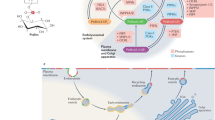
Key Points
-
Ten years ago, the lipid raft field was suffering from ambiguous methodology and imprecise nomenclature.
-
New high-resolution imaging methods are now giving insights into raft dynamics. Together with other studies, this has led to changes in our concept of rafts.
-
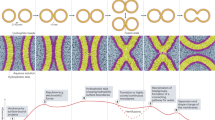
Rafts in plasma membranes can be characterized by three different states: dynamic nanoscale assemblies, raft platforms stabilized by oligomerization and micrometre-scale phase separation.
-
Lipidomics is beginning to give comprehensive views of the lipid composition of raft domains.
-
Three examples of roles that rafts have in cellular function are: T cell signalling, HIV assembly and membrane trafficking.
-
A key open issue for the field is how lipids interact with integral raft proteins.
Abstract
Ten years ago, we wrote a Review on lipid rafts and signalling in the launch issue of Nature Reviews Molecular Cell Biology. At the time, this field was suffering from ambiguous methodology and imprecise nomenclature. Now, new techniques are deepening our insight into the dynamics of membrane organization. Here, we discuss how the field has matured and present an evolving model in which membranes are occupied by fluctuating nanoscale assemblies of sphingolipids, cholesterol and proteins that can be stabilized into platforms that are important in signalling, viral infection and membrane trafficking.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
van Meer, G., Voelker, D. R. & Feigenson, G. W. Membrane lipids: where they are and how they behave. Nature Rev. Mol. Cell Biol. 9, 112–124 (2008).
Singer, S. J. & Nicolson, G. L. The fluid mosaic model of the structure of cell membranes. Science 175, 720–731 (1972).
Simons, K. & Ikonen, E. Functional rafts in cell membranes. Nature 387, 569–572 (1997).
Simons, K. & Toomre, D. Lipid rafts and signal transduction. Nature Rev. Mol. Cell Biol. 1, 31–39 (2000).
Parton, R. G. & Simons, K. The multiple faces of caveolae. Nature Rev. Mol. Cell Biol. 8, 185–194 (2007).
Munro, S. Lipid rafts: elusive or illusive? Cell 115, 377–388 (2003).
Shaw, A. S. Lipid rafts: now you see them, now you don't. Nature Immunol. 7, 1139–1142 (2006).
Lichtenberg, D., Goñi, F. M. & Heerklotz, H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem. Sci. 30, 430–436 (2005).
Lingwood, D. & Simons, K. Detergent resistance as a tool in membrane research. Nature Protoc. 2, 2159–2165 (2007).
Kenworthy, A. K. Have we become overly reliant on lipid rafts? Talking point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 9, 531–535 (2008).
Kalvodova, L. et al. The lipidomes of vesicular stomatitis virus, semliki forest virus, and the host plasma membrane analyzed by quantitative shotgun mass spectrometry. J. Virol. 83, 7996–8003 (2009).
Zidovetzki, R. & Levitan, I. Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. Biochim. Biophys. Acta 1768, 1311–1324 (2007).
Pizzo, P. et al. Lipid rafts and T cell receptor signaling: a critical re-evaluation. Eur. J. Immunol. 32, 3082–3091 (2002).
Kenworthy, A. K. et al. Dynamics of putative raft-associated proteins at the cell surface. J. Cell Biol. 165, 735–746 (2004).
Glebov, O. O. & Nichols, B. J. Lipid raft proteins have a random distribution during localized activation of the T-cell receptor. Nature Cell Biol. 6, 238–243 (2004).
He, H. & Marguet, D. T-cell antigen receptor triggering and lipid rafts: a matter of space and time scales. Talking point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 9, 525–530 (2008).
Jacobson, K., Mouritsen, O. G. & Anderson, R. G. W. Lipid rafts: at a crossroad between cell biology and physics. Nature Cell Biol. 9, 7–14 (2007).
Day, C. A. & Kenworthy, A. K. Tracking microdomain dynamics in cell membranes. Biochim. Biophys. Acta 1788, 245–253 (2009).
Lagerholm, B. C., Weinreb, G. E., Jacobson, K. & Thompson, N. L. Detecting microdomains in intact cell membranes. Annu. Rev. Phys. Chem. 56, 309–336 (2005).
Meyer, B. H. et al. FRET imaging reveals that functional neurokinin-1 receptors are monomeric and reside in membrane microdomains of live cells. Proc. Natl Acad. Sci. USA 103, 2138–2143 (2006).
