Key Points
-
ATP-binding cassette (ABC) transporters constitute a ubiquitous superfamily of integral membrane proteins that are responsible for the ATP-powered translocation of many substrates across membranes.
-
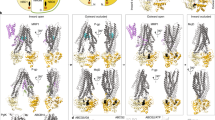
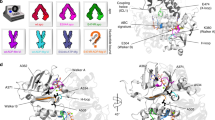
ABC transporters have a characteristic architecture that consists minimally of four domains: two ABC domains (or nucleotide-binding domains) with highly conserved sequence motifs and two transmembrane domains (TMDs). Additional domains can be fused to these core elements to confer regulatory functions, and a periplasmic binding protein is required for ligand delivery to prokaryotic importer members of this family.
-
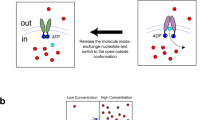
Similarities in the structure of the ABC domains structure support a common mechanism by which ABC transporters, both importers and exporters, orchestrate a series of nucleotide- and substrate-dependent conformational changes that result in substrate translocation across the membrane through an 'alternating-access'-type model. As ABC domains are also integrated into non-transport systems, including proteins that are involved in DNA repair and chromosome maintenance, it is likely that a common set of ATP-dependent conformational changes are relevant to all of these processes.
-
The conformation of ABC transporters that is catalytically competent for nucleotide hydrolysis involves the binding of an ATP molecule between conserved sequence motifs at the interface between the two ABC domains. As a transporter cycles through the different stages of nucleotide binding and hydrolysis, the interface between the two ABC domains switches from the closed state, which is characteristic of ATP binding, to a more open conformation, which is associated with non-ATP states.
-
TMDs are structurally heterogeneous, and three distinct sets of folds have been recognized. Although the detailed folds vary, they all interact with the helical domains of the ABCs through 'coupling helices', which are located in the loops between membrane-spanning helices. These interactions connect the conformations of the TMDs to the nucleotide state of the ABC domains.
-
The activity of ABC transporters can be regulated at the level of protein function through the actions of domains that are fused to the ABCs and/or TMDs. Recent structural studies have established that trans-inhibition, in which the uptake of an extracellular substrate is inhibited by increasing intracellular concentrations of that species, can involve ligand binding to the carboxy-terminal domains of the ABCs that sterically separate these domains and prevent their association, which is required for ATP hydrolysis.
-
Although idealized kinetic models can be described that qualitatively highlight aspects of the transport mechanism, an important goal is to develop quantitative models that detail the kinetic and molecular mechanisms by which ABC transporters couple the binding and hydrolysis of ATP to substrate translocation.
Abstract
ATP-binding cassette (ABC) transporters constitute a ubiquitous superfamily of integral membrane proteins that are responsible for the ATP-powered translocation of many substrates across membranes. The highly conserved ABC domains of ABC transporters provide the nucleotide-dependent engine that drives transport. By contrast, the transmembrane domains that create the translocation pathway are more variable. Recent structural advances with prokaryotic ABC transporters have provided a qualitative molecular framework for deciphering the transport cycle. An important goal is to develop quantitative models that detail the kinetic and molecular mechanisms by which ABC transporters couple the binding and hydrolysis of ATP to substrate translocation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Monod, J. Recherches sur la Croissance des Cultures Bactériennes (Hermann, Editeurs des Sciences et des Arts, Paris, 1942).
Phillips, R. & Quake, S. R. The biological frontier of physics. Phys. Today 59, 38–43 (2006).
Blattner, F. R. et al. The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462 (1997).
Busch, W. & Saier, M. H. Jr. The transporter classification (TC) system. Crit. Rev. Biochem. Mol. Biol. 27, 287–337 (2002).
Stouthamer, A. H. The search for correlation between theoretical and experimental growth yields. Int. Rev. Biochem. 21, 1–47 (1979).
Neijsel, O. M., Teixeira de Mattos, M. J. & Tempest, D. W. in Escherichia coli and Salmonella: Cellular and Molecular Biology (ed. Neidhardt, F. C.) 1283–1692 (ASM, Washington DC, 1996).
Skou, J. C. The identification of the sodium–potassium pump (Nobel lecture). Angew. Chem. Int. Edn Eng. 37, 2320–2328 (1998).
Higgins, C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992).
