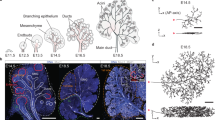
Key Points
-
The tracheal system of Drosophila melanogaster is one of the best characterized multicellular branched organs. Branchless (BNL), a fibroblast growth factor (FGF) ligand, initiates the branching process by triggering cell migration, and functions at the top of a hierarchy of processes that orchestrate branching (for example, branch initiation, branch extension, cell competition, cell intercalation and cell determination).
-
Recent studies have unravelled unexpected similarities in cellular behaviour between tracheal branching in D. melanogaster and angiogenic sprouting in vertebrates. Vascular endothelial growth factor A (VEGFA), encoded by one of the four Vegf genes in mammals, is key to most of the morphogenetic events during angiogenesis that control migration, proliferation and survival of endothelial cells.
-
Tracheal system and vasculature development are conceptually different from lung and kidney development. The lung and kidney occupy a defined volume in an organism and the branching process is essentially limited to a 'bag' of mesenchymal tissue. Lung and kidney branching is controlled by various reciprocal feedback interactions between the branching epithelium and the surrounding mesenchyme. FGF and glial cell-derived neurotrophic factor (GDNF) in lung and kidney, respectively, are specifically expressed by the stroma in regions that prefigure branch outgrowth.
-
Mammary epithelial branching is also regulated by various signals expressed by the epithelium or the stroma, including bone morphogenetic protein (BMP), Wnt and epidermal growth factor (EGF) proteins. Moreover, hormonal control has an important role in mammary gland branching. However, in sharp contrast to the other branching processes, no signal has been identified that is specifically expressed by the stroma in regions that prefigure branch outgrowth. The mammary gland branching process therefore seems to be stochastic.
-
Growing branches are polarized through the establishment of a tip and a stalk. In the fly tracheal system and the vertebrate vasculature, a few cells or a single cell take up the lead position and are followed by stalk cells. Epithelial cells compete for leading positions. The cell interactions that determine the tip and stalk structures depend on Notch-dependent lateral inhibition at the single cell level.
-
In lung, kidney and mammary gland development, the cellular complexity is much higher than in the fly trachea and vertebrate vasculature as the branching tip is composed of many cells, which makes it unlikely that the Notch pathway is involved in the segregation of tip and stalk cells. Cell proliferation is a major factor contributing to elongation and branching in these complex systems.
Abstract
Branched structures are evident at all levels of organization in living organisms. Many organs, such as the vascular system, lung, kidney and mammary gland, are heavily branched. In each of these cases, equally fascinating questions have been put forward, including those that address the cellular and molecular mechanisms that regulate the branching process itself, such as where the branches are initiated and how they extend and grow in the right direction. Recent experiments suggest that cell competition and cell rearrangements might be conserved key features in branch formation and might be controlled by local cell signalling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bryant, D. M. & Mostov, K. E. From cells to organs: building polarized tissue. Nature Rev. Mol. Cell Biol. 9, 887–901 (2008).
Chung, S. & Andrew, D. J. The formation of epithelial tubes. J. Cell Sci. 121, 3501–3504 (2008).
Hogan, B. L. M. & Kolodziej, P. A. Organogenesis: molecular mechanisms of tubulogenesis. Nature Rev. Genet. 3, 513–523 (2002).
Lecuit, T. & Goff, L. L. Orchestrating size and shape during morphogenesis. Nature 450, 189–192 (2007).
Lubarsky, B. & Krasnow, M. A. Tube morphogenesis: making and shaping biological tubes. Cell 112, 19–28 (2003).
Affolter, M. & Caussinus, E. Tracheal branching morphogenesis in Drosophila: new insights into cell behaviour and organ architecture. Development 135, 2055–2064 (2008).
Ghabrial, A., Luschnig, S., Metzstein, M. M. & Krasnow, M. A. Branching morphogenesis of the Drosophila tracheal system. Annu. Rev. Cell Dev. Biol. 19, 623–647 (2003).
Uv, A., Cantera, R. & Samakovlis, C. Drosophila tracheal morphogenesis: intricate cellular solutions to basic plumbing problems. Trends Cell Biol. 13, 301–309 (2003).