Sharma, P. et al. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116, 577–589 (2004). The first demonstration of cholesterol-assisted nanoscale clusters in living cells.
Vyas, N. et al. Nanoscale organization of hedgehog is essential for long-range signaling. Cell 133, 1214–1227 (2008).
Kusumi, A., Koyama-Honda, I. & Suzuki, K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic 5, 213–230 (2004).
Pinaud, F. et al. Dynamic partitioning of a glycosyl-phosphatidylinositol-anchored protein in glycosphingolipid-rich microdomains imaged by single-quantum dot tracking. Traffic 10, 691–712 (2009).
Lenne, P. et al. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J. 25, 3245–3256 (2006).
Schermelleh, L., Heintzmann, R. & Leonhardt, H. A guide to super-resolution fluorescence microscopy. J. Cell Biol. 190, 165–175.
Hell, S. W. Far-field optical nanoscopy. Science 316, 1153–1158 (2007).
Shroff, H., Galbraith, C. G., Galbraith, J. A. & Betzig, E. Live-cell photoactivated localization microscopy of nanoscale adhesion dynamics. Nature Meth. 5, 417–423 (2008).
Rust, M. J., Bates, M. & Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nature Meth. 3, 793–795 (2006).
Eggeling, C. et al. Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457, 1159–1162 (2009). A super-resolution microscopy study showing that sphingolipids and GPI-anchored proteins are transiently trapped in cholesterol-dependent molecular complexes in live cells.
van Zanten, T. S., Cambi, A. & Garcia-Parajo, M. F. A nanometer scale optical view on the compartmentalization of cell membranes. Biochim. Biophys. Acta 1798, 777–787 (2010).
Zhong, L. et al. NSOM/QD-based direct visualization of CD3-induced and CD28-enhanced nanospatial coclustering of TCR and coreceptor in nanodomains in T cell activation. PLoS ONE 4, e5945 (2009).
Lasserre, R. et al. Raft nanodomains contribute to Akt/PKB plasma membrane recruitment and activation. Nature Chem. Biol. 4, 538–547 (2008).
Wawrezinieck, L., Rigneault, H., Marguet, D. & Lenne, P. F. Fluorescence correlation spectroscopy diffusion laws to probe the submicron cell membrane organization. Biophys. J. 89, 4029–4042 (2005).
Wenger, J. et al. Diffusion analysis within single nanometric apertures reveals the ultrafine cell membrane organization. Biophys. J. 92, 913–919 (2007).
Sahl, S. J., Leutenegger, M., Hilbert, M., Hell, S. W. & Eggeling, C. Fast molecular tracking maps nanoscale dynamics of plasma membrane lipids. Proc. Natl Acad. Sci. USA 107, 6829–6834 (2010).
Shevchenko, A. & Simons, K. Lipidomics: coming to grips with lipid diversity. Nature Rev. Mol. Cell Biol. 11, 593–598 (2010).
Dennis, E. A. Lipidomics joins the omics evolution. Proc. Natl Acad. Sci. USA 106, 2089–2090 (2009).
Zech, T. et al. Accumulation of raft lipids in T-cell plasma membrane domains engaged in TCR signalling. EMBO J. 28, 466–476 (2009). The first lipidomic analysis of raft clusters in activated TCR domains.
Klemm, R. W. et al. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J. Cell Biol. 185, 601–612 (2009). The first direct experimental support for a post-Golgi raft pathway that transports proteins towards the plasma membrane.
Brugger, B. et al. The HIV lipidome: a raft with an unusual composition. Proc. Natl Acad. Sci. USA 103, 2641–2646 (2006).
Feigenson, G. W. Phase behavior of lipid mixtures. Nature Chem. Biol. 2, 560–563 (2006).
Jorgensen, K. & Mouritsen, O. G. Phase separation dynamics and lateral organization of two-component lipid membranes. Biophys. J. 69, 942–954 (1995).
Kahya, N. & Schwille, P. Fluorescence correlation studies of lipid domains in model membranes. Mol. Membr. Biol. 23, 29–39 (2006).
Johnston, L. J. Nanoscale imaging of domains in supported lipid membranes. Langmuir 23, 5886–5895 (2007).
Elson, E. L., Fried, E., Dolbow, J. E. & Genin, G. M. Phase separation in biological membranes: integration of theory and experiment. Annu. Rev. Biophys. 39, 207–226 (2010).
