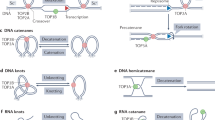
Key Points
-
Topoisomerases are classified into several families, namely, types IA, IB, IC, IIA and IIB. These families have different cellular roles and preferential substrate specificities.
-
Topoisomerases are involved in a range of cellular nucleic acid transactions, including DNA replication, transcription, packaging, compaction, recombination and repair. Topoisomerases interact with various proteins in the cell to accomplish these tasks.
-
Post-translational modification of eukaryotic topoisomerases regulates their activation, localization and destruction.
-
Small molecules and proteins can inhibit topoisomerase activity at different stages of the topoisomerase catalytic cycle. Inhibition of topoisomerases has been exploited in therapeutics and in nature.
Abstract
Topoisomerases are complex molecular machines that modulate DNA topology to maintain chromosome superstructure and integrity. Although capable of stand-alone activity in vitro, topoisomerases are frequently linked to larger pathways and systems that resolve specific DNA superstructures and intermediates arising from cellular processes such as DNA repair, transcription, replication and chromosome compaction. Topoisomerase activity is indispensible to cells, but requires the transient breakage of DNA strands. This property has been exploited, often for significant clinical benefit, by various exogenous agents that interfere with cell proliferation. Despite decades of study, surprising findings involving topoisomerases continue to emerge with respect to their cellular function, regulation and utility as therapeutic targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
07 December 2011
In 'About the authors', James M. Berger's affiliation has been corrected from "California Institute for Quantitative Biology" to "California Institute for Quantitative Biosciences".
References
Forterre, P., Gribaldo, S., Gadelle, D. & Serre, M. C. Origin and evolution of DNA topoisomerases. Biochimie 89, 427–446 (2007). A review that extensively covers the evolution of topoisomerases.
Schoeffler, A. J. & Berger, J. M. DNA topoisomerases: harnessing and constraining energy to govern chromosome topology. Q. Rev. Biophys. 41, 41–101 (2008). A review that comprehensively describes the biochemical and structural nature of topoisomerases.
Lima, C. D., Wang, J. C. & Mondragón, A. Three-dimensional structure of the 67K N-terminal fragment of E. coli DNA topoisomerase I. Nature 367, 138–146 (1994). A report detailing the first crystal structure of a type IA topoisomerase.
Tse, Y. C., Kirkegaard, K. & Wang, J. C. Covalent bonds between protein and DNA. Formation of phosphotyrosine linkage between certain DNA topoisomerases and DNA. J. Biol. Chem. 255, 5560–5565 (1980).
Brown, P. O. & Cozzarelli, N. R. Catenation and knotting of duplex DNA by type 1 topoisomerases: a mechanistic parallel with type 2 topoisomerases. Proc. Natl Acad. Sci. USA 78, 843–847 (1981).
Wang, J. C. Interaction between DNA and an Escherichia coli protein omega. J. Mol. Biol. 55, 523–533 (1971). The first report of a purified topoisomerase activity.
Hiasa, H., DiGate, R. J. & Marians, K. J. Decatenating activity of Escherichia coli DNA gyrase and topoisomerases I and III during oriC and pBR322 DNA replication in vitro. J. Biol. Chem. 269, 2093–2099 (1994).
Wallis, J. W., Chrebet, G., Brodsky, G., Rolfe, M. & Rothstein, R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell 58, 409–419 (1989). An article describing the discovery of eukaryotic topo III and its possible role in homologous recombination.
Harmon, F. G., DiGate, R. J. & Kowalczykowski, S. C. RecQ helicase and topoisomerase III comprise a novel DNA strand passage function: a conserved mechanism for control of DNA recombination. Mol. Cell 3, 611–620 (1999). The first paper to link topo III and RecQ function.
DiGate, R. J. & Marians, K. J. Identification of a potent decatenating enzyme from Escherichia coli. J. Biol. Chem. 263, 13366–13373 (1988). The first study to identify a decatenase activity for topo III.
Lopez, C. R. et al. A role for topoisomerase III in a recombination pathway alternative to RuvABC. Mol. Microbiol. 58, 80–101 (2005).
Kikuchi, A. & Asai, K. Reverse gyrase—a topoisomerase which introduces positive superhelical turns into DNA. Nature 309, 677–681 (1984). An investigation that identifies and characterizes reverse gyrase, a novel type IA topoisomerase that is found in thermophiles.
Hsieh, T. & Plank, J. L. Reverse gyrase functions as a DNA renaturase. J. Biol. Chem. 281, 5640–5647 (2006).
Redinbo, M. R., Stewart, L., Kuhn, P., Champoux, J. J. & Hol, W. G. Crystal structures of human topoisomerase I in covalent and noncovalent complexes with DNA. Science 279, 1504–1513 (1998). A paper that reports the first eukaryotic type IB topoiosmerase structure. In this study, the enzyme is crystallized with and without DNA.
Koster, D. A., Croquette, V., Dekker, C., Shuman, S. & Dekker, N. H. Friction and torque govern the relaxation of DNA supercoils by eukaryotic topoisomerase IB. Nature 434, 671–674 (2005). A report describing the detailed mechanism of topo IB, elucidated through elegant single-molecule experiments.
Champoux, J. J. & Dulbecco, R. An activity from mammalian cells that untwists superhelical DNA—a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay). Proc. Natl Acad. Sci. USA 69, 143–146 (1972). The first paper to report the discovery of topo IB.
Madden, K. R., Stewart, L. & Champoux, J. J. Preferential binding of human topoisomerase I to superhelical DNA. EMBO J. 14, 5399–5409 (1995).
Frohlich, R. F. et al. Tryptophan-205 of human topoisomerase I is essential for camptothecin inhibition of negative but not positive supercoil removal. Nucleic Acids Res. 35, 6170–6180 (2007).
Patel, A., Yakovleva, L., Shuman, S. & Mondragón, A. Crystal structure of a bacterial topoisomerase IB in complex with DNA reveals a secondary DNA binding site. Structure 18, 725–733 (2010).
Stewart, L., Redinbo, M. R., Qiu, X., Hol, W. G. & Champoux, J. J. A model for the mechanism of human topoisomerase I. Science 279, 1534–1541 (1998). Using structural information, the authors propose a detailed mechanism for type IB topoisomerase activity.
Cheng, C., Kussie, P., Pavletich, N. & Shuman, S. Conservation of structure and mechanism between eukaryotic topoisomerase I and site-specific recombinases. Cell 92, 841–850 (1998).
Krogh, B. O. & Shuman, S. A poxvirus-like type IB topoisomerase family in bacteria. Proc. Natl Acad. Sci. USA 99, 1853–1858 (2002).