Ames, G. F., Mimura, C. S., Holbrook, S. R. & Shyamala, V. Traffic ATPases: a superfamily of transport proteins operating from Escherichia coli to humans. Adv. Enzymol. Relat. Areas Mol. Biol. 65, 1–47 (1992).
Holland, I. B., Cole, S. P. C., Kuchler, K. & Higgins, C. F. (eds) ABC proteins: From Bacteria to Man (Academic, London, 2003).
Linton, K. J. & Higgins, C. F. The Escherichia coli ATP-binding cassette (ABC) proteins. Mol. Microbiol. 28, 5–13 (1998).
Dean, M., Rzhetsky, A. & Allikmets, R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 11, 1156–1166 (2001).
Dean, M. & Annilo, T. Evolution of the ATP-binding cassette (ABC) transporter superfamily in vertebrates. Annu. Rev. Genomics Hum. Genet. 6, 123–142 (2005).
Biemans-Oldehinkel, E., Deoven, M. K. & Poolman, B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 580, 1023–1035 (2006).
Ames, G. F. L. Bacterial periplasmic transport systems — structure, mechanism and evolution. Annu. Rev. Biochem. 55, 397–425 (1986).
Heppel, L. A. The effect of osmotic shock on release of bacterial proteins and on active transport. J. Gen. Physiol. 54, 95–113 (1969).
Locher, K. P., Lee, A. T. & Rees, D. C. The E. coli BtuCD structure: a framework for ABC transporter architecture and mechanism. Science 296, 1091–1098 (2002). The first crystal structure of an intact ABC transporter, the vitamin B12 importer BtuCD.
Dawson, R. J. P. & Locher, K. P. Structure of a bacterial multidrug ABC transporter. Nature 443, 180–185 (2006). The first crystal structure of an ABC exporter, the bacterial multidrug transporter Sav1866.
Dawson, R. J. P. & Locher, K. P. Structure of the multidrug ABC transporter Sav1866 from Staphylococcus aureus in complex with AMP-PNP. FEBS Lett. 581, 935–938 (2007).
Pinkett, H. W., Lee, A. T., Lum, P., Locher, K. P. & Rees, D. C. An inward-facing conformation of a putative metal-chelate type ABC transporter. Science 315, 373–377 (2007). The crystal structure of a BtuCD homologue exhibiting an inward-facing conformation.
Hvorup, R. N. et al. Asymmetry in the structure of the ABC transporter binding protein complex BtuCD–BtuF. Science 317, 1387–1390 (2007). The crystal structure of BtuCD complexed to the binding protein BtuF adopts an asymmetric conformation around the translocation pathway.
Hollenstein, K., Frei, D. C. & Locher, K. P. Structure of an ABC transporter in complex with its binding protein. Nature 446, 213–216 (2007). The first structure of a complex between an ABC transporter, ModBC, and its associated binding protein, ModA.
Oldham, M. L., Khare, D., Quiocho, F. A., Davidson, A. L. & Chen, J. Crystal structure of a catalytic intermediate of the maltose transporter. Nature 450, 515–522 (2007). The structure of the maltose ABC transporter–binding protein complex in the ATP-bound state with a maltose trapped in its translocation pathway.
Ward, A., Reyes, C. L., Yu, J., Roth, C. B. & Chang, G. Flexibility in the ABC transporter MsbA: alternating access with a twist. Proc. Natl Acad. Sci. USA 104, 19005–19010 (2007). Structures of the bacterial multidrug transporter homologue MsbA in different conformational states.
Gerber, S., Comellas-Bigler, M., Goetz, B. A. & Locher, K. P. Structural basis of trans-inhibition in a molybdate/tungstate ABC transporter. Science 321, 246–250 (2008). The binding of molybdate and tungstate to regulatory domains of the ABC subunits stabilizes an inward-facing, ATPase-inactive conformation of a ModBC transporter that underllies the phenomenon of trans -inhibition.
Kadaba, N. S., Kaiser, J. T., Johnson, E., Lee, A. & Rees, D. C. The high-affinity E. coli methionine ABC transporter: structure and allosteric regulation. Science 321, 250–253 (2008). The binding of Met to regulatory domains of the ABC subunits stabilizes an inward-facing, ATPase-inactive conformation of a MetNI transporter underlying the phenomenon of trans -inhibition.