Dietrich, C. et al. Lipid rafts reconstituted in model membranes. Biophys. J. 80, 1417–1428 (2001).
Hammond, A. T. et al. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc. Natl Acad. Sci. USA 102, 6320–6325 (2005).
Collins, M. D. & Keller, S. L. Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc. Natl Acad. Sci. USA 105, 124–128 (2008).
Simons, K. & Vaz, W. L. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33, 269–295 (2004).
Baumgart, T. et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc. Natl Acad. Sci. USA 104, 3165–3170 (2007). Shows the presence of liquid-ordered-like and liquid-disordered-like phases in plasma membrane vesicles containing native lipids and proteins.
Levental, I. et al. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem. J. 424, 163–167 (2009).
Lingwood, D., Ries, J., Schwille, P. & Simons, K. Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc. Natl Acad. Sci. USA 105, 10005–10010 (2008). Shows that inflated plasma membranes can separate into large-scale domains at 37°C following cholera toxin cross-linking.
Kaiser, H. J. et al. Order of lipid phases in model and plasma membranes. Proc. Natl Acad. Sci. USA 106, 16645–16650 (2009).
Lingwood, D. & Simons, K. Lipid rafts as a membrane-organizing principle. Science 327, 46–50 (2010).
Kholodenko, B. N., Hancock, J. F. & Kolch, W. Signalling ballet in space and time. Nature Rev. Mol. Cell Biol. 11, 414–426 (2010).
Hancock, J. F. Lipid rafts: contentious only from simplistic standpoints. Nature Rev. Mol. Cell Biol. 7, 456–462 (2006).
Stefanová, I., Horejsí, V., Ansotegui, I. J., Knapp, W. & Stockinger, H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science 254, 1016–1019 (1991).
Seminario, M. C. & Bunnell, S. C. Signal initiation in T-cell receptor microclusters. Immunol. Rev. 221, 90–106 (2008).
Campi, G., Varma, R. & Dustin, M. L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J. Exp. Med. 202, 1031–1036 (2005).
Kaizuka, Y., Douglass, A. D., Varma, R., Dustin, M. L. & Vale, R. D. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc. Natl Acad. Sci. USA 104, 20296–20301 (2007).
Kaizuka, Y., Douglass, A. D., Vardhana, S., Dustin, M. L. & Vale, R. D. The coreceptor CD2 uses plasma membrane microdomains to transduce signals in T cells. J. Cell Biol. 185, 521–534 (2009).
Douglass, A. D. & Vale, R. D. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell 121, 937–950 (2005).
Davis, M. et al. T cells as a self-referential, sensory organ. Annu. Rev. Immunol. 25, 681–695 (2007).
Huang, J. et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature 464, 932–936 (2010).
Huppa, J. et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature 463, 963–967 (2010).
Dunne, P. D. et al. DySCo: quantitating associations of membrane proteins using two-color single-molecule tracking. Biophys. J. 97, L5–L7 (2009).
James, J. R. et al. Single-molecule level analysis of the subunit composition of the T cell receptor on live T cells. Proc. Natl Acad. Sci. USA 104, 17662–17667 (2007).
Lillemeier, B., Pfeiffer, J. R., Surviladze, Z., Wilson, B. S. & Davis, M. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc. Natl Acad. Sci. USA 103, 18992–18997 (2006).
Lillemeier, B. et al. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nature Immunol. 11, 90–96 (2010).
van der Merwe, P. A., Dunne, P. D., Klenerman, D. & Davis, S. J. Taking T cells beyond the diffraction limit. Nature Immunol. 11, 51–52 (2010).
Pralle, A., Keller, P., Florin, E. L., Simons, K. & Horber, J. K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1008 (2000).
Nika, K. et al. Constitutively active Lck kinase in T cells drives antigen receptor signal transduction. Immunity 32, 766–777 (2010).
Anderson, R. G. & Jacobson, K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science 296, 1821–1825 (2002).
Suzuki, K. G. et al. GPI-anchored receptor clusters transiently recruit Lyn and G α for temporary cluster immobilization and Lyn activation: single-molecule tracking study 1. J. Cell Biol. 177, 717–730 (2007). This paper introduced the STALL concept of GPI-anchored protein signalling, which shows that clusters undergoing STALL generate short-lived, digital-like signalling bursts.
Waheed, A. A. & Freed, E. O. Lipids and membrane microdomains in HIV-1 replication. Virus Res. 143, 162–176 (2009).