Forterre, P. DNA topoisomerase V: a new fold of mysterious origin. Trends Biotechnol. 24, 245–247 (2006).
Taneja, B., Schnurr, B., Slesarev, A., Marko, J. F. & Mondragón, A. Topoisomerase V relaxes supercoiled DNA by a constrained swiveling mechanism. Proc. Natl Acad. Sci. USA 104, 14670–14675 (2007).
Slesarev, A. I. et al. DNA topoisomerase V is a relative of eukaryotic topoisomerase I from a hyperthermophilic prokaryote. Nature 364, 735–737 (1993). The study that discovers topo IC.
Taneja, B., Patel, A., Slesarev, A. & Mondragon, A. Structure of the N-terminal fragment of topoisomerase V reveals a new family of topoisomerases. EMBO J. 25, 398–408 (2006).
Belova, G. I. et al. A type IB topoisomerase with DNA repair activities. Proc. Natl Acad. Sci. USA 98, 6015–6020 (2001).
Aravind, L., Leipe, D. D. & Koonin, E. V. Toprim—a conserved catalytic domain in type IA and II topoisomerases, DnaG-type primases, OLD family nucleases and RecR proteins. Nucleic Acids Res. 26, 4205–4213 (1998). Work showing that many nucleotidyl transferases, topoisomerases and nucleases share a catalytic domain known as the Toprim domain.
Berger, J. M., Fass, D., Wang, J. C. & Harrison, S. C. Structural similarities between topoisomerases that cleave one or both DNA strands. Proc. Natl Acad. Sci. USA 95, 7876–7881 (1998). A paper that identifies similarities between the DNA interaction regions of type IA and type II topoisomerases, suggesting that the enzymes have a common DNA cleavage mechanism.
Liu, L. F., Liu, C. C. & Alberts, B. M. Type II DNA topoisomerases: enzymes that can unknot a topologically knotted DNA molecule via a reversible double-strand break. Cell 19, 697–707 (1980).
Brown, P. O. & Cozzarelli, N. R. A sign inversion mechanism for enzymatic supercoiling of DNA. Science 206, 1081–1083 (1979).
Mizuuchi, K., Fisher, L. M., O'Dea, M. H. & Gellert, M. DNA gyrase action involves the introduction of transient double-strand breaks into DNA. Proc. Natl Acad. Sci. USA 77, 1847–1851 (1980).
Gellert, M., Mizuuchi, K., O'Dea, M. H. & Nash, H. A. DNA gyrase: an enzyme that introduces superhelical turns into DNA. Proc. Natl Acad. Sci. USA 73, 3872–3876 (1976). The first investigation to discover a type II topoisomerase.
Goto, T. & Wang, J. C. Yeast DNA topoisomerase II. An ATP-dependent type II topoisomerase that catalyzes the catenation, decatenation, unknotting, and relaxation of double-stranded DNA rings. J. Biol. Chem. 257, 5866–5872 (1982).
Hsieh, T. & Brutlag, D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell 21, 115–125 (1980).
Peng, H. & Marians, K. J. Decatenation activity of topoisomerase IV during oriC and pBR322 DNA replication in vitro. Proc. Natl Acad. Sci. USA 90, 8571–8575 (1993).
Zechiedrich, E. & Cozzarelli, N. Roles of topoisomerase IV and DNA gyrase in DNA unlinking during replication in Escherichia coli. Genes Dev. 9, 2859–2869 (1995). A study that defines distinct roles of topo IV and gyrase during bacterial DNA replication.
Crisona, N. J., Strick, T. R., Bensimon, D., Croquette, V. & Cozzarelli, N. R. Preferential relaxation of positively supercoiled DNA by E. coli topoisomerase IV in single-molecule and ensemble measurements. Genes Dev. 14, 2881–2892 (2000).
McClendon, A. K., Rodriguez, A. C. & Osheroff, N. Human topoisomerase II α rapidly relaxes positively supercoiled DNA: implications for enzyme action ahead of replication forks. J. Biol. Chem. 280, 39337–39345 (2005).
Baxter, J. et al. Positive supercoiling of mitotic DNA drives decatenation by topoisomerase II in eukaryotes. Science 331, 1328–1332 (2011). A paper that suggests that positive supercoiling drives topoisomerase II decatenation activity.
Dong, K. C. & Berger, J. M. Structural basis for gate-DNA recognition and bending by type IIA topoisomerases. Nature 450, 1201–1205 (2007). The first DNA-bound type IIA topoisomerase structure, described in this article, shows a bent DNA conformation, confirming previous models.
Laponogov, I. et al. Structural basis of gate-DNA breakage and resealing by type II topoisomerases. PLoS ONE 5, e11338 (2010). A paper reporting the structure of a ternary topo–DNA–quinolone complex, showing that the quinolone intercalates between the +1 and −1 bases at each of two active sites.
Vologodskii, A. V. et al. Mechanism of topology simplification by type II DNA topoisomerases. Proc. Natl Acad. Sci. USA 98, 3045–3049 (2001). An article that proposes a model for DNA bending by type II topoisomerases to account for topology simplification below the expected thermodynamic equilibrium value.
Charvin, G., Bensimon, D. & Croquette, V. Single-molecule study of DNA unlinking by eukaryotic and prokaryotic type-II topoisomerases. Proc. Natl Acad. Sci. USA 100, 9820–9825 (2003).
Roca, J. & Wang, J. C. The probabilities of supercoil removal and decatenation by yeast DNA topoisomerase II. Genes Cells 1, 17–27 (1996).
Bergerat, A. et al. An atypical topoisomerase II from archaea with implications for meiotic recombination. Nature 386, 414–417 (1997).
Malik, S. B., Ramesh, M. A., Hulstrand, A. M. & Logsdon, J. M. Jr. Protist homologs of the meiotic Spo11 gene and topoisomerase VI reveal an evolutionary history of gene duplication and lineage-specific loss. Mol. Biol. Evol. 24, 2827–2841 (2007).
Bergerat, A., Gadelle, D. & Forterre, P. Purification of a DNA topoisomerase II from the hyperthermophilic archaeon Sulfolobus shibatae. A thermostable enzyme with both bacterial and eucaryal features. J. Biol. Chem. 269, 27663–27669 (1994). A report detailing the discovery of the first type IIB topoisomerase.
Corbett, K. D. & Berger, J. M. Structure of the topoisomerase VI-B subunit: implications for type II topoisomerase mechanism and evolution. EMBO J. 22, 151–163 (2003).
