
Jiang, L. et al. Direct quantification of fusion rate reveals a distal role for AS160 in insulin-stimulated fusion of GLUT4 storage vesicles. J. Biol. Chem. 283, 8508–8516 (2008).
Babbey, C. M., Bacallao, R. L. & Dunn, K. W. Rab10 associates with primary cilia and the exocyst complex in renal epithelial cells. Am. J. Physiol. Renal Physiol. 299, F495–F506 (2010).
Jahn, R. & Scheller, R. H. SNAREs — engines for membrane fusion. Nature Rev. Mol. Cell Biol. 7, 631–643 (2006).
Macaulay, S. L. et al. Functional studies in 3T3L1 cells support a role for SNARE proteins in insulin stimulation of GLUT4 translocation. Biochem. J. 324, 217–224 (1997).
Cheatham, B. et al. Insulin-stimulated translocation of GLUT4 glucose transporters requires SNARE-complex proteins. Proc. Natl Acad. Sci. USA 93, 15169–15173 (1996).
Volchuk, A. et al. Syntaxin 4 in 3T3-L1 adipocytes: regulation by insulin and participation in insulin-dependent glucose transport. Mol. Biol. Cell 7, 1075–1082 (1996).
Bryant, N. J. & Gould, G. W. SNARE proteins underpin insulin-regulated GLUT4 traffic. Traffic 12, 657–664 (2011).
Carr, C. M. & Rizo, J. At the junction of SNARE and SM protein function. Curr. Opin. Cell Biol. 22, 488–495 (2010).
Thurmond, D. C. et al. Regulation of insulin-stimulated GLUT4 translocation by Munc18c in 3T3L1 adipocytes. J. Biol. Chem. 273, 33876–33883 (1998).
Macaulay, S. L. et al. Cellular munc18c levels can modulate glucose transport rate and GLUT4 translocation in 3T3L1 cells. FEBS Lett. 528, 154–160 (2002).
Tamori, Y. et al. Inhibition of insulin-induced GLUT4 translocation by Munc18c through interaction with syntaxin4 in 3T3-L1 adipocytes. J. Biol. Chem. 273, 19740–19746 (1998). Reports a regulatory role for MUNC18C in SNARE-mediated GLUT4 vesicle fusion.
Khan, A. H. et al. Munc18c regulates insulin-stimulated glut4 translocation to the transverse tubules in skeletal muscle. J. Biol. Chem. 276, 4063–4069 (2001).
Kanda, H. et al. Adipocytes from Munc18c-null mice show increased sensitivity to insulin-stimulated GLUT4 externalization. J. Clin. Invest. 115, 291–301 (2005).
Thurmond, D. C., Kanzaki, M., Khan, A. H. & Pessin, J. E. Munc18c function is required for insulin-stimulated plasma membrane fusion of GLUT4 and insulin-responsive amino peptidase storage vesicles. Mol. Cell. Biol. 20, 379–388 (2000).
Oh, E., Spurlin, B. A., Pessin, J. E. & Thurmond, D. C. Munc18c heterozygous knockout mice display increased susceptibility for severe glucose intolerance. Diabetes 54, 638–647 (2005).
Latham, C. F. et al. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic 7, 1408–1419 (2006).
Hu, S. H., Latham, C. F., Gee, C. L., James, D. E. & Martin, J. L. Structure of the Munc18c/Syntaxin4 N-peptide complex defines universal features of the N-peptide binding mode of Sec1/Munc18 proteins. Proc. Natl Acad. Sci. USA 104, 8773–8778 (2007).
Jewell, J. L., Oh, E., Bennett, S. M., Meroueh, S. O. & Thurmond, D. C. The tyrosine phosphorylation of Munc18c induces a switch in binding specificity from syntaxin 4 to Doc2β. J. Biol. Chem. 283, 21734–21746 (2008).
McMahon, H. T., Kozlov, M. M. & Martens, S. Membrane curvature in synaptic vesicle fusion and beyond. Cell 140, 601–605 (2010).
Okada, S. et al. Synip phosphorylation is required for insulin-stimulated Glut4 translocation. Biochem. Biophys. Res. Commun. 356, 102–106 (2007).
Sano, H., Kane, S., Sano, E. & Lienhard, G. E. Synip phosphorylation does not regulate insulin-stimulated GLUT4 translocation. Biochem. Biophys. Res. Commun. 332, 880–884 (2005).