Scheiffele, P., Rietveld, A., Wilk, T. & Simons, K. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274, 2038–2044 (1999).
Grassme, H., Riethmuller, J. & Gulbins, E. Biological aspects of ceramide-enriched membrane domains. Prog. Lipid Res. 46, 161–170 (2007).
Chan, R. et al. Retroviruses human immunodeficiency virus and murine leukemia virus are enriched in phosphoinositides. J. Virol. 82, 11228–11238 (2008).
Lorizate, M. et al. Probing HIV-1 membrane liquid order by Laurdan staining reveals producer cell-dependent differences. J. Biol. Chem. 284, 22238–22247 (2009).
McLaughlin, S., Wang, J., Gambhir, A. & Murray, D. PIP2 and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 (2002).
Tong, J. et al. Role of GAP-43 in sequestering phosphatidylinositol 4,5-bisphosphate to raft bilayers. Biophys. J. 94, 125–133 (2008).
Saad, J. S. et al. Structural basis for targeting HIV-1 Gag proteins to the plasma membrane for virus assembly. Proc. Natl Acad. Sci. USA 103, 11364–11369 (2006).
Wassall, S. R. & Stillwell, W. Polyunsaturated fatty acid-cholesterol interactions: domain formation in membranes. Biochim. Biophys. Acta 1788, 24–32 (2009).
Castillon, G. A., Watanabe, R., Taylor, M., Schwabe, T. M. & Riezman, H. Concentration of GPI-anchored proteins upon ER exit in yeast. Traffic 10, 186–200 (2009).
Fujita, M., Umemura, M., Yoko, O. T. & Jigami, Y. PER1 Is required for GPI-phospholipase A2 activity and involved in lipid remodeling of GPI-anchored proteins. Mol. Biol. Cell 17, 5253–5264 (2006).
Fujita, M., Yoko, O. T. & Jigami, Y. Inositol deacylation by Bst1p is required for the quality control of glycosylphosphatidylinositol-anchored proteins. Mol. Biol. Cell 17, 834–850 (2006).
Kajiwara, K. et al. Yeast ARV1 is required for efficient delivery of an early GPI intermediate to the first mannosyltransferase during GPI assembly and controls lipid flow from the endoplasmic reticulum. Mol. Biol. Cell 19, 2069–2082 (2008).
Barz, W. P. & Walter, P. Two endoplasmic reticulum (ER) membrane proteins that facilitate ER-to-Golgi transport of glycosylphosphatidylinositol-anchored proteins. Mol. Biol. Cell 10, 1043–1059 (1999).
Watanabe, R., Funato, K., Venkataraman, K., Futerman, A. H. & Riezman, H. Sphingolipids are required for the stable membrane association of glycosylphosphatidylinositol-anchored proteins in yeast. J. Biol. Chem. 277, 49538–49544 (2002).
Simons, K. & van Meer, G. Lipid sorting in epithelial cells. Biochemistry 27, 6197–6202 (1988).
Harsay, E. & Bretscher, A. Parallel secretory pathways to the cell surface in yeast. J. Cell Biol. 131, 297–310 (1995).
Proszynski, T. J. et al. A genome-wide visual screen reveals a role for sphingolipids and ergosterol in cell surface delivery in yeast. Proc. Natl Acad. Sci. USA 102, 17981–17986 (2005).
Ejsing, C. et al. Global analysis of the yeast lipidome by quantitative shotgun mass spectrometry. Proc. Natl Acad. Sci. USA 106, 2136–2141 (2009).
Schuck, S. & Simons, K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J. Cell Sci. 117, 5955–5964 (2004).
Klose, C. et al. Yeast lipids can phase separate into micrometer-scale membrane domains. J. Biol. Chem. 20 Jul 2010 (doi: 10.1074/jbc.M110.123554).
Mercer, J., Schelhaas, M. & Helenius, A. Virus entry by endocytosis. Ann. Rev. Biochem. 79, 803–833 (2010).
Sandvig, K. et al. Pathways followed by protein toxins into cells. Int. J. Med. Microbiol. 293, 483–490 (2004).
Ling, H. et al. Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 37, 1777–1788 (1998).
Romer, W. et al. Shiga toxin induces tubular membrane invaginations for its uptake into cells. Nature 450, 670–675 (2007). Shows that Shiga toxin can induce a lipid reorganization in plasma membranes that forms energy-independent endocytic tubules in cells.