Nichols, M. D., DeAngelis, K., Keck, J. L. & Berger, J. M. Structure and function of an archaeal topoisomerase VI subunit with homology to the meiotic recombination factor Spo11. EMBO J. 18, 6177–6188 (1999).
Corbett, K. D., Benedetti, P. & Berger, J. M. Holoenzyme assembly and ATP-mediated conformational dynamics of topoisomerase VI. Nature Struct. Mol. Biol. 14, 611–619 (2007). The first paper to describe a full-length type IIB topoisomerase structure. The study described also uses small-angle X-ray scattering to image nucleotide-mediated conformational changes in the enzyme.
Keeney, S., Giroux, C. N. & Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell 88, 375–384 (1997). Together with reference 46, work linking a topo II-like protein, Spo11 of S. cerevisiae , to the formation of double-strand DNA breaks during meiosis.
Luijsterburg, M. S., White, M. F., van Driel, R. & Dame, R. T. The major architects of chromatin: architectural proteins in bacteria, archaea and eukaryotes. Crit. Rev. Biochem. Mol. Biol. 43, 393–418 (2008).
Boles, T. C., White, J. H. & Cozzarelli, N. R. Structure of plectonemically supercoiled DNA. J. Mol. Biol. 213, 931–951 (1990).
Zechiedrich, E. L. et al. Roles of topoisomerases in maintaining steady-state DNA supercoiling in Escherichia coli. J. Biol. Chem. 275, 8103–8113 (2000).
McClelland, M. et al. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature 413, 852–856 (2001).
Champion, K. & Higgins, N. P. Growth rate toxicity phenotypes and homeostatic supercoil control differentiate Escherichia coli from Salmonella enterica serovar Typhimurium. J. Bacteriol. 189, 5839–5849 (2007). An article showing that distinct differences in overall supercoiling densities between two closely related species are directly linked to small differences in gyrase.
Tretter, E. M., Lerman, J. C. & Berger, J. M. A naturally chimeric type IIA topoisomerase in Aquifex aeolicus highlights an evolutionary path for the emergence of functional paralogs. Proc. Natl Acad. Sci. USA 107, 22055–22059 (2010).
Brochier-Armanet, C. & Forterre, P. Widespread distribution of archaeal reverse gyrase in thermophilic bacteria suggests a complex history of vertical inheritance and lateral gene transfers. Archaea 2, 83–93 (2007).
Forterre, P., Mirambeau, G., Jaxel, C., Nadal, M. & Duguet, M. High positive supercoiling in vitro catalyzed by an ATP and polyethylene glycol-stimulated topoisomerase from Sulfolobus acidocaldarius. EMBO J. 4, 2123–2128 (1985).
Perugino, G., Valenti, A., D'Amaro, A., Rossi, M. & Ciaramella, M. Reverse gyrase and genome stability in hyperthermophilic organisms. Biochem. Soc. Trans. 37, 69–73 (2009).
Charbonnier, F. & Forterre, P. Comparison of plasmid DNA topology among mesophilic and thermophilic eubacteria and archaebacteria. J. Bacteriol. 176, 1251–1259 (1994).
Tadesse, S., Mascarenhas, J., Koesters, B., Hasilik, A. & Graumann, P. L. Genetic interaction of the SMC complex with topoisomerase IV in Bacillus subtilis. Microbiology 151, 1–9 (2005).
Hayama, R. & Marians, K. J. Physical and functional interaction between the condensin MukB and the decatenase topoisomerase IV in Escherichia coli. Proc. Natl Acad. Sci. USA 107, 18826–18831 (2010).
Li, Y. et al. Escherichia coli condensin MukB stimulates topoisomerase IV activity by a direct physical interaction. Proc. Natl Acad. Sci. USA 107, 18832–18837 (2010).
Bhat, M. A., Philp, A. V., Glover, D. M. & Bellen, H. J. Chromatid segregation at anaphase requires the barren product, a novel chromosome-associated protein that interacts with Topoisomerase II. Cell 87, 1103–1114 (1996).
Maeshima, K. & Laemmli, U. K. A two-step scaffolding model for mitotic chromosome assembly. Dev. Cell 4, 467–480 (2003).
Hirano, T. At the heart of the chromosome: SMC proteins in action. Nature Rev. Mol. Cell Biol. 7, 311–322 (2006).
Bhalla, N., Biggins, S. & Murray, A. W. Mutation of YCS4, a budding yeast condensin subunit, affects mitotic and nonmitotic chromosome behavior. Mol. Biol. Cell 13, 632–645 (2002).
Hartung, F. et al. An archaebacterial topoisomerase homolog not present in other eukaryotes is indispensable for cell proliferation of plants. Curr. Biol. 12, 1787–1791 (2002).
Sugimoto-Shirasu, K., Stacey, N. J., Corsar, J., Roberts, K. & McCann, M. C. DNA topoisomerase VI is essential for endoreduplication in Arabidopsis. Curr. Biol. 12, 1782–1786 (2002).
Yin, Y. et al. A crucial role for the putative Arabidopsis topoisomerase VI in plant growth and development. Proc. Natl Acad. Sci. USA 99, 10191–10196 (2002).
Breuer, C. et al. BIN4, a novel component of the plant DNA topoisomerase VI complex, is required for endoreduplication in Arabidopsis. Plant Cell 19, 3655–3668 (2007).
Lee, H. O., Davidson, J. M. & Duronio, R. J. Endoreplication: polyploidy with purpose. Genes Dev. 23, 2461–2477 (2009).
Sugimoto-Shirasu, K. et al. RHL1 is an essential component of the plant DNA topoisomerase VI complex and is required for ploidy-dependent cell growth. Proc. Natl Acad. Sci. USA 102, 18736–18741 (2005).
Rosa, I. D. et al. Adaptation of topoisomerase I paralogs to nuclear and mitochondrial DNA. Nucleic Acids Res. 37, 6414–6428 (2009).
Zhang, H. & Pommier, Y. Mitochondrial topoisomerase I sites in the regulatory D-Loop region of mitochondrial DNA. Biochemistry 47, 11196–11203 (2008).
Kaguni, J. M. & Kornberg, A. Topoisomerase I confers specificity in enzymatic replication of the Escherichia coli chromosomal origin. J. Biol. Chem. 259, 8578–8583 (1984).
Hiasa, H. & Marians, K. J. Topoisomerase IV can support oriC DNA replication in vitro. J. Biol. Chem. 269, 16371–16375 (1994).
Abdurashidova, G. et al. Functional interactions of DNA topoisomerases with a human replication origin. EMBO J. 26, 998–1009 (2007).
Lyu, Y. L. et al. Role of topoisomerase IIβ in the expression of developmentally regulated genes. Mol. Cell. Biol. 26, 7929–7941 (2006).