Klämbt, C., Glazer, L. & Shilo, B. Z. breathless, a Drosophila FGF receptor homolog, is essential for migration of tracheal and specific midline glial cells. Genes Dev. 6, 1668–1678 (1992).
Sutherland, D., Samakovlis, C. & Krasnow, M. A. branchless encodes a Drosophila FGF homolog that controls tracheal cell migration and the pattern of branching. Cell 87, 1091–1101 (1996). A description of the D. melanogaster gene Bnl . BNL functions as a ligand for Breathless (an FGF receptor expressed on developing tracheal cells), is required for tracheal branching and is expressed dynamically in clusters of cells surrounding the tracheal system.
Ghabrial, A. S. & Krasnow, M. A. Social interactions among epithelial cells during tracheal branching morphogenesis. Nature 441, 746–749 (2006). Shows that cell competition creates two distinct classes of cells in developing D. melanogaster tracheal branches. Cells with the highest FGFR activity are at the tip, whereas those with less FGFR activity form the branch stalk.
Ribeiro, C., Neumann, M. & Affolter, M. Genetic control of cell intercalation during tracheal morphogenesis in Drosophila. Curr. Biol. 14, 2197–2207 (2004).
Caussinus, E., Colombelli, J. & Affolter, M. Tip-cell migration controls stalk-cell intercalation during Drosophila tracheal tube elongation. Curr. Biol. 18, 1727–1734 (2008). Identifies the major forces that contribute to D. melanogaster tracheal branch remodelling. One or two leading cells produce enough mechanical power to intercalate many lagging cells.
Jarecki, J., Johnson, E. & Krasnow, M. A. Oxygen regulation of airway branching in Drosophila is mediated by branchless FGF. Cell 99, 211–220 (1999).
Centanin, L. et al. Cell autonomy of HIF effects in Drosophila: tracheal cells sense hypoxia and induce terminal branch sprouting. Dev. Cell 14, 547–558 (2008).
Brodu, V. & Casanova, J. The RhoGAP crossveinless-c links trachealess and EGFR signaling to cell shape remodeling in Drosophila tracheal invagination. Genes Dev. 20, 1817–1828 (2006).
Englund, C., Steneberg, P., Falileeva, L., Xylourgidis, N. & Samakovlis, C. Attractive and repulsive functions of Slit are mediated by different receptors in the Drosophila trachea. Development 129, 4941–4951 (2002).
Kato, K., Chihara, T. & Hayashi, S. Hedgehog and Decapentaplegic instruct polarized growth of cell extensions in the Drosophila trachea. Development 131, 5253–5261 (2004).
Llimargas, M. & Casanova, J. EGF signalling regulates cell invagination as well as cell migration during formation of tracheal system in Drosophila. Dev. Genes Evol. 209, 174–179 (1999).
Vincent, S. et al. DPP controls tracheal cell migration along the dorsoventral body axis of the Drosophila embryo. Development 124, 2741–2750 (1997).
Dickson, B. J. & Gilestro, G. F. Regulation of commissural axon pathfinding by slit and its Robo receptors. Annu. Rev. Cell Dev. Biol. 22, 651–675 (2006).
Leung, D. W., Cachianes, G., Kuang, W. J., Goeddel, D. V. & Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246, 1306–1309 (1989).
Coultas, L., Chawengsaksophak, K. & Rossant, J. Endothelial cells and VEGF in vascular development. Nature 438, 937–945 (2005).
Ferrara, N., Gerber, H.-P. & LeCouter, J. The biology of VEGF and its receptors. Nature Med. 9, 669–676 (2003).
Lohela, M., Bry, M., Tammela, T. & Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell Biol. 21, 154–165 (2009).
Ruhrberg, C. Growing and shaping the vascular tree: multiple roles for VEGF. Bioessays 25, 1052–1060 (2003).
Gerhardt, H. et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 (2003).
Gerhardt, H. VEGF and endothelial guidance in angiogenic sprouting. Organogenesis 4, 241–246 (2008).
Hellström, M. et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445, 776–780 (2007).
Leslie, J. D. et al. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development 134, 839–844 (2007).
Liu, Z.-J. et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol. Cell. Biol. 23, 14–25 (2003).
Shutter, J. R. et al. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 14, 1313–1318 (2000).