Reynwar, B. J. et al. Aggregation and vesiculation of membrane proteins by curvature-mediated interactions. Nature 447, 461–464 (2007).
Windschiegl, B. et al. Lipid reorganization induced by Shiga toxin clustering on planar membranes. PLoS ONE 4, e6238 (2009).
Romer, W. et al. Actin dynamics drive membrane reorganization and scission in clathrin-independent endocytosis. Cell 140, 540–553 (2010).
Ewers, H. et al. GM1 structure determines SV40-induced membrane invagination and infection. Nature Cell Biol. 12, 11–18 (2010).
Bhagatji, P., Leventis, R., Comeau, J., Refaei, M. & Silvius, J. R. Steric and not structure-specific factors dictate the endocytic mechanism of glycosylphosphatidylinositol-anchored proteins. J. Cell Biol. 186, 615–628 (2009).
Sharpe, H. J., Stevens, T. J. & Munro, S. A comprehensive comparison of transmembrane domains reveals organelle-specific properties. Cell 142, 158–169.
Dupuy, A. D. & Engelman, D. M. Protein area occupancy at the center of the red blood cell membrane. Proc. Natl Acad. Sci. USA 105, 2848–2852 (2008).
Niemelä, P. S. et al. Membrane proteins diffuse as dynamic complexes with lipids. J. Am. Chem. Soc. 132, 7574–7575 (2010).
Devaux, P. F. & Morris, R. Transmembrane asymmetry and lateral domains in biological membranes. Traffic 5, 241–246 (2004).
Chen, Y., Veracini, L., Benistant, C. & Jacobson, K. The transmembrane protein CBP plays a role in transiently anchoring small clusters of Thy-1, a GPI-anchored protein, to the cytoskeleton. J. Cell Sci. 122, 3966–3972 (2009).
Suzuki, K. G., Fujiwara, T. K., Edidin, M. & Kusumi, A. Dynamic recruitment of phospholipase C γ at transiently immobilized GPI-anchored receptor clusters induces IP3-Ca2+ signaling: single-molecule tracking study 2. J. Cell Biol. 177, 731–742 (2007).
Goswami, D. et al. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 135, 1085–1097 (2008).
Andrews, N. L. et al. Actin restricts FcɛRI diffusion and facilitates antigen-induced receptor immobilization. Nature Cell Biol. 10, 955–963 (2008).
Viola, A. & Gupta, N. Tether and trap: regulation of membrane-raft dynamics by actin-binding proteins. Nature Rev. Immunol. 7, 889–896 (2007).
Gupta, N. et al. Quantitative proteomic analysis of B cell lipid rafts reveals that ezrin regulates antigen receptor-mediated lipid raft dynamics. Nature Immunol. 7, 625–633 (2006).
Meder, D., Moreno, M. J., Verkade, P., Vaz, W. L. & Simons, K. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc. Natl Acad. Sci. USA 103, 329–334 (2006).
Danielsen, E. M. & Hansen, G. H. Lipid rafts in epithelial brush borders: atypical membrane microdomains with specialized functions. Biochim. Biophys. Acta 1617, 1–9 (2003).
Simons, M. & Trotter, J. Wrapping it up: the cell biology of myelination. Curr. Opin. Neurobiol. 17, 533–540 (2007).
Veatch, S. L., Soubias, O., Keller, S. L. & Gawrisch, K. Critical fluctuations in domain-forming lipid mixtures. Proc. Natl Acad. Sci. USA 104, 17650–17655 (2007).
Honerkamp-Smith, A. R., Veatch, S. L. & Keller, S. L. An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim. Biophys. Acta 1788, 53–63 (2009).
Veatch, S. L. et al. Critical fluctuations in plasma membrane vesicles. ACS Chem. Biol. 3, 287–293 (2008). The first observation of critical behaviour in plasma membranes, suggesting that nanoscale domains form under physiological conditions.
Johnson, S. A. et al. Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles. Biochim. Biophys. Acta 1798, 1427–1435 (2010).
Ejsing, C. S. et al. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal. Chem. 78, 6202–6214 (2006).
Ivanchenko, S. et al. Dynamics of HIV-1 assembly and release. PLoS Pathog. 5, e1000652 (2009).
Nguyen, D. H. & Hildreth, J. E. Evidence for budding of human immunodeficiency virus type 1 selectively from glycolipid-enriched membrane lipid rafts. J. Virol. 74, 3264–3272 (2000).