Yang, X., Li, W., Prescott, E. D., Burden, S. J. & Wang, J. C. DNA topoisomerase IIβ and neural development. Science 287, 131–134 (2000).
Ju, B. G. et al. A topoisomerase IIβ-mediated dsDNA break required for regulated transcription. Science 312, 1798–1802 (2006). A paper that links topo II β-dependent double-strand DNA breaks with the DNA damage and repair enzyme PARP1 in transcription regulation.
Rampakakis, E. & Zannis-Hadjopoulos, M. Transient dsDNA breaks during pre-replication complex assembly. Nucleic Acids Res. 37, 5714–5724 (2009).
Wang, X., Lesterlin, C., Reyes-Lamothe, R., Ball, G. & Sherratt, D. J. Replication and segregation of an Escherichia coli chromosome with two replication origins. Proc. Natl Acad. Sci. USA 108, e243–e250 (2011).
Khodursky, A. B. et al. Analysis of topoisomerase function in bacterial replication fork movement: use of DNA microarrays. Proc. Natl Acad. Sci. USA 97, 9419–9424 (2000).
Hiasa, H. & Marians, K. J. Two distinct modes of strand unlinking during θ-type DNA replication. J. Biol. Chem. 271, 21529–21535 (1996).
Brill, S. J., DiNardo, S., Voelkel-Meiman, K. & Sternglanz, R. Need for DNA topoisomerase activity as a swivel for DNA replication for transcription of ribosomal RNA. Nature 326, 414–416 (1987).
Kim, R. A. & Wang, J. C. Function of DNA topoisomerases as replication swivels in Saccharomyces cerevisiae. J. Mol. Biol. 208, 257–267 (1989).
Yang, L., Wold, M. S., Li, J. J., Kelly, T. J. & Liu, L. F. Roles of DNA topoisomerases in simian virus 40 DNA replication in vitro. Proc. Natl Acad. Sci. USA 84, 950–954 (1987).
Kegel, A. et al. Chromosome length influences replication-induced topological stress. Nature 471, 392–396 (2011). A report presenting the finding that chromosome length determines the need for release and development of superhelical tension in eukaryotes.
Champoux, J. & Been, M. in Mechanistic Studies of DNA Replication and Recombination: ICN–UCLA Symposia on Molecular and Cellular Biology Vol.19 (ed. Alberts, B. M.) 809–815 (Academic, New York, 1980).
Peter, B. J., Ullsperger, C., Hiasa, H., Marians, K. J. & Cozzarelli, N. R. The structure of supercoiled intermediates in DNA replication. Cell 94, 819–827 (1998). Work showing that replication-dependent supercoiling is spread throughout both nascent and unreplicated DNA, and supercoiling is not localized.
Holm, C., Goto, T., Wang, J. C. & Botstein, D. DNA topoisomerase II is required at the time of mitosis in yeast. Cell 41, 553–563 (1985).
Uemura, T. & Yanagida, M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 3, 1737–1744 (1984).
Baxter, J. & Diffley, J. F. Topoisomerase II inactivation prevents the completion of DNA replication in budding yeast. Mol. Cell 30, 790–802 (2008). A study that shows that topo II depletion and inhibition kill eukaryotic cells by two distinct mechanisms.
Uemura, T. et al. DNA topoisomerase II is required for condensation and separation of mitotic chromosomes in S. pombe. Cell 50, 917–925 (1987). Research demonstrating that, in addition to their role in chromosome decatenation, type II topoisomerases are required for condensation of chromosomes prior to segregation.
Adams, D. E., Shekhtman, E. M., Zechiedrich, E. L., Schmid, M. B. & Cozzarelli, N. R. The role of topoisomerase IV in partitioning bacterial replicons and the structure of catenated intermediates in DNA replication. Cell 71, 277–288 (1992). The first paper to report that topo IV is responsible for unlinking of daughter chromosomes prior to cell division in bacteria.
Holm, C., Stearns, T. & Botstein, D. DNA topoisomerase II must act at mitosis to prevent nondisjunction and chromosome breakage. Mol. Cell. Biol. 9, 159–168 (1989).
DiNardo, S., Voelkel, K. & Sternglanz, R. DNA topoisomerase II mutant of Saccharomyces cerevisiae: topoisomerase II is required for segregation of daughter molecules at the termination of DNA replication. Proc. Natl Acad. Sci. USA 81, 2616–2620 (1984). A study that identifies the essential role of type II topoisomerases for resolving sister chromosmes after DNA replication prior to cell segregation in eukaryotes.
Uemura, T. & Yanagida, M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 5, 1003–1010 (1986).
Nurse, P., Levine, C., Hassing, H. & Marians, K. J. Topoisomerase III can serve as the cellular decatenase in Escherichia coli. J. Biol. Chem. 278, 8653–8660 (2003). A paper showing that overexpression of topo III compensates for the loss of topo IV and thus can act as the main cellular decatenase.
Suski, C. & Marians, K. J. Resolution of converging replication forks by RecQ and topoisomerase III. Mol. Cell 30, 779–789 (2008). An article that provides the first evidence that RecQ helicases and topo III collaborate to resolve converging replication forks.
Chang, M. et al. RMI1/NCE4, a suppressor of genome instability, encodes a member of the RecQ helicase/Topo III complex. EMBO J. 24, 2024–2033 (2005).
Xu, D. et al. RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev. 22, 2843–2855 (2008). A study that identifies another single-stranded-DNA-binding protein, RMI1, that associates with the topo III–RecQ helicase complex. The interaction of RMI1 with the topo III–RecQ helicase complex diminishes and regulates sister chromosome exchange during homologous recombination.
Chan, K. L., North, P. S. & Hickson, I. D. BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J. 26, 3397–3409 (2007). A study finding that ultrafine anaphase bridges form at a higher frequency when the helicase BLM is absent, suggesting a role for BLM dependent topo III localization for appropriate segregation of sister chromosomes.
Confalonieri, F. et al. Reverse gyrase: a helicase-like domain and a type I topoisomerase in the same polypeptide. Proc. Natl Acad. Sci. USA 90, 4753–4757 (1993).
Déclais, A. C., Marsault, J., Confalonieri, F., de La Tour, C. B. & Duguet, M. Reverse gyrase, the two domains intimately cooperate to promote positive supercoiling. J. Biol. Chem. 275, 19498–19504 (2000).
Valjavec-Gratian, M., Henderson, T. A. & Hill, T. M. Tus-mediated arrest of DNA replication in Escherichia coli is modulated by DNA supercoiling. Mol. Microbiol. 58, 758–773 (2005).