Siekmann, A. F. & Lawson, N. D. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature 445, 781–784 (2007).
Williams, C. K., Li, J.-L., Murga, M., Harris, A. L. & Tosato, G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood 107, 931–939 (2006).
Dufraine, J., Funahashi, Y. & Kitajewski, J. Notch signaling regulates tumor angiogenesis by diverse mechanisms. Oncogene 27, 5132–5137 (2008).
Roca, C. & Adams, R. H. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 21, 2511–2524 (2007).
Fraisl, P., Mazzone, M., Schmidt, T. & Carmeliet, P. Regulation of angiogenesis by oxygen and metabolism. Dev. Cell 16, 167–179 (2009).
Carmeliet, P. & Tessier-Lavigne, M. Common mechanisms of nerve and blood vessel wiring. Nature 436, 193–200 (2005).
Larrivée, B., Freitas, C., Suchting, S., Brunet, I. & Eichmann, A. Guidance of vascular development: lessons from the nervous system. Circ. Res. 104, 428–441 (2009).
Metzger, R. J., Klein, O. D., Martin, G. R. & Krasnow, M. A. The branching programme of mouse lung development. Nature 453, 745–750 (2008). Reconstructs the complete 3D branching pattern and lineage of the mouse bronchial tree, up to the pseudoglandular stage, which turns out to be remarkably stereotyped.
Ackerman, K. G. et al. Gata4 is necessary for normal pulmonary lobar development. Am. J. Respir. Cell Mol. Biol. 36, 391–397 (2007).
Cardoso, W. V. & Lü, J. Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611–1624 (2006).
Horowitz, A. & Simons, M. Branching morphogenesis. Circ. Res. 103, 784–795 (2008).
Bellusci, S., Grindley, J., Emoto, H., Itoh, N. & Hogan, B. L. Fibroblast growth factor 10 (FGF10) and branching morphogenesis in the embryonic mouse lung. Development 124, 4867–4878 (1997).
Moerlooze, L. D. et al. An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal–epithelial signalling during mouse organogenesis. Development 127, 483–492 (2000).
Sekine, K. et al. Fgf10 is essential for limb and lung formation. Nature Genet. 21, 138–141 (1999).
Mailleux, A. A. et al. Evidence that SPROUTY2 functions as an inhibitor of mouse embryonic lung growth and morphogenesis. Mech. Dev. 102, 81–94 (2001).
Tefft, J. D. et al. Conserved function of mSpry-2, a murine homolog of Drosophila sprouty, which negatively modulates respiratory organogenesis. Curr. Biol. 9, 219–222 (1999).
Bellusci, S., Henderson, R., Winnier, G., Oikawa, T. & Hogan, B. L. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development 122, 1693–1702 (1996).
Eblaghie, M. C., Reedy, M., Oliver, T., Mishina, Y. & Hogan, B. L. Evidence that autocrine signaling through Bmpr1a regulates the proliferation, survival and morphogenetic behavior of distal lung epithelial cells. Dev. Biol. 291, 67–82 (2006).
Lebeche, D., Malpel, S. & Cardoso, W. V. Fibroblast growth factor interactions in the developing lung. Mech. Dev. 86, 125–136 (1999).
Chuang, P.-T., Kawcak, T. N. & McMahon, A. P. Feedback control of mammalian Hedgehog signaling by the Hedgehog-binding protein, Hip1, modulates Fgf signaling during branching morphogenesis of the lung. Genes Dev. 17, 342–347 (2003).
Liu, Y. et al. Novel role for Netrins in regulating epithelial behavior during lung branching morphogenesis. Curr. Biol. 14, 897–905 (2004).
Mucenski, M. L. et al. β-Catenin is required for specification of proximal/distal cell fate during lung morphogenesis. J. Biol. Chem. 278, 40231–40238 (2003).
Shu, W. et al. Wnt/β-catenin signaling acts upstream of N-myc, BMP4, and FGF signaling to regulate proximal–distal patterning in the lung. Dev. Biol. 283, 226–239 (2005).
Dressler, G. R. The cellular basis of kidney development. Annu. Rev. Cell Dev. Biol. 22, 509–529 (2006).
Kopan, R., Cheng, H.-T. & Surendran, K. Molecular insights into segmentation along the proximal–distal axis of the nephron. J. Am. Soc. Nephrol 18, 2014–2020 (2007).