Acknowledgements
We thank H. He for critically reading the manuscript and the members of the K.S. laboratory for input, especially D. Lingwood and I. Levental. Work in the K.S. laboratory was supported by the EUFP6 PRISM grant LSHB-CT2007–037,740, DFG Schwerpunktprogramm1175, the BMBF BioChance Plus grant 0313,827 and the BMBF ForMaT grant 03FO1212.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
Kai Simons is a co-founder of the biotechnology company JADO Technologies, which specializes in membrane invention technologies, including lipid raft modulation.
Related links
Glossary
- Caveola
-
A 50–80-nm, flask-shaped pit that forms in the plasma membrane and is enriched in caveolins, cavins, sphingolipids and cholesterol.
- Förster resonance energy transfer
-
A fluorescence-based method for detecting interactions between fluorophores that are <10 nm apart. It is dependent on the spectral overlap between donor and acceptor chromophores and uses non-radiative energy transfer from an excited donor molecule to excite an acceptor molecule.
- Fluorescence polarization anisotropy
-
A technique to measure rotational diffusion using changes in fluorescence polarization that are due to fluorophore rotation.
- GPI-anchored protein
-
(Glycosyl phosphatidylinositol-anchored protein). One of a class of proteins that become post-translationally linked to GPI in the lumen of the ER.
- Total internal reflection fluorescence (TIRF) microscopy
-
An optical technique based on evanescent wave illumination (∼150 nm into the sample) that is created by a totally internally reflected beam at the glass–water interface.
- Quantum dot
-
A nanoscale semiconductor crystal used as a label in fluorescence microscopy owing to its high emission and photostability.
- Fluorescence correlation spectroscopy
-
A technique that measures diffusion by correlating the fluorescence signal of a diffusing fluorophore with time.
- Stimulated emission depletion
-
A nanoscopic technique that uses a red-shifted beam to deplete the emission of the periphery of the excitation spot and create a smaller excitation region, thus overcoming the diffraction limit.
- PALM and STORM
-
(Photoactivated localization microscopy and stochastic optical reconstruction microscopy). Super-resolution microscopy techniques that use stochastically photoactivated fluorescent probes to reconstitute the full image from individual point spread functions.
- Near-field scanning optical microscopy
-
A super-resolution technique that exploits the evanescent wave near the surface of the sample by placing the detector close to the sample.
- Glycosphingolipid
-
A lipid that contains at least one sugar residue and a ceramide (N-acylated sphingoid).
- Palmitoylation
-
The reversible covalent attachment of fatty acids to Cys residues of membrane proteins, which promotes their membrane association.
- Major histocompatibility complex
-
A complex of genetic loci in higher vertebrates that encodes a family of cellular antigens that allow the immune system to recognize self from non-self.
- Lipid shell
-
A model proposing that specific membrane proteins bind to complexes of cholesterol and sphingolipids or laterally organize specific contacting lipids.
- STALL
-
(Stimulation-induced temporary arrest of lateral diffusion). The cholesterol-assisted, sub-second trapping of GPI-anchored signalling proteins with downstream signalling proteins.
- Myristate
-
A group that is attached to a protein through an amide bond by the irreversible, co-translational process of myristoylation. This is an important modification for membrane targeting.
- Shiga toxin
-
One of a family of protein toxins produced by bacteria that can cause dysentery.
- Line energy
-
The energy arising from the unfavourable interaction or 'tension' of two phase- segregating membrane domains. It is the product of line tension and interaction length.
Rights and permissions
About this article
Cite this article
Simons, K., Gerl, M. Revitalizing membrane rafts: new tools and insights. Nat Rev Mol Cell Biol 11, 688–699 (2010). https://doi.org/10.1038/nrm2977
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrm2977
This article is cited by
-
Choline supplementation mitigates effects of bilirubin in cerebellar granule neurons in vitro
Pediatric Research (2024)
-
Single-molecule localization microscopy reveals STING clustering at the trans-Golgi network through palmitoylation-dependent accumulation of cholesterol
Nature Communications (2024)
-
Lipids as mediators of cancer progression and metastasis
Nature Cancer (2024)
-
Lipid Raft Facilitated Receptor Organization and Signaling: A Functional Rheostat in Embryonic Development, Stem Cell Biology and Cancer
Stem Cell Reviews and Reports (2023)
-
Fluorescent GD2 analog for single-molecule imaging
Glycoconjugate Journal (2023)