Fachinetti, D. et al. Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol. Cell 39, 595–605 (2010).
Crozat, E. & Grainge, I. FtsK DNA translocase: the fast motor that knows where it's going. Chembiochem 11, 2232–2243 (2010).
Bigot, S. & Marians, K. J. DNA chirality-dependent stimulation of topoisomerase IV activity by the C-terminal AAA+ domain of FtsK. Nucleic Acids Res. 38, 3031–3040 (2010).
Espeli, O., Lee, C. & Marians, K. J. A physical and functional interaction between Escherichia coli FtsK and topoisomerase IV. J. Biol. Chem. 278, 44639–44644 (2003).
Madabhushi, R. & Marians, K. J. Actin homolog MreB affects chromosome segregation by regulating topoisomerase IV in Escherichia coli. Mol. Cell 33, 171–180 (2009).
Coelho, P. A. et al. Dual role of topoisomerase II in centromere resolution and aurora B activity. PLoS Biol. 6, e207 (2008).
Li, H., Wang, Y. & Liu, X. Plk1-dependent phosphorylation regulates functions of DNA topoisomerase IIα in cell cycle progression. J. Biol. Chem. 283, 6209–6221 (2008).
Espeli, O., Levine, C., Hassing, H. & Marians, K. J. Temporal regulation of topoisomerase IV activity in E. coli. Mol. Cell 11, 189–201 (2003).
Kang, S., Han, J., Park, J., Skarstad, K. & Wang, D. H. SeqA protein stimulates the relaxing and decatenating activities of topoisomerase IV. J. Biol. Chem. 278, 48779–48785 (2003).
Liu, L. F. & Wang, J. C. Supercoiling of the DNA template during transcription. Proc. Natl Acad. Sci. USA 84, 7024–7027 (1987). A paper presenting the first evidence of DNA supercoiling induced by a translocating motor protein.
Wu, H. Y., Shyy, S. H., Wang, J. C. & Liu, L. F. Transcription generates positively and negatively supercoiled domains in the template. Cell 53, 433–440 (1988). An article providing experimental evidence that transcription results in the formation of positive supercoils ahead of the RNA polymerase, and negative supercoils behind it.
Blot, N., Mavathur, R., Geertz, M., Travers, A. & Muskhelishvili, G. Homeostatic regulation of supercoiling sensitivity coordinates transcription of the bacterial genome. EMBO Rep. 7, 710–715 (2006).
Drolet, M., Bi, X. & Liu, L. F. Hypernegative supercoiling of the DNA template during transcription elongation in vitro. J. Biol. Chem. 269, 2068–2074 (1994).
Tuduri, S. et al. Topoisomerase I suppresses genomic instability by preventing interference between replication and transcription. Nature Cell Biol. 11, 1315–1324 (2009).
Drolet, M. et al. Overexpression of RNase H partially complements the growth defect of an Escherichia coli ΔtopA mutant: R-loop formation is a major problem in the absence of DNA topoisomerase I. Proc. Natl Acad. Sci. USA 92, 3526–3530 (1995).
Massé, E. & Drolet, M. Relaxation of transcription-induced negative supercoiling is an essential function of Escherichia coli DNA topoisomerase I. J. Biol. Chem. 274, 16654–16658 (1999).
Cheng, B., Zhu, C. X., Ji, C., Ahumada, A. & Tse-Dinh, Y. C. Direct interaction between Escherichia coli RNA polymerase and the zinc ribbon domains of DNA topoisomerase I. J. Biol. Chem. 278, 30705–30710 (2003).
Merino, A., Madden, K. R., Lane, W. S., Champoux, J. J. & Reinberg, D. DNA topoisomerase I is involved in both repression and activation of transcription. Nature 365, 227–232 (1993).
Mondal, N. et al. Elongation by RNA polymerase II on chromatin templates requires topoisomerase activity. Nucleic Acids Res. 31, 5016–5024 (2003).
Durand-Dubief, M., Persson, J., Norman, U., Hartsuiker, E. & Ekwall, K. Topoisomerase I regulates open chromatin and controls gene expression in vivo. EMBO J. 29, 2126–2134 (2010).
Lotito, L. et al. Global transcription regulation by DNA topoisomerase I in exponentially growing Saccharomyces cerevisiae cells: activation of telomere-proximal genes by TOP1 deletion. J. Mol. Biol. 377, 311–322 (2008).
Rossi, F. et al. Specific phosphorylation of SR proteins by mammalian DNA topoisomerase I. Nature 381, 80–82 (1996). The first report of a topo IB-associated kinase activity for SR proteins.
Juge, F., Fernando, C., Fic, W. & Tazi, J. The SR protein B52/SRp55 is required for DNA topoisomerase I recruitment to chromatin, mRNA release and transcription shutdown. PLoS Genet. 6, e1001124 (2010).
Malanga, M., Czubaty, A., Girstun, A., Staron, K. & Althaus, F. R. Poly(ADP-ribose) binds to the splicing factor ASF/SF2 and regulates its phosphorylation by DNA topoisomerase I. J. Biol. Chem. 283, 19991–19998 (2008).
Mcnamara, S., Wang, H., Hanna, N. & Miller, W. Topoisomerase IIβ negatively modulates retinoic acid receptor a function: a novel mechanism of retinoic acid resistance. Mol. Cell. Biol. 28, 2066–2077 (2008).
Peciña, A. et al. Targeted stimulation of meiotic recombination. Cell 111, 173–184 (2002).
Keeney, S. Spo11 and the formation of DNA double-strand breaks in meiosis. Genome Dyn. Stab. 2, 81–123 (2008).
Neale, M. J., Pan, J. & Keeney, S. Endonucleolytic processing of covalent protein-linked double-strand breaks. Nature 436, 1053–1057 (2005). An article describing how Spo11 is removed from chromosome ends to permit homologous recombination to occur in S. cerevisiae.
Li, W. & Ma, H. Double-stranded DNA breaks and gene functions in recombination and meiosis. Cell Res. 16, 402–412 (2006).
Baudat, F., Manova, K., Yuen, J. P., Jasin, M. & Keeney, S. Chromosome synapsis defects and sexually dimorphic meiotic progression in mice lacking Spo11. Mol. Cell 6, 989–998 (2000).
Hartsuiker, E. et al. Ctp1 CtIP and Rad32 Mre11 nuclease activity are required for Rec12 Spo11 removal, but Rec12 Spo11 removal is dispensable for other MRN-dependent meiotic functions. Mol. Cell. Biol. 29, 1671–1681 (2009).