Quaggin, S. E. & Kreidberg, J. A. Development of the renal glomerulus: good neighbors and good fences. Development 135, 609–620 (2008).
Costantini, F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation 74, 402–421 (2006).
Watanabe, T. & Costantini, F. Real-time analysis of ureteric bud branching morphogenesis in vitro. Dev. Biol. 271, 98–108 (2004).
al Awqati, Q. & Goldberg, M. R. Architectural patterns in branching morphogenesis in the kidney. Kidney Int. 54, 1832–1842 (1998).
Costantini, F. & Shakya, R. GDNF/Ret signaling and the development of the kidney. Bioessays 28, 117–127 (2006).
Shakya, R., Watanabe, T. & Costantini, F. The role of GDNF/Ret signaling in ureteric bud cell fate and branching morphogenesis. Dev. Cell 8, 65–74 (2005).
Chi, X. et al. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev. Cell 17, 199–209 (2009).
Shakya, R. et al. The role of GDNF in patterning the excretory system. Dev. Biol. 283, 70–84 (2005). Shows the contribution of RET-dependent cell movements and RET-independent epithelial transitions in the Wolffian duct in the formation of the ureteric bud.
Grieshammer, U. et al. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev. Cell 6, 709–717 (2004).
Basson, M. A. et al. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev. Cell 8, 229–239 (2005).
Majumdar, A., Vainio, S., Kispert, A., McMahon, J. & McMahon, A. P. Wnt11 and Ret/Gdnf pathways cooperate in regulating ureteric branching during metanephric kidney development. Development 130, 3175–3185 (2003).
Michos, O. et al. Reduction of BMP4 activity by gremlin 1 enables ureteric bud outgrowth and GDNF/WNT11 feedback signalling during kidney branching morphogenesis. Development 134, 2397–2405 (2007).
Michos, O. et al. Gremlin-mediated BMP antagonism induces the epithelial–mesenchymal feedback signaling controlling metanephric kidney and limb organogenesis. Development 131, 3401–3410 (2004).
Miyazaki, Y., Oshima, K., Fogo, A., Hogan, B. L. & Ichikawa, I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J. Clin. Invest. 105, 863–873 (2000).
Ohuchi, H. et al. FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643–649 (2000).
Qiao, J. et al. FGF-7 modulates ureteric bud growth and nephron number in the developing kidney. Development 126, 547–554 (1999).
Zhao, H. et al. Role of fibroblast growth factor receptors 1 and 2 in the ureteric bud. Dev. Biol. 276, 403–415 (2004).
Bénazet, J.-D. et al. A self-regulatory system of interlinked signaling feedback loops controls mouse limb patterning. Science 323, 1050–1053 (2009). An interesting example of epithelial–mesenchymal feedback loops between SHH and FGF signalling, involving the BMP antagonist gremlin 1. This self-regulatory signalling network results in the robust regulation of mouse distal limb development.
Chu, E. Y. et al. Canonical WNT signaling promotes mammary placode development and is essential for initiation of mammary gland morphogenesis. Development 131, 4819–4829 (2004).
Veltmaat, J. M. et al. Gli3-mediated somitic Fgf10 expression gradients are required for the induction and patterning of mammary epithelium along the embryonic axes. Development 133, 2325–2335 (2006).
Hinck, L. & Silberstein, G. B. Key stages in mammary gland development: the mammary end bud as a motile organ. Breast Cancer Res. 7, 245–251 (2005).
Sternlicht, M. D., Kouros-Mehr, H., Lu, P. & Werb, Z. Hormonal and local control of mammary branching morphogenesis. Differentiation 74, 365–381 (2006).
Watson, C. J. & Khaled, W. T. Mammary development in the embryo and adult: a journey of morphogenesis and commitment. Development 135, 995–1003 (2008).
Hens, J. R. et al. BMP4 and PTHrP interact to stimulate ductal outgrowth during embryonic mammary development and to inhibit hair follicle induction. Development 134, 1221–1230 (2007).
Hens, J. R. & Wysolmerski, J. J. Key stages of mammary gland development: molecular mechanisms involved in the formation of the embryonic mammary gland. Breast Cancer Res. 7, 220–224 (2005).