Fujita, Y. et al. Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neurotransmitter release process. Neuron 20, 905–915 (1998).
Martens, S., Kozlov, M. M. & McMahon, H. T. How synaptotagmin promotes membrane fusion. Science 316, 1205–1208 (2007).
Lipschutz, J. H. & Mostov, K. E. Exocytosis: the many masters of the exocyst. Curr. Biol. 12, R212–R214 (2002).
Rosen, E. D. & Spiegelman, B. M. Adipocytes as regulators of energy balance and glucose homeostasis. Nature 444, 847–853 (2006).
Herman, M. A. & Kahn, B. B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 116, 1767–1775 (2006).
Acknowledgements
This work was supported by a US National Institutes of Health (NIH) grant R01DK076906. The authors thank M. Uhm and D. Bridges for their critical reading and discussions of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Related links
Glossary
- Gluconeogenesis
-
De novo synthesis of glucose from non-carbohydrate carbon sources.
- Trans-Golgi network
-
(TGN). The terminal Golgi stack where proteins are sorted and packaged into vesicles for delivery to their cellular destination.
- Insulin resistance
-
Physiological condition that is defined by a failure of tissues and organs to respond to normal concentrations of insulin.
- Type 2 diabetes mellitus
-
A chronic metabolic disorder that is characterized by increased plasma glucose levels that result from an inability of tissues to respond to insulin.
- Anabolic hormone
-
Secreted peptide that signals to cells to upregulate metabolic processes that convert simple energy sources into macromolecules.
- Adipokines
-
Hormones and cytokines that are released by adipocytes and signal to other tissues to alter feeding behaviour and metabolism.
- Small GTPases
-
20–35 kDa guanine nucleotide-binding proteins that switch between an inactive GDP-bound conformation and an active GTP-bound conformation.
- Guanine nucleotide exchange factors
-
(GEFs). A family of enzymes that activate GTPases by catalysing GDP release, thus allowing cytoplasmic GTP to bind to the GTPase.
- GTPase-activating proteins
-
(GAPs). A family of enzymes that inactive GTPases by catalysing GTP hydrolysis.
- SNARE regulatory proteins
-
(Soluble N-ethylmaleimide-sensitive factor attachment protein receptor regulatory proteins). A family of small helical proteins that bridge two membranes and drive membrane fusion events.
- Lipid rafts
-
Rigid regions of the plasma membrane that are enriched in cholesterol and glycosphingolipids.
- Effector
-
A protein that preferentially binds to an activated small GTPase.
- Exocyst
-
An evolutionarily conserved protein complex that consists of eight subunits and targets exocytic vesicles to sites of docking and fusion at the plasma membrane.
- Hypoglycaemia
-
Physiological condition that is defined by abnormally low blood glucose levels.
- Clathrin-mediated endocytosis
-
A mechanism for internalizing extracellular molecules and portions of the plasma membrane. This pathway is dependent on the membrane curvature-inducing coat protein clathrin.
- Cholesterol-dependent endocytosis
-
A clathrin-independent mechanism for internalizing molecules. This mechanism is blocked by drugs that deplete cellular cholesterol and often requires the lipid raft protein caveolin.
- Sorting endosome
-
A membrane compartment that is localized close to the cell surface where recently endocytosed proteins are delivered and sorted for degradation or recycling.
- Recycling endosomes
-
Membrane compartments that many recycling proteins pass through before returning to the cell surface.
Rights and permissions
About this article
Cite this article
Leto, D., Saltiel, A. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat Rev Mol Cell Biol 13, 383–396 (2012). https://doi.org/10.1038/nrm3351
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3351
This article is cited by
-
Bisphenol a downregulates GLUT4 expression by activating aryl hydrocarbon receptor to exacerbate polycystic ovary syndrome
Cell Communication and Signaling (2024)
-
Complex rearrangement in TBC1D4 in an individual with diabetes due to severe insulin resistance syndrome
European Journal of Human Genetics (2024)
-
Oligodendrocyte–axon metabolic coupling is mediated by extracellular K+ and maintains axonal health
Nature Neuroscience (2024)
-
Diabetic Encephalopathy: Role of Oxidative and Nitrosative Factors in Type 2 Diabetes
Indian Journal of Clinical Biochemistry (2024)
-
Deletion of Tbc1d4/As160 abrogates cardiac glucose uptake and increases myocardial damage after ischemia/reperfusion
Cardiovascular Diabetology (2023)