Lu, W. J., Chapo, J., Roig, I. & Abrams, J. M. Meiotic recombination provokes functional activation of the p53 regulatory network. Science 328, 1278–1281 (2010). A recent paper that highlights the role of p53 in SPO11-mediated homologous recombination.
Cliby, W. A. S. Phase and G2 arrests induced by topoisomerase I poisons are dependent on ATR kinase function. J. Biol. Chem. 277, 1599–1606 (2002).
Treszezamsky, A. D. et al. BRCA1- and BRCA2-deficient cells are sensitive to etoposide-induced DNA double-strand breaks via topoisomerase II. Cancer Res. 67, 7078–7081 (2007).
Lin, C. P., Ban, Y., Lyu, Y. L. & Liu, L. F. Proteasome-dependent processing of topoisomerase I-DNA adducts into DNA double strand breaks at arrested replication forks. J. Biol. Chem. 284, 28084–28092 (2009).
Mao, Y., Sun, M., Desai, S. D. & Liu, L. F. SUMO-1 conjugation to topoisomerase I: a possible repair response to topoisomerase-mediated DNA damage. Proc. Natl Acad. Sci. USA 97, 4046–4051 (2000).
Mao, Y., Desai, S. D., Ting, C. Y., Hwang, J. & Liu, L. F. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J. Biol. Chem. 276, 40652–40658 (2001).
Pouliot, J. J., Yao, K. C., Robertson, C. A. & Nash, H. A. Yeast gene for a Tyr-DNA phosphodiesterase that repairs topoisomerase I complexes. Science 286, 552–555 (1999). A paper reporting the discovery of yeast Tdp1, a repair protein that removes covalently attached topo IB from DNA ends.
Nitiss, K. C., Malik, M., He, X., White, S. W. & Nitiss, J. L. Tyrosyl-DNA phosphodiesterase (Tdp1) participates in the repair of Top2-mediated DNA damage. Proc. Natl Acad. Sci. USA 103, 8953–8958 (2006).
Cortes Ledesma, F., El Khamisy, S. F., Zuma, M. C., Osborn, K. & Caldecott, K. W. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature 461, 674–678 (2009).
Zeng, Z., Cortes-Ledesma, F., El Khamisy, S. F. & Caldecott, K. W. TDP2/TTRAP is the major 5′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J. Biol. Chem. 286, 403–409 (2011).
Mao, Y., Desai, S. D. & Liu, L. F. SUMO-1 conjugation to human DNA topoisomerase II isozymes. J. Biol. Chem. 275, 26066–26073 (2000).
Desai, S. D., Liu, L. F., Vazquez-Abad, D. & D'Arpa, P. Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J. Biol. Chem. 272, 24159–24164 (1997).
Sordet, O. et al. Hyperphosphorylation of RNA polymerase II in response to topoisomerase I cleavage complexes and its association with transcription- and BRCA1-dependent degradation of topoisomerase I. J. Mol. Biol. 381, 540–549 (2008).
Desai, S. D. et al. Transcription-dependent degradation of topoisomerase I-DNA covalent complexes. Mol. Cell. Biol. 23, 2341–2350 (2003).
Xiao, H. et al. The topoisomerase IIβ circular clamp arrests transcription and signals a 26S proteasome pathway. Proc. Natl Acad. Sci. USA 100, 3239–3244 (2003).
Datta, A. & Jinks-Robertson, S. Association of increased spontaneous mutation rates with high levels of transcription in yeast. Science 268, 1616–1619 (1995).
Lippert, M. J. et al. Role for topoisomerase 1 in transcription-associated mutagenesis in yeast. Proc. Natl Acad. Sci. USA 108, 698–703 (2011).
Takahashi, T., Burguiere-Slezak, G., Van der Kemp, P. A. & Boiteux, S. Topoisomerase 1 provokes the formation of short deletions in repeated sequences upon high transcription in Saccharomyces cerevisiae. Proc. Natl Acad. Sci. USA 108, 692–697 (2011). Together with reference 157, the first reports on transcription-mediated genomic deletions that are a result of topo IB action.
Kim, N. et al. Mutagenic processing of ribonucleotides in DNA by yeast topoisomerase I. Science 332, 1561–1564 (2011). A recent paper that is the first to report the mutagenic role of topo IB in the removal of genomically misincorpated ribonucleotides.
Wang, Y., Knudsen, B. R., Bjergbaek, L., Westergaard, O. & Andersen, A. H. Stimulated activity of human topoisomerases IIα and IIβ on RNA-containing substrates. J. Biol. Chem. 274, 22839–22846 (1999).
Deweese, J. E. & Osheroff, N. Coordinating the two protomer active sites of human topoisomerase IIα: nicks as topoisomerase II poisons. Biochemistry 48, 1439–1441 (2009).
Sayer, J., Jerina, D. & Shuman, S. Individual nucleotide bases, not base pairs, are critical for triggering site-specific DNA cleavage by vaccinia topoisomerase. J. Biol. Chem. 39718–39726 (2004).
Kingma, P. S. & Osheroff, N. Apurinic sites are position-specific topoisomerase II poisons. J. Biol. Chem. 272, 1148–1155 (1997).
Kingma, P. S. & Osheroff, N. Spontaneous DNA damage stimulates topoisomerase II-mediated DNA cleavage. J. Biol. Chem. 272, 7488–7493 (1997).
Ira, G., Malkova, A., Liberi, G., Foiani, M. & Haber, J. E. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115, 401–411 (2003). An investigation which shows that interactions between RecQ helicases and topo III restrict crossover events during repair of double-strand breaks.
Wu, L. & Hickson, I. D. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature 426, 870–874 (2003). A report demonstrating that topo III and a RecQ helicase interact to resolve double Holliday junctions, resulting in limited crossover during homologous recombination.
Plank, J. L., Wu, J. & Hsieh, T. S. Topoisomerase IIIα and Bloom's helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc. Natl Acad. Sci. USA 103, 11118–11123 (2006). A study which finds that topo III collaborates with a RecQ-like helicase and RPA to remove double Holliday junctions, using a convergent migration mechanism to collapse the junctions and produce non-crossover products.
Harmon, F. G., Brockman, J. P. & Kowalczykowski, S. C. RecQ helicase stimulates both DNA catenation and changes in DNA topology by topoisomerase III. J. Biol. Chem. 278, 42668–42678 (2003). The first paper to show the ability of a RecQ helicase to stimulate topo III, and to suggest that the interaction is important for recombination.
Singh, T. R. et al. BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase–double Holliday junction dissolvasome. Genes Dev. 22, 2856–2868 (2008).
Cejka, P. et al. DNA end resection by Dna2–Sgs1–RPA and its stimulation by Top3–Rmi1 and Mre11–Rad50–Xrs2. Nature 467, 112–116 (2010). A demonstration that nucleases required for end resectioning may be recruited to broken ends by topo III in vivo.