Hollenstein, K., Dawson, R. J. P. & Locher, K. P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 17, 412–418 (2007).
Linton, K. J. Structure and function of ABC transporters. Physiology (Bethesda) 22, 122–130 (2007).
Davidson, A. L., Dassa, E., Orelle, C. & Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 72, 317–364 (2008).
Davidson, A. L. & Maloney, P. C. ABC transporters: how small molecules do a big job. Trends Microbiol. 15, 448–455 (2007).
Dawson, R. J. P., Hollenstein, K. & Locher, K. P. Uptake or extrusion: crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 65, 250–257 (2007).
Oldham, M. L., Davidson, A. L. & Chen, J. Structural insights into ABC transporter mechanism. Curr. Opin. Struct. Biol. 18, 726–733 (2008).
Locher, K. P. Structure and mechanism of ATP-binding cassette transporters. Phil. Trans. R. Soc. Lond. B 364, 239–245 (2008).
Schmitt, L., Benabdelhak, H., Blight, M. A., Holland, I. B. & Stubbs, M. T. Crystal structure of the nucleotide-binding domain of the ABC-transporter haemolysin B: identification of a variable region within ABC helical domains. J. Mol. Biol. 330, 333–342 (2003).
Karpowich, N. et al. Crystal structures of the MJ1267 ATP binding cassette reveal an induced-fit effect at the ATPase active site of an ABC transporter. Structure 9, 571–586 (2001).
Jones, P. M. & George, A. M. Subunit interactions in ABC transporters: towards a functional architecture. FEMS Microbiol. Lett. 179, 187–202 (1999).
Hopfner, K.-P. et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell 101, 789–800 (2000).
Smith, P. C. et al. ATP binding to the motor domain from an ABC transporter drives formation of a nucleotide sandwich dimer. Mol. Cell 10, 139–149 (2002).
Saurin, W., Koster, W. & Dassa, E. Bacterial binding protein-dependent permeases — characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol. Microbiol. 12, 993–1004 (1994).
Mourez, M., Hofnung, N. & Dassa, E. Subunit interactions in ABC transporters: a conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 16, 3066–3077 (1997).
Jones, P. M. & George, A. M. Mechanism of ABC transporters: a molecular dynamics simulation of a well characterized nucleotide-binding subunit. Proc. Natl Acad. Sci. USA 99, 12639–12644 (2002).
Dassa, E. & Bouige, E. The ABC of ABCs: a phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 152, 211–229 (2001).
Steward, J. B. & Hermodson, M. A. Topology of RbsC, the membrane component of the Escherichia coli ribose transporter. J. Bacteriol. 185, 5234–5239 (2003).
Fukami-Kobayashi, K., Tateno, Y. & Nishikawa, K. Domain dislocation: a change of core structure in periplasmic binding proteins in their evolutionary history. J. Mol. Biol. 286, 279–290 (1999).
Quiocho, F. A. & Ledvina, P. Atomic structure and specificity of bacterial periplasmic receptors for active transport and chemotaxis: variations of common themes. Mol. Microbiol. 20, 17–25 (1996).
Wilkinson, A. J. & Verschueren, K. H. G. in ABC Proteins: From Bacteria to Man (eds Holland, I. B. et al.) 647 (Academic, London, 2003).
Widdas, W. F. Inability of diffusion to account for placental glucose transfer in the sheep and consideration of the kinetics of a possible carrier transfer. J. Physiol. 118, 23–39 (1952).
Jardetsky, O. Simple allosteric model for membrane pumps. Nature 211, 969–970 (1966).
Poolman, B. et al. Functional analysis of detergent solubilized and membrane-reconstituted ABC transporters. Methods Enzymol. 400, 429–459 (2005).
Patzlaff, J. S., van der Heide, T. & Poolman, B. The ATP/substrate stoichiometry of the ATP-binding cassette (ABC) transporter OpuA. J. Biol. Chem. 278, 29546–29551 (2003). A careful measurement of the ATP-to-translocated ligand ratio for the tightly coupled OpuA transporter.
Davidson, A. L. & Nikaido, H. Overproduction, solubilization, and reconstitution of the maltose transport system from Escherichia coli. J. Biol. Chem. 265, 4254–4260 (1990).