Moraes, R. C. et al. Constitutive activation of smoothened (SMO) in mammary glands of transgenic mice leads to increased proliferation, altered differentiation and ductal dysplasia. Development 134, 1231–1242 (2007).
Bocchinfuso, W. P. et al. Induction of mammary gland development in estrogen receptor-α knockout mice. Endocrinology 141, 2982–2994 (2000).
Brisken, C. et al. A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl Acad. Sci. USA 95, 5076–5081 (1998).
Feng, Y., Manka, D., Wagner, K.-U. & Khan, S. A. Estrogen receptor-α expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proc. Natl Acad. Sci. USA 104, 14718–14723 (2007).
Mallepell, S., Krust, A., Chambon, P. & Brisken, C. Paracrine signaling through the epithelial estrogen receptor α is required for proliferation and morphogenesis in the mammary gland. Proc. Natl Acad. Sci. USA 103, 2196–2201 (2006).
Silberstein, G. B. Postnatal mammary gland morphogenesis. Microsc. Res. Tech. 52, 155–162 (2001).
Nelson, C. M., Vanduijn, M. M., Inman, J. L., Fletcher, D. A. & Bissell, M. J. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 314, 298–300 (2006). Shows that the geometry of mammary tubules dictates the position of branches. Mammary branches initiate at sites with a local minimum concentration of autocrine inhibitory morphogens, such as TGFβ.
Sagasti, A., Guido, M. R., Raible, D. W. & Schier, A. F. Repulsive interactions shape the morphologies and functional arrangement of zebrafish peripheral sensory arbors. Current biology 15, 804–814 (2005).
Daniel, C. W., Robinson, S. & Silberstein, G. B. The role of TGF-β in patterning and growth of the mammary ductal tree. J. Mammary Gland Biol. Neoplasia 1, 331–341 (1996).
Ewan, K. B. et al. Latent transforming growth factor-β activation in mammary gland: regulation by ovarian hormones affects ductal and alveolar proliferation. Am. J. Pathol. 160, 2081–2093 (2002).
Ewald, A. J., Brenot, A., Duong, M., Chan, B. S. & Werb, Z. Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570–581 (2008). Reports that mammary gland branching results from the active motility of both luminal and myoepithelial cells. Luminal epithelial cells advance collectively, whereas myoepithelial cells seem to restrain elongating ducts.
Parsa, S. et al. Terminal end bud maintenance in mammary gland is dependent upon FGFR2b signaling. Dev. Biol. 317, 121–131 (2008).
Lu, P., Ewald, A. J., Martin, G. R. & Werb, Z. Genetic mosaic analysis reveals FGF receptor 2 function in terminal end buds during mammary gland branching morphogenesis. Dev. Biol. 321, 77–87 (2008).
Lecuit, T. & Lenne, P.-F. Cell surface mechanics and the control of cell shape, tissue patterns and morphogenesis. Nature Rev. Mol. Cell Biol. 8, 633–644 (2007).
Farhadifar, R., Röper, J.-C., Aigouy, B., Eaton, S. & Jülicher, F. The influence of cell mechanics, cell–cell interactions, and proliferation on epithelial packing. Curr. Biol. 17, 2095–2104 (2007).
Rauzi, M., Verant, P., Lecuit, T. & Lenne, P.-F. Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nature Cell Biol. 10, 1401–1410 (2008).
Desprat, N., Supatto, W., Pouille, P.-A., Beaurepaire, E. & Farge, E. Tissue deformation modulates twist expression to determine anterior midgut differentiation in Drosophila embryos. Dev. Cell 15, 470–477 (2008).
Colombelli, J. et al. Mechanosensing in actin stress fibers revealed by a close correlation between force and protein localization. J. Cell Sci. 122, 1665–1679 (2009).
Hutson, M. S. et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 300, 145–149 (2003).
Kiehart, D. P., Galbraith, C. G., Edwards, K. A., Rickoll, W. L. & Montague, R. A. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J. Cell Biol. 149, 471–490 (2000).
Vogel, A. & Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 103, 577–644 (2003).
Solon, J., Kaya-Copur, A., Colombelli, J. & Brunner, D. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137, 1331–1342 (2009).