Niu, H. et al. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature 467, 108–111 (2010).
Wells, N. J., Addison, C. M., Fry, A. M., Ganapathi, R. & Hickson, I. D. Serine 1524 is a major site of phosphorylation on human topoisomerase II α protein in vivo and is a substrate for casein kinase II in vitro. J. Biol. Chem. 269, 29746–29751 (1994).
Choudhary, C. et al. Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325, 834–840 (2009).
Ryu, H., Furuta, M., Kirkpatrick, D., Gygi, S. P. & Azuma, Y. PIASy-dependent SUMOylation regulates DNA topoisomerase II α activity. J. Cell Biol. 191, 783–794 (2010).
Dawlaty, M. M. et al. Resolution of sister centromeres requires RanBP2-mediated SUMOylation of topoisomerase IIα. Cell 133, 103–115 (2008).
Bachant, J., Alcasabas, A., Blat, Y., Kleckner, N. & Elledge, S. J. The SUMO-1 isopeptidase Smt4 is linked to centromeric cohesion through SUMO-1 modification of DNA topoisomerase II. Mol. Cell 9, 1169–1182 (2002).
Shapiro, P. et al. Extracellular signal-regulated kinase activates topoisomerase IIα through a mechanism independent of phosphorylation. Mol. Cell. Biol. 19, 3551–3560 (1999).
Plo, I. et al. Overexpression of the atypical protein kinase C ζ reduces topoisomerase II catalytic activity, cleavable complexes formation, and drug-induced cytotoxicity in monocytic U937 leukemia cells. J. Biol. Chem. 277, 31407–31415 (2002).
Kimura, K., Saijo, M., Tanaka, M. & Enomoto, T. Phosphorylation-independent stimulation of DNA topoisomerase II α activity. J. Biol. Chem. 271, 10990–10995 (1996).
Bernard, P. & Couturier, M. Cell killing by the F plasmid CcdB protein involves poisoning of DNA-topoisomerase II complexes. J. Mol. Biol. 226, 735–745 (1992).
Smith, A. B. & Maxwell, A. A strand-passage conformation of DNA gyrase is required to allow the bacterial toxin, CcdB, to access its binding site. Nucleic Acids Res. 34, 4667–4676 (2006).
Vizán, J., Hernández-Chico, C., del Castillo, I. & Moreno, F. The peptide antibiotic microcin B17 induces double-strand cleavage of DNA mediated by E. coli DNA gyrase. EMBO J. 10, 467–476 (1991).
Hegde, S. et al. A fluoroquinolone resistance protein from Mycobacterium tuberculosis that mimics DNA. Science 308, 1480–1483 (2005). The study that identifies and structurally characterizes a DNA-mimic protein in Mycobacterium tuberculosis that provides quinolone resistance to gyrase.
Staker, B. L. et al. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc. Natl Acad. Sci. USA 99, 15387–15392 (2002).
Lewis, R. J. et al. The nature of inhibition of DNA gyrase by the coumarins and the cyclothialidines revealed by X-ray crystallography. EMBO J. 15, 1412–1420 (1996).
Tsai, F. T. et al. The high-resolution crystal structure of a 24-kDa gyrase B fragment from E. coli complexed with one of the most potent coumarin inhibitors, clorobiocin. Proteins 28, 41–52 (1997).
Classen, S., Olland, S. & Berger, J. M. Structure of the topoisomerase II ATPase region and its mechanism of inhibition by the chemotherapeutic agent ICRF-187. Proc. Natl Acad. Sci. USA 100, 10629–10634 (2003).
Corbett, K. D. & Berger, J. M. Structural basis for topoisomerase VI inhibition by the anti-Hsp90 drug radicicol. Nucleic Acids Res. 34, 4269–4277 (2006).
Edwards, M. J. et al. A crystal structure of the bifunctional antibiotic simocyclinone D8, bound to DNA gyrase. Science 326, 1415–1418 (2009).
Bax, B. D. et al. Type IIA topoisomerase inhibition by a new class of antibacterial agents. Nature 466, 935–940 (2010). Work showing that bacterial type IIA topoisomerases are inhibited by a novel compound that binds across the dimer interface and intercalates into the DNA.
Cheng, B. et al. Asp-to-Asn substitution at the first position of the DxD TOPRIM motif of recombinant bacterial topoisomerase I is extremely lethal to E. coli. J. Mol. Biol. 385, 558–567 (2009). A paper reporting how a single active-site mutation turns a type IA topo into a highly lethal machine, suggesting that this previously unrealized drug target may become a viable future target.
Dwyer, D. J., Kohanski, M. A., Hayete, B. & Collins, J. J. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Mol. Syst. Biol. 3, 91 (2007).
Kohanski, M. A., Dwyer, D. J., Hayete, B., Lawrence, C. A. & Collins, J. J. A common mechanism of cellular death induced by bactericidal antibiotics. Cell 130, 797–810 (2007).
Wang, X., Zhao, X., Malik, M. & Drlica, K. Contribution of reactive oxygen species to pathways of quinolone-mediated bacterial cell death. J. Antimicrob. Chemother. 65, 520–524 (2010).
Bowman, K. J., Newell, D. R., Calvert, A. H. & Curtin, N. J. Differential effects of the poly (ADP-ribose) polymerase (PARP) inhibitor NU1025 on topoisomerase I and II inhibitor cytotoxicity in L1210 cells in vitro. Br. J. Cancer 84, 106–112 (2001). An article which shows that the PARP1 inhibitor NU1025 enhances the toxicity of the topo IB inhibitor camptothecin, suggesting that this kind of synergy with topo inhibitors may be a promising approach for the treatment of cancer and microbial infections.
Wesierska-Gadek, J., Schloffer, D., Gueorguieva, M., Uhl, M. & Skladanowski, A. Increased susceptibility of poly(ADP-ribose) polymerase-1 knockout cells to antitumor triazoloacridone C-1305 is associated with permanent G2 cell cycle arrest. Cancer Res. 64, 4487–4497 (2004).
Munoz-Gamez, J. A. et al. Inhibition of poly (ADP-ribose) polymerase-1 enhances doxorubicin activity against liver cancer cells. Cancer Lett. 301, 47–56.
Austin, C., Sng, J., Patel, S. & Fisher, L. Novel HeLa topoisomerase II is the IIβ isoform: complete coding sequence and homology with other type II topoisomerases. Biochim. Biophys. Acta 1172, 283–291 (1993).