Chen, J., Sharma, S., Quiocho, F. A. & Davidson, A. L. Trapping the transition state of an ATP-binding cassette transporter: evidence for a concerted mechanism of maltose transport. Proc. Natl Acad. Sci. USA 98, 1525–1530 (2001). ATP–vanadate is used to trap a complex between the maltose transporter and the binding protein.
Borths, E. L., Poolman, B., Hvorup, R. N., Locher, K. P. & Rees, D. C. In vitro functional characterization of BtuCD-F, the Escherichia coli ABC transporters for vitamin B12 uptake. Biochemistry 44, 16301–16309 (2005).
Nikaido, K. & Ames, G. F. L. One intact ATP-binding subunit is sufficient to support ATP hydrolysis and translocation in an ABC transporter, the histidine permease. J. Biol. Chem. 274, 26727–26735 (1999).
Walker, J. E., Saraste, M., Runswick, M. J. & Gay, N. J. Distantly related sequences in the α- and β-subunits of ATP–synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1, 945–951 (1982).
Maegley, K. A., Admiraal, S. J. & Herschlag, D. Ras-catalyzed hydrolysis of GTP: a new perspective from model studies. Proc. Natl Acad. Sci. USA 93, 8160–8166 (1996).
Matte, A., Tari, L. W. & Delbaere, L. T. J. How do kinases transfer phosphoryl groups? Structure 6, 413–419 (1998).
Geourjon, C. et al. A common mechanism for ATP hydrolysis in ABC transporter and helicase superfamilies. Trends Biochem. Sci. 25, 539–544 (2001).
Moody, J. E., Millen., L., Binns, D., Hunt, J. F. & Thomas, P. J. Cooperative, ATP-dependent association of the nucleotide binding casettes during the catalytic cycle of ATP-binding cassette transporters. J. Biol. Chem. 277, 21111–21114 (2002).
Zaitseva, J. et al. H662 is the linchpin of ATP hydrolysis in the nucleotide-binding domain of the ABC transporter HlyB. EMBO J. 24, 1901–1910 (2005).
Menz, R. I., Walker, J. E. & Leslie, A. G. W. Structure of bovine mitochondrial F1-ATPase with nucleotide bound to all three catalytic sites: implications for the mechanism of rotary catalysis. Cell 106, 331–341 (2001).
Schindelin, H., Kisker, C., Schlessman, J. L., Howard, J. B. & Rees, D. C. Structure of ADP–AlF4-stabilized nitrogenase complex and its implications for signal transduction. Nature 387, 370–376 (1997).
Leipe, D. D., Koonin, E. V. & Aravind, L. Evolution and classification of P-loop kinases and related proteins. J. Mol. Biol. 333, 781–815 (2003).
Erzberger, J. P. & Berger, J. M. Evolutionary relationships and structural mechanisms of AAA+ proteins. Annu. Rev. Biophys. Biomol. Struct. 35, 93–114 (2006).
Scheffzek, K. et al. The Ras–RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 (1997).
Enemark, E. J. & Joshua-Tor, L. On helicases and other motor proteins. Curr. Opin. Struct. Biol. 18, 243–257 (2008).
Wittinghofer, A. Signaling mechanistics: aluminum fluoride for molecule of the year. Curr. Biol. 7, R682–R685 (1997).
Rees, D. C. & Howard, J. B. Structural bioenergetics and energy transduction mechanisms. J. Mol. Biol. 293, 343–350 (1999).
Chen, J., Lu, G., Lin, J., Davidson, A. L. & Quiocho, F. A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 12, 651–661 (2003).
Orelle, C., Ayvaz, T., Everly, R. M., Klug, C. S. & Davidson, A. L. Both maltose-binding protein and ATP are required for nucleotide-binding domain closure in the intact maltose ABC transporter. Proc. Natl Acad. Sci. USA 105, 12837–12842 (2008).
Goetz, B. A., Perozo, E. & Locher, K. P. Distinct gate conformations of the ABC transporter BtuCD revealed by electron spin resonance spectroscopy and chemical cross-linking. FEBS Lett. 583, 266–270 (2009).
Kadner, R. J. Regulation of methionine transport activity in Escherichia coli. J. Bacteriol. 122, 110–119 (1975). An exquisite in vivo analysis of the trans -inhibition of Met transport activity by intracellular Met.