Bénazet, J. D. & Zeller, R. Vertebrate limb development: moving from classical morphogen gradients to an integrated 4D patterning system. Cold Spring Harb. Perspect. Biol. 1 a001339 (2009).
Scherz, P. J., Harfe, B. D., McMahon, A. P. & Tabin, C. J. The limb bud Shh–Fgf feedback loop is terminated by expansion of former ZPA cells. Science 305, 396–399 (2004).
Verheyden, J. M. & Sun, X. An Fgf/Gremlin inhibitory feedback loop triggers termination of limb bud outgrowth. Nature 454, 638–641 (2008). An interesting example of a self-promoting and self-terminating circuit that might be used to attain proper tissue size in a range of developmental and regenerative settings.
Smet, I. D. & Jürgens, G. Patterning the axis in plants — auxin in control. Curr. Opin. Genet. Dev. 17, 337–343 (2007).
Bayer, E. M. et al. Integration of transport-based models for phyllotaxis and midvein formation. Genes Dev. 23, 373–384 (2009).
Kuhlemeier, C. Phyllotaxis. Trends Plant Sci. 12, 143–150 (2007).
Reinhardt, D. et al. Regulation of phyllotaxis by polar auxin transport. Nature 426, 255–260 (2003).
Smith, R. S. et al. A plausible model of phyllotaxis. Proc. Natl Acad. Sci. USA 103, 1301–1306 (2006).
Acknowledgements
We thank H. Gerhardt, R. Metzger, Z. Werb, F. Costantini, C. Kuehlemeier and R. Smith for stimulating discussions. We apologize for not being able to cite all relevant primary publications because of space restrictions.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Endothelial tissue
-
A tissue made up of flattened endothelial cells that forms the interior surface of blood vessels.
- E-cadherin
-
The major Ca2+-dependent cell–cell adhesion molecule involved in the establishment of embryonic epithelium and in ensuring that epithelial cells remain bound together.
- Ectoderm
-
The epithelium that covers the body surface of the early embryo.
- Actin–myosin filaments
-
The parallel arrangement of actin and myosin filaments. Using the hydrolysis of ATP, myosin can make the two types of filament slide on each other to shorten the structure as a whole.
- Tracheal sac
-
A cluster of about 80 ectodermal cells that have invaginated within the embryo and formed an epithelial sac.
- Filopodium
-
A thin, dynamic cytoplasmic projection covered with cell membrane that extends from the leading edge of migrating cells. Filopodia contain actin filament bundles and are presumably involved in exploring the cell environment.
- Intercalation
-
The process during which cells insert between cells that are already in contact with each other.
- Chemoattractant
-
A molecule with a chemotaxis-inducing effect on motile cells, which migrate towards its source.
- Hypoxia
-
The lack of an adequate oxygen supply to an area of the body.
- Angiogenic sprouting
-
The growth of new blood vessels from pre-existing vessels.
- Astrocyte
-
A star-shaped cell that provides support and protection for neurons in the central nervous system.
- Intersegmental vessel
-
A vessel that carries blood from the dorsal aorta between somites to the dorsal side of the neural tube.
- Axonal growth cone
-
The motile extension of a developing axon. Axonal growth cones use external cues to guide their movements.
- Apical ectodermal ridge
-
The thickening of the ectoderm rim at the tip of a developing limb bud in a vertebrate embryo.
Rights and permissions
About this article
Cite this article
Affolter, M., Zeller, R. & Caussinus, E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol 10, 831–842 (2009). https://doi.org/10.1038/nrm2797
Issue Date:
DOI: https://doi.org/10.1038/nrm2797
This article is cited by
-
Toward Measuring the Mechanical Stresses Exerted by Branching Embryonic Airway Epithelial Explants in 3D Matrices of Matrigel
Annals of Biomedical Engineering (2022)
-
The miR-200 family in normal mammary gland development
BMC Developmental Biology (2021)
-
Programmed and self-organized flow of information during morphogenesis
Nature Reviews Molecular Cell Biology (2021)
-
Image-based modeling of kidney branching morphogenesis reveals GDNF-RET based Turing-type mechanism and pattern-modulating WNT11 feedback
Nature Communications (2019)
-
Anxa4 mediated airway progenitor cell migration promotes distal epithelial cell fate specification
Scientific Reports (2018)