Linka, R. M. et al. C-terminal regions of topoisomerase IIα and IIβ determine isoform-specific functioning of the enzymes in vivo. Nucleic Acids Res. 35, 3810–3822 (2007).
Ye, J. et al. TRF2 and apollo cooperate with topoisomerase 2 α to protect human telomeres from replicative damage. Cell 142, 230–242 (2010).
Niimi, A., Suka, N., Harata, M., Kikuchi, A. & Mizuno, S. Co-localization of chicken DNA topoisomerase IIa, but not β, with sites of DNA replication and possible involvement of a C-terminal region of a through its binding to PCNA. Chromosoma 110, 102–114 (2001).
Grue, P. et al. Essential mitotic functions of DNA topoisomerase IIα are not adopted by topoisomerase IIβ in human H69 cells. J. Biol. Chem. 273, 33660–33666 (1998).
Peng, H. & Marians, K. J. Escherichia coli topoisomerase IV. Purification, characterization, subunit structure, and subunit interactions. J. Biol. Chem. 268, 24481–24490 (1993).
Kato, J. et al. New topoisomerase essential for chromosome segregation in E. coli. Cell 63, 393–404 (1990). The discovery of topo IV and its importance for chromosome segregation in E. coli.
Kampranis, S. C. & Maxwell, A. Conversion of DNA gyrase into a conventional type II topoisomerase. Proc. Natl Acad. Sci. USA 93, 14416–14421 (1996).
Corbett, K. D., Schoeffler, A. J., Thomsen, N. D. & Berger, J. M. The structural basis for substrate specificity in DNA topoisomerase IV. J. Mol. Biol. 351, 545–561 (2005).
Hsieh, T.-J., Farh, L., Huang, W. M. & Chan, N.-L. Structure of the topoisomerase IV C-terminal domain: a broken β-propeller implies a role as geometry facilitator in catalysis. J. Biol. Chem. 279, 55587–55593 (2004).
Corbett, K. D., Shultzaberger, R. K. & Berger, J. M. The C-terminal domain of DNA gyrase A adopts a DNA-bending β-pinwheel fold. Proc. Natl Acad. Sci. USA 101, 7293–7298 (2004). The first high-resolution structure of the C-terminal domain from bacterial type IIA topoisomerase.
Ruthenburg, A. J., Graybosch, D. M., Huetsch, J. C. & Verdine, G. L. A superhelical spiral in the Escherichia coli DNA gyrase A C-terminal domain imparts unidirectional supercoiling bias. J. Biol. Chem. 280, 26177–26184 (2005).
Changela, A., DiGate, R. J. & Mondragón, A. Crystal structure of a complex of a type IA DNA topoisomerase with a single-stranded DNA molecule. Nature 411, 1077–1081 (2001).
Schmidt, B., Burgin, A., Deweese, J., Osheroff, N. & Berger, J. A novel and unified two-metal mechanism for DNA cleavage by type II and IA topoisomerases. Nature 465, 641–644 (2010).
Acknowledgements
The authors thank K. Drlica for thoughtful discussions and editing. This work was supported by a US National Science Foundation pre-doctoral fellowship (to S.M.V.) and the US National Cancer Institute (grant CA077373 to J.M.B.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Supercoil
-
The result of duplex DNA twisting on itself in three-dimensional space.
- Paralogues
-
Homologous genes separated by a duplication event that have evolved new functions.
- Domains of life
-
The three major evolutionary branches (Bacteria, Archaea and Eukarya) of modern-day cellular lineages.
- Negatively supercoiled DNA
-
DNA that is under-twisted (wound to <10.5 base pairs per turn). Negative supercoiling destabilizes the DNA, allowing complementary strands to be more easily denatured. By contrast, DNA becomes more resistant to denaturation when positively supercoiled (wound to >10.5 base pairs per turn).
- Superfamily 2 helicase
-
A member of a diverse group of ATP-dependent nucleic acid helicases and translocases that share a conserved domain architecture. Many members of this family can locally melt or unwind short duplex regions.
- Abasic lesions
-
Regions of DNA damage in which the nucleobase has been excised from the sugar backbone.
- Catenanes
-
Topologically interlinked duplex DNA rings.
- Plectonemic supercoiling
-
The natural tendency of supercoiled DNA to wrap back on itself, forming intramolecularly wound structures known as plectonemes. Because DNA is itself a chiral molecule, negatively and positively supercoiled states cause the DNA to coil in opposite directions (in the form of right- and left-handed supertwists, respectively).
- Superhelical densities
-
Measurements of the over- or under-twistedness of DNA, generally expressed as the ratio by which the twist of the supercoiled state differs from that of the relaxed state.
- ATP-binding-cassette ATPases
-
(ABC ATPases). A family of proteins that include membrane-bound transporters, DNA repair factors and structural maintenance of chromosomes (SMC) proteins. ABC ATPases possess a conserved ATPase site that is often formed at dimer interfaces. ATP binding results in conformational changes (often dimerization) that affect the associated partner proteins and substrates.
- Origin
-
A chromosomal site for replisome assembly.
- Origin recognition complex
-
A multisubunit protein complex that localizes to origins to initiate DNA replication in eukaryotes.
- Poly(ADP-ribose) polymerase 1
-
An enzyme that adds chains of ADP-ribose to proteins as a response to DNA damage and cell death.
- Precatenanes
-
Entangled daughter duplexes that are formed behind a replication fork during strand synthesis.
- Hemicatenane
-
A junction between two double-stranded DNA molecules, in which one strand of one DNA molecule forms a duplex with the complementary strand on the other DNA molecule.
- Scissile phosphate
-
The phosphate at which a nucleic acid backbone is broken by nucleophilic attack.
- E3 ligases
-
Enzymes that attach ubiquitin or ubiquitin-like proteins to target proteins.
Rights and permissions
About this article
Cite this article
Vos, S., Tretter, E., Schmidt, B. et al. All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol 12, 827–841 (2011). https://doi.org/10.1038/nrm3228
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm3228
This article is cited by
-
The Bisdioxopiperazine ICRF-193 Attenuates LPS-induced IL-1β Secretion by Macrophages
Inflammation (2024)
-
Chromatinization modulates topoisomerase II processivity
Nature Communications (2023)
-
Unraveling topoisomerase IA gate dynamics in presence of PPEF and its preclinical evaluation against multidrug-resistant pathogens
Communications Biology (2023)
-
ATM inhibitor KU60019 synergistically sensitizes lung cancer cells to topoisomerase II poisons by multiple mechanisms
Scientific Reports (2023)
-
The TDRD3-USP9X complex and MIB1 regulate TOP3B homeostasis and prevent deleterious TOP3B cleavage complexes
Nature Communications (2023)