Jacquet, E. et al. ATP hydrolysis and pristinamycin IIA inhibition of the Staphylococcus aureus Vga(A), a dual ABC protein involved in streptogramin A resistance. J. Biol. Chem. 283, 25332–25339 (2008).
Hopfner, K. P. & Tainer, J. A. Rad50/SMC proteins and ABC transporters: unifying concepts from high-resolution structures. Curr. Opin. Struct. Biol. 13, 249–255 (2003).
Ambudkar, S. V., Kimchi-Sarfaty, C., Sauna, Z. E. & Gottesman, M. M. P-glycoprotein: from genomics to mechanism. Oncogene 22, 7468–7485 (2003).
Higgins, C. F. Multiple molecular mechanisms for multidrug resistance transporters. Nature 446, 749–757 (2007).
Kraulis, P. J. MOLSCRIPT: a program to produce both detailed and schematic plots of protein structures. J. Appl. Crystallogr. 24, 946–950 (1991).
Merritt, E. A. & Murphy, M. E. P. Raster3D Version 2.0 — a program for photorealistic molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 50, 869–873 (1994).
Berman, H. M. et al. The Protein Data Bank. Nucleic Acids Res. 28, 235–242 (2000).
Schmid, B. et al. Biochemical and structural characterization of the crosslinked complex of nitrogenase: comparison to the ADP–AlF4-stabilized structure. Biochemistry 41, 15557–15565 (2002).
Acknowledgements
We thank A.T. Lee and J.B. Howard for discussions and their long-term contributions to our research in this area. The support of the Fulbright Foundation and Jane Coffin Childs Memorial Funds for Medical Research (O.L.) and National Institutes of Health grant GM45162 (D.C.R) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Glossary
- ABC
-
(ATP-binding cassette). A set of conserved sequence motifs that defines an eponymous family of ATPases. ABC is often used interchangeably with nucleotide-binding domain (NBD).
- BtuCD
-
The vitamin B12 uptake system that is composed of the integral membrane protein subunit BtuC and the ATP-binding cassette subunit BtuD.
- Sav1866
-
A bacterial ATP-binding cassette exporter from Staphylococcus aureus that is homologous to eukaryotic multidrug resistance transporters.
- MalFGK2
-
A maltose transporter, which is composed of two homologous integral membrane subunits — MalF and MalG — and two copies of the ATP-binding cassette subunit MalK.
- F1-ATPase
-
A stable, water-soluble subassembly of the membrane-bound ATP synthase, of which the active site for ATP synthesis and hydrolysis is located at the interface between homologous α- and β-subunits.
- Nitrogenase
-
The enzyme system that catalyses the ATP-dependent reduction of dinitrogen to ammonia during the process of biological nitrogen fixation. The nitrogenase Fe-protein contains the binding site for ATP.
- G protein
-
One of a family of GTP-binding proteins that are involved in signal transduction, in which GTP binding and hydrolysis and subsequent nucleotide exchange drive a series of conformational changes that are used in signalling cascades.
- AAA+ ATPase
-
(ATPases associated with diverse cellular activities). A large family of structurally conserved ATPases in which assembly of the ATPase active site requires subunit oligomerization.
- MetNI
-
A Met transporter, which is composed of the integral membrane protein MetI and the ATP-binding cassette subunit MetN.
Rights and permissions
About this article
Cite this article
Rees, D., Johnson, E. & Lewinson, O. ABC transporters: the power to change. Nat Rev Mol Cell Biol 10, 218–227 (2009). https://doi.org/10.1038/nrm2646
Issue Date:
DOI: https://doi.org/10.1038/nrm2646
This article is cited by
-
Enhancing coevolutionary signals in protein–protein interaction prediction through clade-wise alignment integration
Scientific Reports (2024)
-
Insights into the molecular mechanisms underlying the different heat tolerance of the scleractinian coral Pavona decussata
Coral Reefs (2024)
-
Genome-wide transcriptional response of Escherichia coli O157:H7 to light-emitting diodes with various wavelengths
Scientific Reports (2023)
-
Structural remodelling of the carbon–phosphorus lyase machinery by a dual ABC ATPase
Nature Communications (2023)
-
Whole-genome sequencing and comparative genomic analysis of a pathogenic Enterocytozoon hepatopenaei strain isolated from Litopenaeus vannamei
Aquaculture International (2023)